Abstract
Background
Provider-initiated HIV testing and counselling (PITC) is promoted as a means to increase HIV case finding. We assessed the effectiveness of PITC to increase HIV testing rate and HIV case finding among outpatients in Rwandan health facilities (HF).
Methods
PITC was introduced in six HFs in 2009-2010. HIV testing rate and case finding were compared between phase 1 (pre-PITC) and phase 3 (PITC period) for outpatient-department (OPD) attendees only, and for OPD and voluntary counseling & testing (VCT) departments combined.
Results
Out of 26,367 adult OPD attendees in phase 1, 4.7 % were tested and out of 29,864 attendees in phase 3, 17.0 % were tested (p < 0.001). The proportion of HIV cases diagnosed was 0.25 % (67/26,367) in phase 1 and 0.46 % (136/29864) in phase 3 (p < 0.001). In multivariable analysis, both testing rate and case finding were significantly higher in phase 3 for OPD attendees. In phase 1 most of the HIV testing was done in VCT departments rather than at the OPD (78.6 % vs 21.4 % respectively); in phase 3 this was reversed (40.0 % vs 60.0 %; p < 0.001). In a combined analysis of VCT and OPD attendees, testing rate increased from 18.7 % in phase 1 to 25.4 % in phase 3, but case finding did not increase. In multivariable analysis, testing rate was significantly higher in phase 3 (OR 1.67; 95 % CI 1.60-1.73), but case finding remained stable (OR 1.09; 95 % CI 0.93-1.27).
Conclusion
PITC led to a shift of HIV testing from VCT department to the OPD, a higher testing rate, but no additional HIV case finding.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-016-1355-z) contains supplementary material, which is available to authorized users.
Keywords: HIV testing rate, HIV case finding, PITC, Rwanda
Background
Sub-Saharan Africa has the greatest burden of HIV with 25 million people living with HIV by the end of 2012 [1]. Knowledge of HIV status is imperative for prevention and timely start of HIV care [2–4]. About 30 % of people in sub-Saharan countries have never been tested for HIV [1]. To achieve universal HIV testing, the World Health Organization (WHO) recommends provider initiated testing and counselling (PITC) to facilitate timely diagnosis and access to HIV related services [2]. According to this policy all patients presenting at health facilities (HFs) in generalized HIV epidemics, regardless of signs or symptoms, should be offered an HIV test on an opt-out basis, making it a standard component of medical care [2].
PITC has mainly been implemented in antenatal (ANC) and tuberculosis (TB) clinics in sub-Saharan countries, with high overall testing rate levels [5–12]. Studies from several countries (Kenya, Ethiopia, Uganda) have reported high levels of acceptability of PITC, increased HIV testing rate, and linkage to care after HIV diagnosis in outpatient departments (OPDs) [13–16]. Rwanda is a country with a generalised epidemic [1], but the adult HIV prevalence (3 %) [17] is low compared to that of most sub-Saharan African countries. Rwanda has a dense network of health facilities that offer HIV testing and antiretroviral treatment, and the coverage of cART (80 %) is higher than in most countries in the region [18].
The Rwanda Ministry of Health (MOH) adopted PITC as a policy to increase the opportunity for HIV testing and ensure timely HIV diagnosis among HF attendees. We implemented PITC in 2009/2010 and assessed whether PITC was an effective strategy to increase HIV testing rate and HIV case finding in outpatient departments of six Rwandan HFs. In order to examine whether PITC led to a shift from testing at voluntary counseling and testing (VCT) clinics to testing at OPDs, we also collected data on testing at VCT departments.
Methods
Setting
Four HFs in Musanze district (North-West Rwanda) and four HFs in Gasabo district (Central Rwanda; area of the capital) were purposefully selected for this study, ensuring inclusion of urban and rural HFs with sufficient numbers of attendees. All included HFs had a complete range of HIV testing, care and treatment services.
Study design
The study consisted of three phases: in phase 1 (routine care period; March-May 2009) PITC was not operational; in phase 2 (preparation phase; June-November 2009) PITC was introduced; and in phase 3 (PITC intervention period; December 2009-February 2010) PITC was operational. PITC was introduced in six HFs while two served as controls.
Intervention
Biomedical materials for PITC were delivered to six HFs. Health care workers (HCWs) were trained to offer PITC, administer HIV testing, and use registers adapted for the study to record testing. In TB and ANC departments PITC was already commonly practiced prior to the start of the study, following national policy. With the exception of Ruhengeri hospital, VCT departments were operational in the other HFs, where attendees could go and be tested on their own accord, or where they could be referred to by health staff from other departments. During phase 3, PITC was newly operational in the OPD. HCWs informed clinic attendees that it was MOH-recommended policy to offer HIV testing to all clients [19]. PITC was offered in three ways. Option 1 involved a rapid test by the HCW using a finger-prick blood sample in the consultation department. Option 2 involved a test on a venous blood sample drawn by the HCW and sent to the laboratory for rapid testing. Option 3 involved the HCW offering the test, and upon consent sending the attendee to the laboratory for a venous blood draw and rapid testing. In all options, HCWs provided post-test counseling. Each option was implemented at two intervention sites (Table 1).
Table 1.
Characteristics of health facilities included in the PITC study, Rwanda 2009-10
| Study site | Province | Level of urbanization | Type | Total attendeesa | Interventionb | Remarks |
|---|---|---|---|---|---|---|
| Rwaza | Northern | Rural | Health center | 7,147 | Option 1 | HF staff started some form of PITC already prior to phase 1 |
| Kinyinya | Kigali City | Urban | Health center | 7,964 | Option 1 | |
| Ruhengeri | Northern | Semi-urban | Hospital | 9,191 | Option 2 | No VCT department at this HF; clients were referred to Muhoza |
| Muhoza | Northern | Semi-urban | Health center | 26,125 | Option 2 | HF located next to Ruhengeri hospital (500 m). |
| Kibagabaga | Kigali City | Urban | Hospital | 6,771 | Option 3 | This hospital was affected by managerial changes during phase 3 |
| Kimironko | Kigali City | Urban | Health center | 13,135 | Option 3 | |
| Gasiza (c) | Northern | Rural | Health center | 7,535 | Control | |
| Kabuye (c) | Kigali City | Semi-urban | Health center | 6,980 | Control | Staff initiated some form of PITC during phase 3 on their own initiative. |
aTotal attendees: people that sought health services in all departments (Antenatal Care, Family Planning, Out-patients department, Tuberculosis department, Voluntary counseling and testing) at the study sites during the study periods, March-May 2009 and December 2009-February 2010
bOption 1 involved a rapid test by the health care worker (HCW) using a finger-prick blood sample in the consultation department. Option 2 involved a test on a venous blood sample drawn by the HCW and sent to the laboratory for rapid testing. Option 3 involved the HCW offering the test, and upon consent sending the attendee to the laboratory for a venous blood draw and rapid testing. In all options, HCWs provided post-test counseling
Abbreviations: HF Health Facility, PITC Provider Initiated testing and Counseling, VCT Voluntary Counseling and testing
HIV testing
Both in the routine care and the intervention period, a serial rapid test algorithm was used to test for HIV. The first test used in the rapid test algorithm was Determine HIV-1/2/O (Abbott Laboratories, Abbott Park, Illinois, USA). If the Determine was negative, no further testing was done and the patient was diagnosed as HIV negative. If positive, the UniGold (Trinity Biotech, Bray, Ireland) test was done. In case Determine and UniGold had discrepant results, a third test, the Capillus HIV-1/HIV-2 (Cambridge Biotech Corp. Worcester, MA, USA), was done. Capillus acted as a tiebreaker in order to reach a final HIV result.
Data abstraction
Trained field workers abstracted data from registers in the laboratory, OPD and VCT using structured forms in phase 1 and phase 3. To enable linkage between registers in different departments in phase 1, some adaptations were made to the registers. During phase 2 registers from the OPD were adapted to capture additional data on PITC implementation. The variables introduced to the existing registers were: HIV test offer, test acceptance, reason for refusal, test result received, history of fever and number of HIV tests done in the preceding two years. To facilitate linkage between registers from different departments, patient reference numbers on stickers were inserted in the registers starting from the initial point of entry into the clinic. The following data were abstracted: age, sex, study site, tested, and test result. Data on test offer, test acceptance, reasons for refusal, receipt of result, history of fever and number of previous HIV tests in preceding 2 years were abstracted only during phase 3 in the OPD.
Outcome measures
Our study had two primary outcomes: HIV testing rate and HIV case finding. HIV testing rate was defined as the proportion of individuals tested for HIV out of the total number of attendees of a department. HIV case finding was defined as the proportion of individuals that were diagnosed with HIV, out of the total number of attendees of a department. Secondary outcome measures were test offer, test acceptance, test result received and reasons for refusing an HIV test during the intervention phase. A “test offer” consisted of explicitly stating to a patient that an HIV test would be done unless he/she declined. “Test acceptance” indicates that the patient consented to be tested for HIV after having been offered a test. “Test result received” means the patient obtained the result of the HIV test after being tested. If clinic attendees declined to be tested for HIV, they were asked for the reason to decline (“reasons for refusal”).
Data management and analysis
Our analyses were limited to attendees aged 15 years or above. The characteristics of the HF attendees were described using percentages, medians and interquartile ranges [IQR]. Multivariable logistic regression models were used to establish whether PITC (i.e. phase 3) was an independent determinant for the two main outcomes, HIV testing rate and HIV case finding, when adjusted for age and sex of attendees, and study site. Robust standard errors were used to take account of clustering effect of site. P values of <0.05 were considered statistically significant. For all analyses Stata version 11.2 was used (Stata Corp; College Station, TX, USA).
Ethics
The Rwanda National Ethics Committee and the research committee of the Academic Medical Center, Amsterdam, provided approval for this study. All patients were aware that as part of clinical care their demographic and clinical data were registered in paper-based clinic registers. As only such routinely collected data were abstracted from clinic registers, and no names were entered into the electronic study database, the ethics committees did not require that written informed consent was sought from patients.
Results
Characteristics of OPD attendees
During phase 1 of the study (March-May 2009), a total of 31,204 adult attendees were registered at the OPDs of the eight HFs included in this study. The majority (64 %) were women, and the median age was 28 years (IQR 22–41). The number of attendees ranged from 1,878 in Kabuye to 10,745 in Muhoza (Additional file 1: Table S1). At the same HFs, 34,512 adult attendees were registered during phase 3 (December 2009-February 2010). The median age of OPD attendees in phase 3 was also 28 years (IQR 22–40) and a similar proportion was female (61 %). Again Kabuye registered the smallest number of attendees (2,137) and Muhoza the largest number (8,147) (Additional file 1: Table S1).
HIV testing rate at OPD
Out of 26,367 attendees in phase 1 at the six intervention sites, 4.7 % (1,234) were tested for HIV. During phase 3, out of 29,864 attendees 17.0 % (5,065) tested for HIV (Table 2, Fig. 1a). The difference in testing rate between the phases was 12.3 % (p < 0.001). Important differences were observed between the sites: in Muhoza and Kimironko testing rate increased with 20.2 % and 15.9 % respectively during phase 3, but in Kibagabaga the testing rate decreased with 7.8 %. Rwaza had the highest testing rate in both phase 1 and phase 3. Here, clinic staff had introduced a form of PITC on their own initiative prior to phase 1.
Table 2.
HIV testing rate and HIV case finding during phase 1 and phase 3 by study site in outpatient departments in eight health facilities, PITC study, Rwanda 2009-2010
| Phase 1 | Phase 3 | Differences between phases 3 and 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study site | N | HIV testing rate n | HIV testing rate % | HIV+ n | HIV+ % (of all tested) | HIV+ % (of all eligible) | N | HIV testing rate n | HIV testing rate % | HIV+ n | HIV+ % (of all tested) | HIV+ % (of all eligible) | Difference in testing rate | Difference in HIV case finding |
| A | B | C = B/A | D | E = D/B | F = D/A | G | H | I = H/G | J | K = J/H | L = J/G | M = I - C | N = L - F | |
| Rwaza | 2,270 | 615 | 27.1 % | 10 | 1.6 % | 0.44 % | 3,654 | 1,255 | 34.3 % | 3 | 0.24 % | 0.08 % | 7.3 % | -0.36 % |
| Kinyinya | 2,584 | 34 | 1.3 % | 6 | 17.6 % | 0.23 % | 3,488 | 311 | 8.9 % | 19 | 6.1 % | 0.54 % | 7.6 % | 0.31 % |
| Ruhengeri | 3,829 | 195 | 5.1 % | 8 | 4.1 % | 0.21 % | 5,259 | 612 | 11.6 % | 11 | 1.8 % | 0.21 % | 6.5 % | 0.00 % |
| Muhoza | 10,745 | 50 | 0.5 % | 6 | 12.0 % | 0.06 % | 8,147 | 1,682 | 20.6 % | 47 | 2.8 % | 0.58 % | 20.2 % | 0.52 % |
| Kibagabaga | 2,131 | 313 | 14.7 % | 30 | 9.6 % | 1.41 % | 3,465 | 240 | 6.9 % | 11 | 4.6 % | 0.32 % | -7.8 % | -1.09 % |
| Kimironko | 4,808 | 27 | 0.6 % | 7 | 25.9 % | 0.15 % | 5,851 | 965 | 16.5 % | 45 | 4.7 % | 0.77 % | 15.9 % | 0.62 % |
| Total | 26,367 | 1,234 | 4.7 % | 67 | 5.4 % | 0.25 % | 29,864 | 5,065 | 17.0 % | 136 | 2.7 % | 0.46 % | 12.3 % | 0.21 % |
| Gasiza (c) | 2,959 | 41 | 1.4 % | 4 | 9.8 % | 0.14 % | 2,511 | 22 | 0.9 % | 4 | 18.2 % | 0.16 % | -0.5 % | 0.02 % |
| Kabuye (c) | 1,878 | 201 | 10.7 % | 5 | 2.5 % | 0.27 % | 2,137 | 605 | 28.3 % | 13 | 2.1 % | 0.61 % | 17.6 % | 0.34 % |
| Total | 4,837 | 242 | 5.0 % | 9 | 3.7 % | 0.19 % | 4,648 | 627 | 13.5 % | 17 | 2.7 % | 0.37 % | 8.5 % | 0.18 % |
HIV + = HIV positive individuals. (c) Control site
Fig. 1.
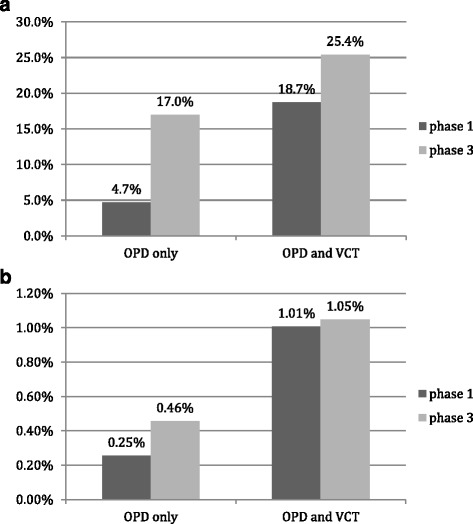
a. HIV testing rate at OPD. b. HIV case finding at OPD
In the two control sites, testing rate increased from 5.0 % to 13.5 % (p < 0.001). One control site (Gasiza) did not experience a change in testing rate, but the other site (Kabuye) experienced an increase in testing rate of 17.6 % (Table 2). In this latter clinic the staff initiated a form of PITC on their own initiative between phase 1 and 3. In multivariable analysis (adjusting for age, sex and site), an HIV test was significantly more likely to be done in phase 3 compared to phase 1 in both the intervention (adjusted Odds Ratio [aOR] 4.57; 95 % Confidence Interval [CI] 4.26–4.90; Table 3) and control sites (aOR 2.76; 95 % CI 2.34–3.24; Additional file 1: Table S2).
Table 3.
Multivariable logistic regression to determine the association of the PITC intervention with HIV testing rate and HIV case finding in the outpatient department of 6 health facilities (upper panel) and in the outpatient and voluntary counseling and testing departments combined of the same 6 health facilities (lower panel); Rwanda, 2009-2010
| HIV testing rate | HIV case finding | |||||||
|---|---|---|---|---|---|---|---|---|
| OPD only | n/N (%) | aOR | (95 % CI) | P | n/N (%) | aOR | (95 % CI) | P |
| Study phase | ||||||||
| Phase 1 | 1,234/26,367 (4.7 %) | 1 | <0.001 | 67/26,367 (0.25 %) | 1 | <0.001 | ||
| Phase 3 | 5,065/29,864 (17 %) | 4.57 | (4.26-4.90) | 136/29,864 (0.46 %) | 1.97 | (1.41-2.76) | ||
| Study site | ||||||||
| Ruhengeri | 807/9,088 (8.9 %) | 1 | <0.001 | 19/9,088 (0.21 %) | 1 | <0.001 | ||
| Muhoza | 1,732/18,892 (9.2 %) | 1.18 | (1.08-1.30) | 53/18,892 (0.28 %) | 1.87 | (1.06-3.31) | ||
| Kibagabaga | 553/5,596 (9.9 %) | 0.79 | (0.69-0.89) | 41/5,596 (0.73 %) | 3.10 | (1.69-5.68) | ||
| Kinyinya | 345/6,072 (5.7 %) | 0.55 | (0.48-0.63) | 25/6,072 (0.41 %) | 2.32 | (1.24-4.35) | ||
| Kimironko | 992/10,659 (9.3 %) | 0.97 | (0.88-1.08) | 52/10,659 (0.49 %) | 3.13 | (1.78-5.51) | ||
| Rwaza | 1,870/5,924 (31.6 %) | 4.65 | (4.21-5.13) | 13/5,924 (0.22 %) | 1.19 | (0.57-2.49) | ||
| Sex | ||||||||
| Females | 3,685/34,610 (10.7 %) | 1 | <0.001 | 111/34,610 (0.32 %) | 1 | 0.078 | ||
| Males | 2,597/21,035 (12.4 %) | 1.19 | (1.13-1.26) | 92/21,035 (0.44 %) | 1.30 | (0.98-1.73) | ||
| Age group (years) | ||||||||
| 15 - 24 | 2,275/19,643 (11.6 %) | 1 | <0.001 | 35/19,643 (0.18 %) | 1 | <0.001 | ||
| 25 - 34 | 1,732/16,687 (10.4 %) | 0.93 | (0.87-1.00) | 65/16,687 (0.39 %) | 2.09 | (1.38-3.16) | ||
| 35 - 44 | 863/7,648 (11.3 %) | 0.92 | (0.84-1.01) | 48/7,648 (0.63 %) | 3.73 | (2.41-5.78) | ||
| ≥45 | 1,270/11,470 (11.1 %) | 0.77 | (0.71-0.83) | 42/11,470 (0.37 %) | 2.38 | (1.52-3.71) | ||
| OPD & VCT combined | n/N (%) | aOR | (95 % CI) | P | n/N (%) | aOR | (95 % CI) | P |
| Study phase | ||||||||
| Phase 1 | 5,779/30,914 (18.7 %) | 1 | <0.001 | 311/30,914 (1.01 %) | 1 | <0.001 | ||
| Phase 3 | 8,442/33,242 (25.4 %) | 1.67 | 1.60-1.73 | 348/33,242 (1.05 %) | 1.09 | 0.93-1.28 | ||
| Study site | ||||||||
| Ruhengeri | 807/9,088 (8.9 %) | 1 | <0.001 | 19/9,088 (0.21 %) | 1 | <0.001 | ||
| Muhoza | 6,673/23,833 (28.0 %) | 3.95 | 3.65-4.28 | 270/23,833 (1.13 %) | 6.26 | 3.78-10.38 | ||
| Kibagabaga | 959/6,004 (16.0 %) | 1.49 | 1.34-1.66 | 104/6,004 (1.73 %) | 8.27 | 4.87-14.06 | ||
| Kinyinya | 1,223/6,950 (17.6 %) | 1.91 | 1.73-2.10 | 94/6,950 (1.35 %) | 7.22 | 4.25-12.28 | ||
| Kimironko | 1,763/11,431 (15.4 %) | 1.58 | 1.44-1.73 | 153/11,431 (1.34 %) | 7.39 | 4.41-12.38 | ||
| Rwaza | 2,796/6,850 (40.8 %) | 7.04 | 6.43-7.70 | 19/6,850 (0.28 %) | 1.57 | 0.81-3.06 | ||
| Sex | 0.417 | |||||||
| Females | 8,242/39,170 (21.0 %) | 1 | <0.001 | 413/39,170 (1.05 %) | 1 | |||
| Males | 5,959/24,397 (24.4 %) | 1.34 | 1.29-1.39 | 246/24,397 (1.01 %) | 0.93 | 0.80-1.10 | ||
| Age group (years) | ||||||||
| 15 - 24 | 5,498/22,867 (24.0 %) | 1 | <0.001 | 157/22,867 (0.69 %) | 1 | <0.001 | ||
| 25 - 34 | 4,728/19,685 (24.0 %) | 1.11 | 1.06-1.16 | 270/19,685 (1.37 %) | 2.02 | 1.65-2.46 | ||
| 35 - 44 | 1,887/8,672 (21.8 %) | 0.91 | 0.85-0.96 | 128/8,672 (1.48 %) | 2.42 | 1.91-3.06 | ||
| ≥45 | 1,933/12,133 (15.9 %) | 0.54 | 0.51-0.58 | 90/12,133 (0.74 %) | 1.34 | 1.03-1.73 | ||
Abbreviations: aOR adjusted Odds ratio, CI confidence interval, OPD outpatient department, VCT voluntary counselling and testing
Upper panel, OPD only data; information on age was missing from 783 attendees and information on sex from 586 attendees
Lower panel, OPD & VCT data combined: information on age was missing from 799 attendees and information on sex from 589 attendees
Adjusted odds ratios are adjusted for the other variables in the Table
During phase 3, out of 29,863 eligible OPD attendees in the intervention sites, 92.9 % (27, 753) were offered an HIV test, 19.6 % of those accepted; of those who accepted 93.3 % were tested, of whom 92.2 % received results (Additional file 1: Figure S1). Among the 27,753 attendees in phase 3 who were offered an HIV test and who declined, a reason for refusal was noted for 5,876 (21.2 %). The most common reasons reported for refusing an HIV test were previous knowledge of HIV status (33.4 %; 1,963/5,876) and lack of interest (25.3 %; 1,486/5,876) (Additional file 1: Figure S2).
HIV case finding at OPD
The proportion of HIV positive attendees identified in phase 1 at the intervention sites was 0.25 % (67/26,367) while it was 0.46 % (136/29,864) during phase 3, an absolute increase of 0.21 % (p < 0.001; Table 2, Fig. 1b). Important differences were seen between sites. Rwaza and Kibagabaga experienced a decrease in HIV case finding. Ruhengeri did not show a change in case finding and Muhoza and Kimironko showed an increase of 0.52 % and 0.62 % respectively. In multivariable analysis (adjusting for age, sex and site), HIV case finding in the intervention sites was significantly higher in phase 3 (aOR 1.97; 95 % CI 1.41–2.76) compared to phase 1 (Table 3).
In the control sites the percentage of HIV positive attendees increased by 0.18 % (from 0.19 % to 0.37 %; p = 0.094). There was no change in Gasiza and an increase of 0.34 % in Kabuye. The aOR for the control sites in phase 3 was 1.85 (95 % CI 0.83–4.12) (Additional file 1: Table S2) compared to phase 1.
Characteristics of OPD and VCT attendees
In a secondary analysis we examined HIV testing rate and case finding in the combined attendee populations of OPD and VCT departments. The VCT departments of the eight health facilities registered 6,541 new clients in phase 1 and 5,129 in phase 3. During phase 1, a total of 37,747 adult attendees were registered at the OPD and VCT departments of the eight HFs (Additional file 1: Table S3). The majority (63 %) were women, and the median age was 28 years (interquartile range [IQR] 22–40). The number of attendees ranged from 2,381 in Kibagabaga to 13,614 in Muhoza. At the same HFs, 39,642 adult attendees were registered during phase 3. The median age of attendees in phase 3 was also 28 years (IQR 22–39) and a similar proportion was female (60 %). Gasiza registered the smallest number of attendees (3,154) and Muhoza the largest number (10,219) (Additional file 1: Table S3).
HIV testing rate at OPD and VCT combined
Out of 30,914 attendees at OPDs and VCT departments in phase 1 at the six intervention sites, 18.7 % (5,779) were tested for HIV; during phase 3, out of 33,242 attendees, 25.4 % (8,442) were tested for HIV (p < 0.001, Table 4, Fig. 1a). In phase 1, 78.6 % of tested attendees (4,545/5779) were tested at the VCT; in phase 3 this had declined to 40.0 % (3,377/8,442; p < 0.001). So there was both a relative and an absolute decline in the number of attendees tested at VCT departments.
Table 4.
HIV testing rate and HIV case finding during phase 1 and 3 by study site in outpatient and voluntary counseling and testing departments in eight health facilities, PITC study, Rwanda 2009-2010
| Phase 1 | Phase 3 | Differences between phases 3 and 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study site | N | HIV testing rate n | HIV testing rate % | HIV + n | HIV+ % (% of testing rate) | HIV+ % (% of eligible) | N | HIV testing rate n | HIV testing rate % | HIV+ n | HIV+ % (% of testing rate) | HIV+ % (% of eligible) | Difference in testing rate | Difference in HIV case finding |
| A | B | C = B/A | D | E = D/B | F = D/A | G | H | I = H/G | J | K = J/H | L = J/G | M = I - C | N = L - F | |
| Rwaza | 2,863 | 1208 | 42.2 % | 14 | 1.2 % | 0.49 % | 3,987 | 1,588 | 39.8 % | 5 | 0.31 % | 0.13 % | -2.4 % | -0.36 % |
| Kinyinya | 3,072 | 522 | 17.0 % | 30 | 5.7 % | 0.98 % | 3,878 | 701 | 18.1 % | 64 | 9.1 % | 1.65 % | 1.1 % | 0.67 % |
| Ruhengeri | 3,829 | 195 | 5.1 % | 8 | 4.1 % | 0.21 % | 5,259 | 612 | 11.6 % | 11 | 1.8 % | 0.21 % | 6.5 % | 0.00 % |
| Muhoza | 13,614 | 2919 | 21.4 % | 128 | 4.4 % | 0.94 % | 10,219 | 3,754 | 36.7 % | 142 | 3.8 % | 1.39 % | 15.3 % | 0.45 % |
| Kibagabaga | 2,381 | 561 | 23.6 % | 72 | 12.8 % | 3.02 % | 3,623 | 398 | 11.0 % | 32 | 8.0 % | 0.88 % | -12.6 % | -2.14 % |
| Kimironko | 5,155 | 374 | 7.3 % | 59 | 15.8 % | 1.14 % | 6,276 | 1389 | 22.1 % | 94 | 6.8 % | 1.50 % | 14.88 % | 0.35 % |
| Total | 30,914 | 5,779 | 18.7 % | 311 | 5.4 % | 1.01 % | 33,242 | 8,442 | 25.4 % | 348 | 4.1 % | 1.05 % | 6.70 % | 0.04 % |
| Gasiza (c) | 3,602 | 684 | 19.0 % | 16 | 2.3 % | 0.44 % | 3,154 | 665 | 21.1 % | 5 | 0.8 % | 0.16 % | 2.09 % | -0.29 % |
| Kabuye (c) | 3,231 | 1554 | 48.1 % | 35 | 2.3 % | 1.08 % | 3,246 | 1714 | 52.8 % | 60 | 3.5 % | 1.85 % | 4.71 % | 0.77 % |
| Total | 6,833 | 2,238 | 32.8 % | 51 | 2.3 % | 0.75 % | 6,400 | 2,379 | 37.2 % | 65 | 2.7 % | 1.02 % | 4.42 % | 0.27 % |
Abbreviations: HIV+: HIV positive individuals, c control
In multivariable analysis (adjusting for age, sex and site), the testing rate was significantly increased in phase 3 in both the intervention (aOR 1.67; 95 % CI 1.60–1.73; Table 3) and control sites (aOR 1.14; 95 % CI 1.06–1.24; Additional file 1: Table S4), compared to the rate in phase 1.
HIV case finding at OPD and VCT combined
The proportion of HIV positive attendees identified in phase 1 at intervention sites was 1.01 % (311/30,914) while it was 1.05 % (348/33,242) during phase 3, an increase of 0.04 % (p = 0.608; Table 4, Fig. 1b). In multivariable analysis (adjusting for age, sex and study site), case finding was not significantly increased in phase 3 in intervention sites (aOR 1.09; 95 % CI 0.93–1.28) compared to phase 1 (Table 3).
In the control sites the percentage of HIV positive attendees increased by 0.27 % (from 0.75 % to 1.02 %; p = 0.094). The aOR for HIV case finding at the control sites in phase 3 was 1.29 (95 % CI 0.89–1.86) compared to phase 1 (Additional file 1: Table S4).
Discussion
Main findings
PITC was associated with a higher HIV testing rate among attendees of OPDs of six Rwandan HFs, but the increase in HIV case finding was limited. The increase in HIV testing at the OPD was accompanied by a decrease in HIV testing at the VCT departments. In a combined analysis of attendees of OPD and VCT departments, PITC was associated with an increase in HIV testing rate but not with additional HIV case finding. The findings from our study may be applicable to other settings with a similar HIV epidemiologic profile, such as a relatively low prevalence and high cART coverage, and thus may inform decision-making in Rwanda and elswhere.
HIV testing rate
The implementation of PITC in the OPD led to a 4.5 fold increase in testing rate during the intervention phase compared to routine care period when studying the OPD only. A combined analysis of the OPD and VCT showed a 1.7 fold increase in testing rate. Our data indicate a shift of testing from VCT to OPD after the introduction of PITC: both the absolute number of HIV tests done at the VCT declined, and the proportion of HIV tests done at the VCT (from 79 % to 40 %). This may indicate that patients who were tested in phase 3 at OPD clinics might have been tested in the VCT department in the absence of PITC. Alternatively, perhaps patients preferred to be tested in the OPD to avoid stigma associated with VCT departments [20]. A study from Zambia found that introducing PITC in nine OPDs directly increased HIV testing rates compared with the number tested under VCT in the same month; so PITC provided an additional testing route rather than replacing VCT [15]. A study from Botswana reported an increase of HIV testing rate following the nationwide introduction of PITC [21]. A study from Gauteng Province, South Africa reported a 2.9 fold increase in HIV testing rate under PITC compared to VCT referral model [16]. Another study from Durban, South Africa, compared a period of standard care to a subsequent period when all patients registered at the OPD were given an educational intervention and offered a rapid HIV test as a routine; both HIV testing rate and HIV case finding increased strongly (5-fold higher weekly HIV case finding) [22]. This report did not mention the impact of the intervention on the total number of VCT clients/tests, and whether a similar shifting phenomenon occurred as in our setting.
Attendees who declined testing during the intervention phase were asked by health care workers for the reasons for refusing an HIV test. In line with Cunningham et al. previous knowledge of HIV status and perceived lack of interest were the most common reasons for refusing an HIV test [23]. Previous knowledge as one of the common reasons for declining may imply that relatively many people were aware of their HIV status. This finding must be seen in the context of results from a recent global report on sub-Saharan African countries, including Rwanda, which indicated that in Rwanda an estimated 39 % of people were tested within the last year and thus are likely to know their HIV status [1]. The Rwandan demographic and health survey reported similar findings (39 % of women and 38 % of men received results from an HIV test taken during the 12 months prior to the survey) [17]. A South African study reported that 31 % declined testing because they were uncomfortable or afraid of an HIV test and 19 % reported not feeling the need to be tested [16]. Although OPD attendees did not incur costs to get tested in our study, a Ugandan study reported that the cost of testing and lack of perceived risk were the reasons attributed to prior lack of testing [24]. Strategies such as intensifying health education for patients that aim to remove misconceptions about HIV are needed [25]. The importance of retesting despite previous negative tests should be emphasized during post-test counseling. Another strategy may be to establish a stigma-free and enabling environment at health facilities by re-arranging the patient flow and fully integrating HIV testing into other services, instead of stand-alone HIV clinics or laboratories.
HIV case finding
Increasing HIV case finding is critical towards achieving universal access to care and treatment services and PITC could be an effective approach [15]. In our study, the absolute difference in HIV case finding between phase 3 and phase 1 was 0.21 % in the OPD, which represents a limited increase. There were important differences seen between sites; some had a limited increase while others experienced a decrease in case finding. In one health facility (Kibagabaga) this decrease was explained by the decrease of HIV testing rate due to management changes at this site. In another facility (Rwaza) this decrease might be explained by the already high HIV testing rate in phase 1. The analysis of the combined VCT and OPD populations demonstrated that PITC was not significantly associated with increased HIV case finding. In Zambia, the integration of PITC into routine OPD care substantially increased case finding of HIV positive patients [15, 26]. Systematic reviews have demonstrated the importance of PITC in identifying undiagnosed HIV infections [27, 28]. Studies have also reported other benefits of routine HIV testing for identification of previously undiagnosed HIV positive cases, i.e. increased knowledge of HIV status and reduction of risk behavior [14, 22, 29, 30]. So our data are different from findings from other published studies regarding the effect of PITC on HIV case finding. The differences in HIV prevalence and the level of HIV services delivery may explain this discrepancy. Rwanda has a relatively low prevalence [17], a decreasing trend of new infections and a high ART coverage (above 80 %) compared to the countries in which other PITC studies were done [18]. PITC led to an increase in the HIV testing rate, but not to an increase in case finding. This would suggest that in these settings PITC at general OPDs is not contributing to reaching the 90-90-90 goals.
Limitations
Our study had some limitations. The study was conducted in eight clinics that were purposely chosen to be different in several respects (urban-rural, relatively high vs. relatively low HIV prevalence, small versus very large clinics). Rwanda is a relatively small country with rather similar levels of health services provision across the nation, albeit it with some variation between urban and rural settings. Therefore we believe the results may be applied to national scale. We implemented a before-and-after-intervention study design; this lacks the strengths of a randomized clinical trial and therefore the study results should be interpreted with caution. Although we introduced and operationalized PITC, the implementation was not fully rigorous; health workers reported that they had offered an HIV test to 93 % of the attendees and that 19 % of these accepted the test. The variation between sites in characteristics and result did not allow to draw conclusions regarding the three different testing options. Phase 3, during which PITC was implemented was a different season than phase 1; this may have affected the composition of the group of attendees, and hence testing history and willingness to be tested. However, seasonal effects have a larger impact on the composition of pediatric clinic populations than on that of adult clinic populations. As our study population was limited to those aged 15 years or above, we think seasonal effects have not played a large role in the observed differences.
Conclusions
PITC led to an increase in HIV testing rate and a limited additional HIV case finding among OPD attendees at intervention sites compared with the routine care period. Previous knowledge of HIV status and perceived lack of interest were the most common reasons to decline testing. Introducing PITC in the OPDs led to a shift of HIV testing from the VCT department to the OPD at intervention sites. HIV testing is important in prevention, and PITC may play a large role here. However, in our setting PITC was not effective in increasing HIV case finding. We recommend further research taking into consideration differences in HIV prevalence, population HIV testing rates, and ART coverage.
Acknowledgements
We would like to thank all study participants, INTERACT staff, clinic staff of participating health care facilities and community workers. We thank Inge Krul, Mara van Duin, Hadassa Fikse, Carmen Franse, Annemarie Griffioen, and Maxime Hofman for their help in fieldwork and Rolina Meijering for her help in data management. We thank Frank Cobelens (AIGHD, AMC) for helpful discussions and feedback on a draft of this manuscript. We acknowledge and thank the Infectious Disease Network for Treatment and Research in Africa (INTERACT) for their technical and logistical support. This study was financed by The Netherlands Organization for Scientific Research/Netherlands Foundation for the Advancement of Tropical Research (NWO-WOTRO/NACCAP: W070520100) and the European Union (SANTE/2006/105–316).
Abbreviations
- ANC
Antenatal care
- aOR
Adjusted Odds Ratio
- cART
Combination antiretroviral therapy
- CI
Confidence Interval
- HCW
Health care worker
- HF
Health facility
- HIV
Human Immunodeficiency virus
- IQR
Interquartile ranges
- MOH
Ministry of health
- OPD
Outpatient department
- PITC
Provider-initiated HIV testing and counselling
- TB
Tuberculosis
- VCT
Voluntary counselling and testing
- WHO
World health organization
Additional file
Characteristics of 65,716 clinic attendees of outpatient departments in eight health facilities by study phase and site, PITC study, Rwanda 2009–2010. Table S2. Multivariable logistic regression to determine the association of study phase with HIV testing rate and HIV case finding in the outpatient department of 2 control health facilities in Rwanda, 2009–2010. Table S3. Characteristics of 77,389 clinic attendees of outpatient and voluntary counseling and testing departments in eight health facilities by study phase and site, PITC study, Rwanda 2009–2010. Table S4. Multivariable logistic regression to determine the association of study phase with HIV testing rate and HIV case finding in the outpatient and voluntary counseling and testing departments of 2 control health facilities in Rwanda, 2009–2010. Figure S1. HIV testing rate and testing cascade in OPD in phase 3 at intervention sites, Rwanda, 2009–10. Figure S2. Reasons for refusing an HIV test in phase 3 at intervention sites, Rwanda, 2009–10. (DOC 311 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-016-1355-z) contains supplementary material, which is available to authorized users.
Competing interest
The authors declare that they have no competinng interest.
Authors’ contributions
MSVDL, MB, FK, VM, LDN designed the study and AA, JL, EB, advised on study concept and design. FK, EB, led data collection and all authors advised on data collection. DKVS, FK, MB, MSDVL did the statistical analyses. All authors contributed to the interpretation of findings. DKVS wrote the first draft of the manuscript, FK wrote subsequent versions and all authors made critical revisions and all approved the final version.
Contributor Information
Felix R. Kayigamba, Email: fkaigamba@gmail.com
Daniëla Van Santen, Email: dvsanten@ggd.amsterdam.nl.
Mirjam I. Bakker, Email: M.Bakker@kit.nl
Judith Lammers, Email: judith.lammers@amc.uva.nl.
Veronicah Mugisha, Email: vm2208@columbia.edu.
Emmanuel Bagiruwigize, Email: emmanuelb@theaccessproject.com.
Ludwig De Naeyer, Email: ldnaeyer@yahoo.com.
Anita Asiimwe, Email: anita.asiimwe@gmail.com.
Maarten F. Schim Van Der Loeff, Email: mschimvdloeff@ggd.amsterdam.nl
References
- 1.UNAIDS . Global Report. UNAIDS Report on the global AIDS epidemic 2013. Geneva: Joint United Nations Programme on HIV/AIDS; 2013. [Google Scholar]
- 2.WHO . Guidance on Provider-Initiated HIV Testing and Counselling in Health Facilities. Strengthening health services to fight HIV/AIDS. Geneva: World Health Organization; 2007. [Google Scholar]
- 3.WHO . Policy on collaborative TB/HIV activities Guidelines for national programmes and other stakeholders. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 4.WHO . Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 5.Ministry of Health, Rwanda . Annual Report 2011-2012. Kigali: Ministry of Health, Rwanda; 2012. [Google Scholar]
- 6.Ujiji OA, Rubenson B, Ilako F, Marrone G, Wamalwa D, Wangalwa G, et al. Is ‘Opt-Out HIV Testing’ a real option among pregnant women in rural districts in Kenya? BMC Public Health. 2011;8:11–151. doi: 10.1186/1471-2458-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hensen B, Baggaley R, Wong VJ, Grabbe KL, Shaffer N, Lo YR, et al. Universal voluntary HIV testing in antenatal care settings: a review of the contribution of provider-initiated testing & counselling. Trop Med Int Health. 2012;17:59–70. doi: 10.1111/j.1365-3156.2011.02893.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Rie A, Sabue M, Jarrett N, Westreich D, Behets F, Kokolomani J, et al. Counselling and testing TB patients for HIV: evaluation of three implementation models in Kinshasa. Congo Int J Tuberc Lung Dis. 2008;12:73–8. [PubMed] [Google Scholar]
- 9.Bock NN, Nadol P, Rogers M, Fenley MA, Moore J, Miller B. Provider-initiated HIV testing and counseling in TB clinical settings: tools for program implementation. Int J Tuberc Lung Dis. 2008;12:69–72. [PubMed] [Google Scholar]
- 10.Pope DS, Deluca AN, Kali P, Hausler H, Sheard C, Hoosain E, et al. A Cluster-Randomized Trial of Provider-Initiated (Opt-Out) HIV Counseling and Testing of Tuberculosis Patients in South Africa. J Acquir Immune Defic Syndr. 2008;48:190–5. doi: 10.1097/QAI.0b013e3181775926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corneli A, Jarrett NM, Sabue M, Duvall S, Bahati E, Behets F, et al. Patient and provider perspectives on implementation models of HIV counseling and testing for patients with TB. Int J Tuberc Lung Dis. 2008;12:79–84. [PubMed] [Google Scholar]
- 12.Odhiambo J, Kizito W, Njoroge A, Wambua N, Nganga L, Mburu M, et al. Provider-initiated HIV testing and counselling for TB patients and suspects in Nairobi. Kenya Int J Tuberc Lung Dis. 2008;12:63–8. [PubMed] [Google Scholar]
- 13.Girma S, Enquselassie F. Uptake of provider initiated HIV counseling and testing (PICT) among out patient department (OPD) clients with possible clinical signs of HIV infection in Addis Ababa. Ethiop Med J. 2009;47:245–54. [PubMed] [Google Scholar]
- 14.Kiene SM, Bateganya M, Wanyenze R, Lule H, Nantaba H, Stein MD. Initial outcomes of provider-initiated routine HIV testing and counseling during outpatient care at a rural Ugandan hospital: risky sexual behavior, partner HIV testing, disclosure, and HIV care seeking. AIDS Patient Care STDS. 2010;24:117–26. doi: 10.1089/apc.2009.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topp SM, Chipukuma JM, Chiko MM, Wamulume CS, Bolton-Moore C, Reid SE. Opt-out provider-initiated HIV testing and counselling in primary care outpatient clinics in Zambia. Bull World Health Organ. 2011;89:328–35. doi: 10.2471/BLT.10.084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalal S, Lee CW, Farirai T, Schilsky A, Goldman T, Moore J, et al. Provider-initiated HIV testing and counseling: increased uptake in two public community health centers in South Africa and implications for scale-up. PLoS One. 2011;6:e27293. doi: 10.1371/journal.pone.0027293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Statistics of Rwanda (NISR), MEASURE DHS ICF International Calverton, Maryland USA, Ministry of Health (MOH). Rwanda Demographic and Health Survey. Kigali: NSIR/MEASURE DHS/Rwanda Ministry of Health, 2011.
- 18.WHO . Global AIDS Response. Epidemic Update and health sector progress towards Universal Access. Progress report. Geneva: World Health Organization; 2011. [Google Scholar]
- 19.Kayigamba RF, Bakker IM, Lammers L, Mugisha V, Bagiruwigize E, Asiimwe A, et al. Provider-initiated HIV testing and counselling in Rwanda: acceptability among clinic attendees and workers, reasons for testing and predictors of testing. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topp SM, Chipukuma JM, Giganti M, Mwango LK, Chiko LM, Tambatamba-Chapula B, et al. Strengthening health systems at facility-level: feasibility of integrating antiretroviral therapy into primary health care services in Lusaka. Zambia PLoS One. 2010;5 doi: 10.1371/journal.pone.0011522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steen TW, Seipone K, Gomez Fde L, Anderson MG, Kejelepula M, Keapoletswe K, et al. Two and a half years of routine HIV testing in Botswana. J Acquir Immune Defic Syndr. 2007;44:484–8. doi: 10.1097/QAI.0b013e318030ffa9. [DOI] [PubMed] [Google Scholar]
- 22.Bassett IV, Giddy J, Nkera J, Wang B, Losina E, Lu Z, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46:181–6. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham CO, Doran B, DeLuca J, Dyksterhouse R, Asgary R, Sacajiu G. Routine opt-out HIV testing in an urban community health center. AIDS Patient Care STDS. 2009;23:619–23. doi: 10.1089/apc.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanjako D, Kamya M, Daniel K, Mayanja-Kizza H, Freers J, Whalen C, et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala. Uganda AIDS Behav. 2007;11:753–8. doi: 10.1007/s10461-006-9180-9. [DOI] [PubMed] [Google Scholar]
- 25.Awosan KJ, Ibrahim MTO, Ali AI. Human immunodeficiency virus/Acquired immune deficiency syndrome (HIV/AIDS), Related knowledge, risk perception and practice of confidential counseling and testing for HIV among patients in a tertiary health institution in North Western Nigeria. J AIDS and HIV Res. 2013;11:430–5. [Google Scholar]
- 26.Silvestri DM, Modjarrad K, Blevins ML, Halale E, Vermund SH, McKinzie JP. A comparison of HIV detection rates using routine opt-out provider-initiated HIV testing and counseling versus a standard of care approach in a rural African setting. J Acquir Immune Defic Syndr. 2011;56:e9–32. doi: 10.1097/QAI.0b013e3181fdb629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roura M, Watson-Jones D, Kahawita TM, Ferguson L, Ross DA. Provider-initiated testing and counselling programmes in sub-Saharan Africa: a systematic review of their operational implementation. AIDS. 2013;27:617–26. doi: 10.1097/QAD.0b013e32835b7048. [DOI] [PubMed] [Google Scholar]
- 29.Wanyenze RK, Nawavvu C, Namale AS, Mayanja B, Bunnell R, Abang B, et al. Acceptability of routine HIV counselling and testing, and HIV seroprevalence in Ugandan hospitals. Bull World Health Organ. 2008;86:302–9. doi: 10.2471/BLT.07.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett IV, Walensky RP. Integrating HIV screening into routine health care in resource-limited settings. Clin Infect Dis. 2010;50:S77–8. doi: 10.1086/651477. [DOI] [PMC free article] [PubMed] [Google Scholar]


