Abstract
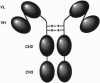
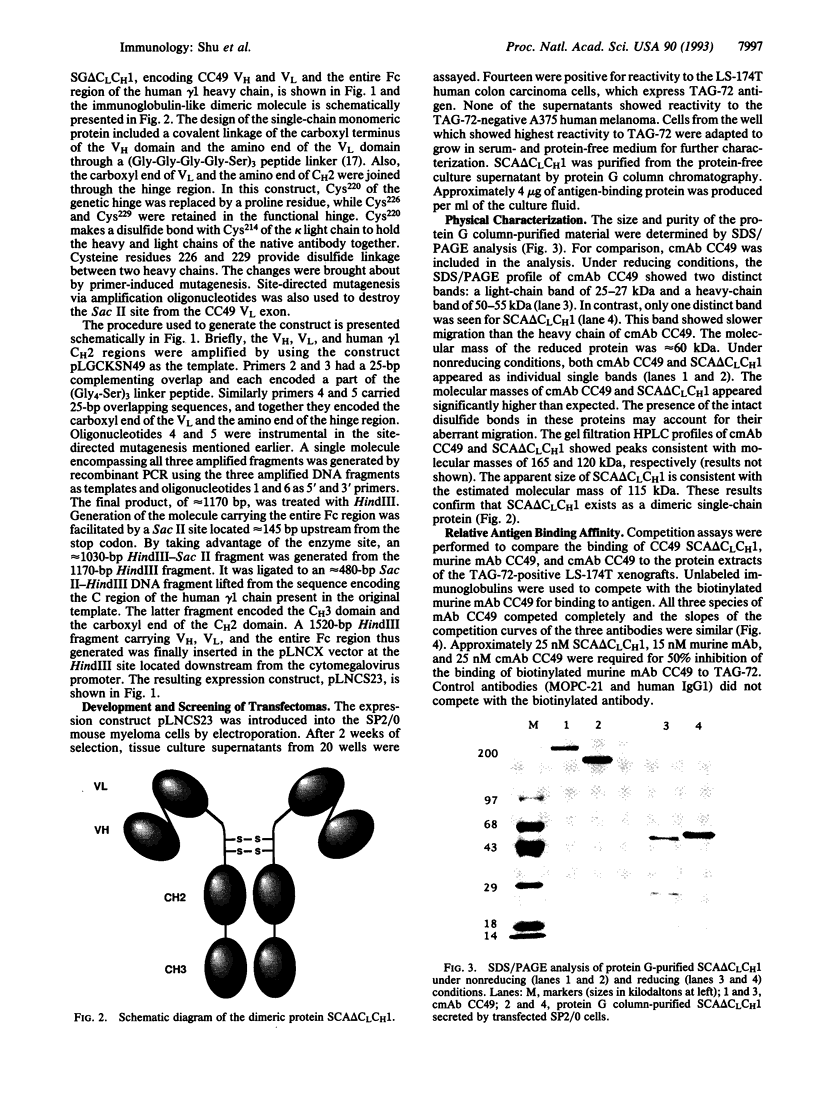
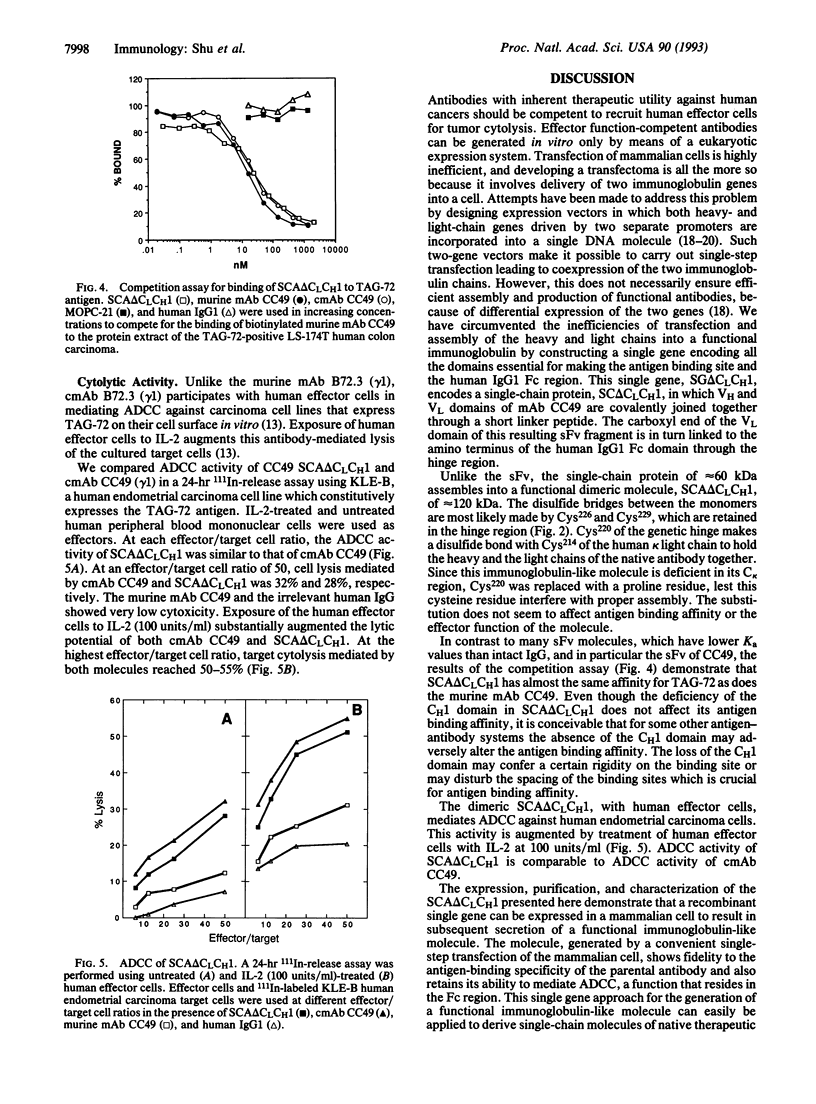
We describe construction of a single gene encoding a single-chain immunoglobulin-like molecule. This single-gene approach circumvents inefficiencies inherent in delivering two genes into a mammalian cell and in the assembly of a functional immunoglobulin molecule. It would also facilitate ex vivo transfection of cells for gene-therapy protocols. SP2/0 murine myeloma cells transfected with the single gene SG delta CLCH1 expressed a single-chain protein, SC delta CLCH1, comprising approximately 60 kDa of the anti-carcinoma monoclonal antibody (mAb) CC49. The single-chain protein consisted of the heavy- and light-chain variable (VH and VL) domains of the mAb covalently joined through a short linker peptide, while the carboxyl end of the VL domain was linked to the amino terminus of the human gamma 1 Fc region through the hinge region. The single-chain protein assembled into a dimeric molecule, termed SCA delta CLCH1, of approximately 120 kDa and was secreted into the tissue culture fluid. SDS/PAGE analysis of the secreted immunoglobulin purified by protein G affinity chromatography confirmed the size of the molecule. The native mAb CC49 and SCA delta CLCH1 of CC49 showed similar binding to the tumor-associated glycoprotein TAG-72, and the chimeric mAb CC49 and SCA delta CLCH1 showed similar cytotoxic activity. This single-gene construct approach provides a way of generating an immunoglobulin-like molecule which retains the specificity, binding properties, and cytolytic activity of the chimeric mAb CC49. The immunoglobulin-like molecule SCA delta CLCH1 is potentially a therapeutic and diagnostic reagent against a range of human carcinomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrn R. A., Mordenti J., Lucas C., Smith D., Marsters S. A., Johnson J. S., Cossum P., Chamow S. M., Wurm F. M., Gregory T. Biological properties of a CD4 immunoadhesin. Nature. 1990 Apr 12;344(6267):667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- Colcher D., Hand P. H., Nuti M., Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Minelli M. F., Roselli M., Muraro R., Simpson-Milenic D., Schlom J. Radioimmunolocalization of human carcinoma xenografts with B72.3 second generation monoclonal antibodies. Cancer Res. 1988 Aug 15;48(16):4597–4603. [PubMed] [Google Scholar]
- Dorai H., Mueller B. M., Reisfeld R. A., Gillies S. D. Aglycosylated chimeric mouse/human IgG1 antibody retains some effector function. Hybridoma. 1991 Apr;10(2):211–217. doi: 10.1089/hyb.1991.10.211. [DOI] [PubMed] [Google Scholar]
- Hand P. H., Calvo B., Milenic D., Yokota T., Finch M., Snoy P., Garmestani K., Gansow O., Schlom J., Kashmiri S. V. Comparative biological properties of a recombinant chimeric anti-carcinoma mAb and a recombinant aglycosylated variant. Cancer Immunol Immunother. 1992;35(3):165–174. doi: 10.1007/BF01756183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman N., Gill L. L., De Winter R. F., Wagner K., Hollis M., Businger F., Ammaturo D., Buchegger F., Mach J. P., Heusser C. Generation of a recombinant mouse-human chimaeric monoclonal antibody directed against human carcinoembryonic antigen. Int J Cancer. 1989 Sep 15;44(3):424–433. doi: 10.1002/ijc.2910440308. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzell P., Kashmiri S., Colcher D., Primus F. J., Hand P. H., Roselli M., Finch M., Yarranton G., Bodmer M., Whittle N. Generation and characterization of a recombinant/chimeric B72.3 (human gamma 1). Cancer Res. 1991 Jan 1;51(1):181–189. [PubMed] [Google Scholar]
- Johnson V. G., Schlom J., Paterson A. J., Bennett J., Magnani J. L., Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986 Feb;46(2):850–857. [PubMed] [Google Scholar]
- Kasid A., Morecki S., Aebersold P., Cornetta K., Culver K., Freeman S., Director E., Lotze M. T., Blaese R. M., Anderson W. F. Human gene transfer: characterization of human tumor-infiltrating lymphocytes as vehicles for retroviral-mediated gene transfer in man. Proc Natl Acad Sci U S A. 1990 Jan;87(1):473–477. doi: 10.1073/pnas.87.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi N. F. A chimeric IL-2/Ig molecule possesses the functional activity of both proteins. J Immunol. 1991 Feb 1;146(3):915–919. [PubMed] [Google Scholar]
- Liu A. Y., Robinson R. R., Hellström K. E., Murray E. D., Jr, Chang C. P., Hellström I. Chimeric mouse-human IgG1 antibody that can mediate lysis of cancer cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3439–3443. doi: 10.1073/pnas.84.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A. Y., Robinson R. R., Murray E. D., Jr, Ledbetter J. A., Hellström I., Hellström K. E. Production of a mouse-human chimeric monoclonal antibody to CD20 with potent Fc-dependent biologic activity. J Immunol. 1987 Nov 15;139(10):3521–3526. [PubMed] [Google Scholar]
- Milenic D. E., Yokota T., Filpula D. R., Finkelman M. A., Dodd S. W., Wood J. F., Whitlow M., Snoy P., Schlom J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991 Dec 1;51(23 Pt 1):6363–6371. [PubMed] [Google Scholar]
- Molinolo A., Simpson J. F., Thor A., Schlom J. Enhanced tumor binding using immunohistochemical analyses by second generation anti-tumor-associated glycoprotein 72 monoclonal antibodies versus monoclonal antibody B72.3 in human tissue. Cancer Res. 1990 Feb 15;50(4):1291–1298. [PubMed] [Google Scholar]
- Muraro R., Kuroki M., Wunderlich D., Poole D. J., Colcher D., Thor A., Greiner J. W., Simpson J. F., Molinolo A., Noguchi P. Generation and characterization of B72.3 second generation monoclonal antibodies reactive with the tumor-associated glycoprotein 72 antigen. Cancer Res. 1988 Aug 15;48(16):4588–4596. [PubMed] [Google Scholar]
- Primus F. J., Pendurthi T. K., Hutzell P., Kashmiri S., Slavin D. C., Callahan R., Schlom J. Chimeric B72.3 mouse/human (IgG1) antibody directs the lysis of tumor cells by lymphokine-activated killer cells. Cancer Immunol Immunother. 1990;31(6):349–357. doi: 10.1007/BF01741406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin-Chiorini D. C., Horan Hand P. H., Kashmiri S. V., Calvo B., Zaremba S., Schlom J. Biologic properties of a CH2 domain-deleted recombinant immunoglobulin. Int J Cancer. 1993 Jan 2;53(1):97–103. doi: 10.1002/ijc.2910530119. [DOI] [PubMed] [Google Scholar]
- Tao M. H., Morrison S. L. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989 Oct 15;143(8):2595–2601. [PubMed] [Google Scholar]
- Tsang K. Y., Kashmiri S. V., De Filippi R., Qi C. F., Calvo B., Shu L., Nieroda C. A., Greiner J. W., Schlom J. A human T cell line engineered to secrete chimeric monoclonal antibody. J Immunother Emphasis Tumor Immunol. 1993 Apr;13(3):143–152. doi: 10.1097/00002371-199304000-00001. [DOI] [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]