Abstract
The sensitivity of carotid body chemoreceptors to hypoxia is low just after birth and increases over the first few weeks of the postnatal period. At present, it is believed that the hypoxia-induced excitation of carotid body glomus cells begins with the inhibition of the outward K+ current via one or more O2 sensors. Although the nature of the O2 sensors and their signals that inhibit the K+ current are not well defined, studies suggest that the postnatal maturation of the glomus cell response to hypoxia is largely due to the increased sensitivity of K+ channels to hypoxia. As KV, BK and TASK channels that are O2-sensitive contribute to the K+ current, it is important to identify the O2 sensor and the signaling molecule for each of these K+ channels. Various O2 sensors (mitochondrial hemeprotein, hemeoxygenase-2, NADPH oxidase) and associated signals have been proposed to mediate the inhibition of K+ channels by hypoxia. Studies suggest that a mitochondrial hemeprotein is likely to serve as an O2 sensor for K+ channels, particularly for TASK, and that multiple signals may be involved. Thus, changes in the sensitivity of the mitochondrial O2 sensor to hypoxia, the sensitivity of K+ channels to signals generated by mitochondria, and/or the expression levels of K+ channels are likely to account for the postnatal maturation of O2 sensing by glomus cells.
Keywords: Carotid body, hypoxia, K+ channel, postnatal maturation, O2 sensor, background K+ channel
1. Introduction
A hypoxic stress imposed, for instance, by breathing air with a reduced O2 partial pressure elicits a feedback mechanism that increases ventilation and restores the arterial O2 tension to more normal levels. In the newborn animal, chemoreceptor and ventilatory responses to acute hypoxia are weak, but become stronger during the first few weeks after birth as the animal breathes normoxic air. This postnatal maturation in the ventilatory response to hypoxia is believed to be due to an increased sensitivity of the chemoreceptors in the carotid body glomus cells to low arterial oxygen pressure. When the newborn animal is exposed to the hypoxic or hyperoxic environment during the critical postnatal period, the carotid body fails to mature properly and the animal exhibits a diminished ventilatory response to hypoxia even later in life (Donnelly et al., 2005). Thus, breathing air with a normal O2 pressure during the first few weeks of life is crucial for the normal postnatal development of the carotid body glomus cell response to hypoxia. As one can imagine, the cellular and molecular events that occur during the process of maturation of the carotid body response to hypoxia are probably as complex as the physiology of the peripheral chemoreceptor system itself. A detailed knowledge of the mechanisms by which hypoxia increases ventilation in the adult is necessary to identify which O2 sensing pathways are underdeveloped in the newborn.
The currently accepted scheme of O2 sensing begins with the inhibition of K+ channels expressed in the plasma membrane of glomus cells via O2 sensors. The reduction of K+ current results in glomus cell depolarization that opens voltage-dependent Ca2+ channels. The enhanced Ca2+ influx and the subsequent rise in intracellular [Ca2+] stimulate the secretion of transmitters that act on postsynaptic receptors present at the carotid sinus afferent nerve terminals. The electrical signal in the form of action potentials travels to the brainstem and regulates respiratory and cardiovascular functions.
The developmental change in the carotid body response to hypoxia can potentially occur at multiple sites. Interestingly, the postnatal maturation of hypoxia-induced excitation of glomus cells followed a time course similar to the maturation of ventilatory and chemoreceptor responses to acute hypoxia (Eden and Hanson, 1987; Kholwadwala and Donnelly, 1992). The degree of catecholamine secretion from glomus cells during the early postnatal period was also well correlated with the change in carotid sinus afferent nerve activity during the same period (Donnelly, 2005). Furthermore, the magnitude of hypoxia-induced depolarization and elevation of intracellular [Ca2+] measured in isolated glomus cells were small just after birth but increased 4 over the next 2–3 weeks (Bamford et al., 1999; Wasicko et al., 1999). Thus, one major site at which the postnatal maturation of O2 sensing occurs is the glomus cell itself (Carroll and Kim, 2005). Interestingly, elevation of extracellular [K+] produced similar levels of depolarization and increase in intracellular [Ca2+] in glomus cells from newborn and older animals (Wasicko et al., 1999). These findings suggest that the developmental increase in the O2 sensitivity of glomus cells occurs at steps prior to the depolarization-induced Ca2+ influx. Therefore, the postnatal development of glomus cell O2 sensing is most likely to occur at an early critical step involving inhibition of the K+ current by hypoxia.
To define the role of K+ channels in the postnatal development of glomus cell response to hypoxia, all K+ channels present in the plasma membrane must be identified and characterized. Both O2-sensitive and O2-insensitive K+ channels can be involved in the postnatal maturation of O2 sensing by glomus cells. For example, the O2-sensitive K+ channels could become more sensitive to hypoxia resulting in progressively stronger depolarization of glomus cells over the period of several weeks after birth. A decrease in the level of expression and activity of O2-insensitive K+ channels with or without an increase in the sensitivity of O2-sensitive K+ channels should also contribute to the postnatal maturation of glomus cell response to hypoxia.
Although K+ channels are generally considered to be the main modulators of glomus cell excitation by hypoxia, other types of ion channels may be involved in the postnatal maturation of O2 sensing, if they are directly or indirectly modulated by hypoxia. For example, hypoxia could potentially increase the background inward Na+ current, although no such phenomenon has been reported so far. At rest, a balance between cation influx and K+ efflux sets the resting Em at approximately −60 mV. The depolarization induced by K+ channel inhibition by hypoxia is not only the result of the inhibition of K+ efflux, but also dependent on the net cation influx largely carried by Na+. Thus, although the initial cause of depolarization is the inhibition of K+ current, the resting (background) Na+ influx, presumably TTX-insensitive, is critical for the depolarization. The TTX-sensitive Na+ current is not affected by hypoxia and therefore unlikely to be involved in the depolarization elicited by hypoxia (Lopez-Lopez et al., 1989; Lopez-Lopez et al., 1997). An increase in O2 sensitivity of K+ channels may not necessarily lead to increased depolarization, unless the resting Na+ influx remains largely unchanged or increased by hypoxia. The resting Na+ current has been recorded in rat glomus cells (Buckler and Vaughan-Jones, 1994a; Carpenter and Peers, 2001), but the basic biophysical property of the Na+ channel that gives rise to the resting Na+ current has not yet been characterized.
Glomus cells also express Cl−- and HCO3−-permeable ion channels that may modulate the degree of hypoxia-induced depolarization. Ion channels that are sensitive to hypercapnia and protons may also participate in the postnatal maturation of O2 response by glomus cells, as the interaction between CO2 and O2 may change during the early postnatal period (Bamford et al., 1999). All of these mechanisms need to be elucidated to understand the process of postnatal maturation of O2 sensing by glomus cells. This article reviews the current knowledge of the role of K+ channels in O2 sensing by glomus cells and in the postnatal development of glomus cell response to hypoxia. The focus is on the cellular and ionic events that occur at the level of the glomus cell where the initial O2 sensing step is thought to take place.
2. K+ channels in glomus cells
Glomus cells express many different types of K+ channels. Comparison of native and cloned K+ channels and the use of ion channel blockers have led to the molecular identification of most K+ channels expressed in glomus cells. These include the classical voltage-gated K+ channels (KV), ether-a-go-go-related K+ channel (HERG), Ca2+-activated K+ channel (BK or maxi-K), inwardly rectifying K+ channels (Kir) and two-pore domain K+ (K2P) channels. Whole-cell and single channel recordings have confirmed the presence of KV, BK, HERG and K2P channels in glomus cells. Within the K2P channel family, TASK has been found in rat, mouse and human glomus cells, and TREK has been identified in rat glomus cells. Although Kir4.1 and Kir5.1 have been detected in rat glomus cells by immunohistochemistry (Yamamoto et al., 2008), the corresponding currents have not yet been identified. Single channel recording and immunocytochemistry have shown that an ATP-sensitive K+ channel that consists of Kir6 and SUR subunits are expressed in rat glomus cells (Kim et al., 2011a).
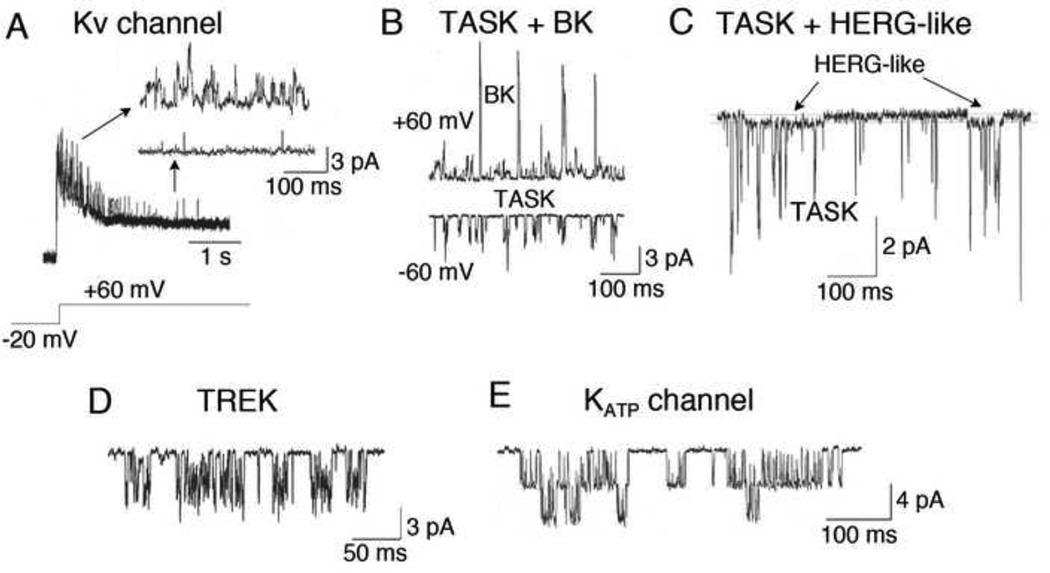
Many excitable cells express G protein-gated K+ channels that are modulated by receptor agonists via direct action of G protein subunits. Glomus cells express a number of G protein-coupled receptors, but a G protein-gated K+ channel has not yet been described. Fig. 1 shows different K+ channels that can be recorded from rat glomus cells and their presumed molecular identities, based on single channel kinetics and/or pharmacological properties. Glomus cells in non-rodent mammals such as rabbit may also express K+ channels other than those shown in Fig. 1 (Ganfornina and Lopez-Barneo, 1992). The density and activity of different K+ channels in glomus cells may vary among species (Shirahata and Sham, 1999). The expression levels of different K+ channels may change with age, particularly during the first few weeks after birth, and this may contribute to the postnatal maturation of O2 sensing by glomus cells. This is further discussed in Sections 4–6 below.
Figure 1.
Single K+ channels recorded from cell-attached and inside-out patches of rat glomus cells. K+ channels with properties similar to those of KV, BK, TASK-1/3, HERG, TREK and KATP channels are shown. (A–C) Pipette solution contained (mM) 140 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3) and the bath perfusion solution contained (mM) 117 NaCl, 23 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 glucose (pH 7.3). (A) Cell-attached patch: K+ channels were activated upon depolarization from −20 mV to +60 mV. Activation of single channels was followed by inactivation. This K+ channel probably represents a slowly inactivating KV channel. (B) Cell-attached patches were formed with pipette potential set at 0 mV. TASK (~36-pS) and BK (~220-pS) were observed in cell-attached patches at membrane potentials indicated. TASK was present in almost every patch at both −60 mV and +60 mV, whereas BK was observed only at depolarized potentials. (C) In some cell-attached patches, a channel with low single channel conductance (~12-pS) was observed together with TASK, and showed kinetic properties similar to those of cloned HERG (~12-pS) that has long openings. (D) TREK was recorded from an inside-out patch (pipette potential is set at +60 mV) after application of negative pressure. The bath solution was same as the pipette solution. (E) Opening of an ATP-sensitive (KATP) K+ channel (~72-pS) was observed in some inside out patches. The detailed description of recording single channels can be found in recent reports (Kim et al., 2009a; Kim et al., 2011a).
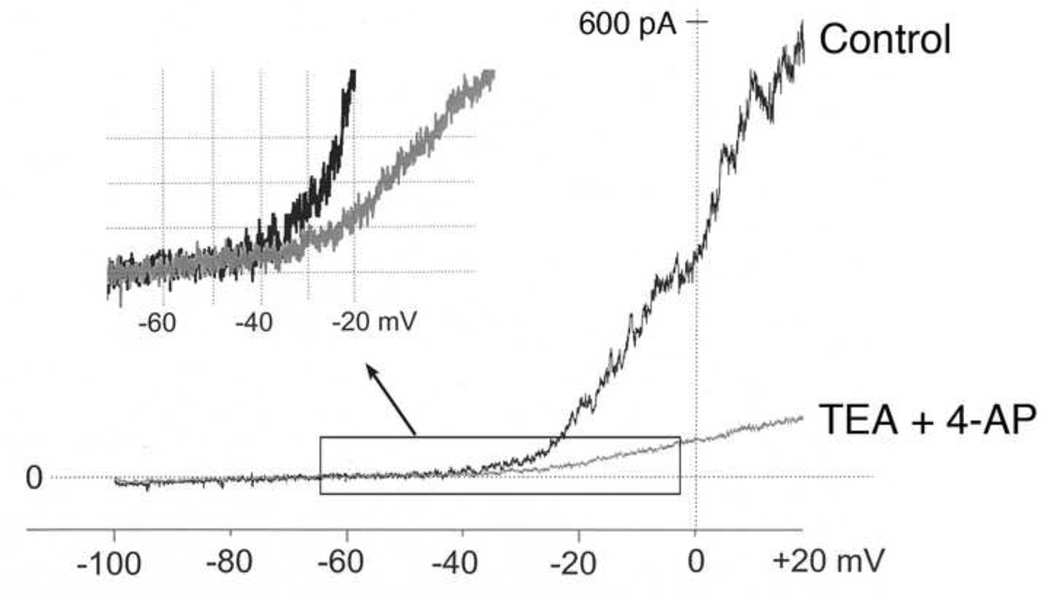
Studies that have recorded whole-cell K+ currents from rabbit glomus cells have shown that the outward K+ current is mainly from KV and BK channels, as tetraethylammonium (TEA) and 4-aminopyridine (4-AP) nearly completely blocked the outward current (Urena et al., 1989). In many early studies, leak subtraction protocol was often used when recording whole-cell currents. This would eliminate the contribution from background K+ channels such as TASK. Based on single channel recordings in cell-attached patches, however, TASK is predicted to provide a significant amount of the outward K+ current, at least in rat glomus cells (Buckler et al., 2000; Kim et al., 2009a). In a preliminary experiment, we recorded whole-cell currents from 14-day old rat glomus cells without leak subtraction, and applied TEA (10 mM) and 4-AP (2 mM) to the perfusion solution to block KV and BK channels. Under this condition, a significant TEA/4-AP-resistant outward current was present, which most likely was due to TASK (Fig. 2). On average, at membrane potentials (−70 mV to −50 mV) close to the resting Em (approximately −60 mV), TEA/4-AP had no effect on the whole-cell current. At −30 mV, the sizes of the TEA/4-AP-sensitive and insensitive currents were about equal, suggesting that ~50% of the whole-cell current was TEA/4-AP-insensitive. Thus, the TASK current was dominant near the resting Em with little or no contributions from KV and BK channels in rat glomus cells. At depolarized potentials, the highly voltage-dependent KV and BK channels become much more active than TASK. It would be interesting to know whether the TEA/4-AP-resistant, TASK-like whole-cell current can be recorded from rabbit and cat glomus cells without leak subtraction, and whether TASK-like single channels are present in cell-attached patches. Such basic information would be necessary for identifying which K+ channels and which O2 sensing mechanisms are modulated during the early postnatal period in different species.
Figure 2.
Effect of TEA and 4-AP on the whole-cell current from a glomus cell isolated from 14-day old rat without leak subtraction. In the whole-cell mode, the membrane potential was held at −60 mV, and a ramp voltage applied (−100 mV to +20 mV; 800 ms duration). Pipette solution contained (mM) 140 KCl, 4 mM ATP, 100 µM GTP, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3) and the bath perfusion solution contained (mM) 117 NaCl, 23 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 glucose (pH 7.3). TEA (10 mM) and 4-AP (2 mM) was applied to the bath solution to block Kv and BK currents. On the upper left corner, current tracings between −65 mV and −20 mV (enclosed by a rectangle) are magnified to show the difference between control (black line) and after TEA/4-AP treatment (gray line). Note that the two currents begin to diverge at approximately −40 mV.
3. O2 sensors and signals that inhibit K+ channels
To understand the mechanism of postnatal maturation of O2 sensing by glomus cells, all O2 sensors and their signals need to be identified for each O2-sensitive K+ channel. All three major K+ channels (KV, BK and TASK) expressed in glomus cells are believed to be O2-sensitive. Different mechanisms by which hypoxia excites glomus cells have been proposed by various research groups, suggesting involvement of multiple processes for O2 sensing. The O2 sensors proposed so far are mitochondrial hemeproteins, NADPH oxidase, hypoxia-inducible factor (HIF), and hemeoxygenase-2 (HO-2). To understand the role of these O2 sensors, knockout mice lacking or partially lacking a putative O2 sensor have been used to test the O2 sensitivity of the whole animal or glomus cells.
In heterozygous SDHD+/− mice (129SvJ strain) partially lacking the mitochondrial succinate dehydrogenase, the secretory response (as determined by an amperometric measurement of catecholamine secretion) of single cells in carotid body slices to hypoxia was found to be normal (Piruat et al., 2004). The secretory response of HO-2−/− mice (C57BL/6J strain) to hypoxia was also normal (Adachi et al., 2004; Ortega-Saenz et al., 2006). In mice (C57BL/6 strain) with a disrupted gp91 phagocytic oxidase (gp91phox), a gene that codes for a subunit of the neutrophilic form of NADPH oxidase, the carotid sinus nerve activity in response to hypoxia was similar to that of wild type mice (He et al., 2002). A study using mice with partial HIF-1α deficiency showed normal acute ventilatory responses to hypoxia and hypercapnia (Kline et al., 2002). Interestingly, in HIF-1α+/− mice, the carotid sinus nerve activity in response to hypoxia was lost, and O2 sensing in these mice was found to involve a non-carotid body pathway that was sensitive to vagotomy. Further studies using HIF-1α+/− mice showed that HIF-1α activation was responsible for chronic intermittent hypoxia-induced changes in cardio-respiratory response (Peng et al., 2006). In contrast, HIF-2α+/− mice showed an augmented response to acute hypoxia, and this was attributed to an increased oxidative stress (Peng et al., 2011). In general, interpretations of studies obtained using knockout mice are somewhat problematic for study of acute O2 sensing, because compensatory mechanisms are likely to be elicited. Interestingly, knockout mice lacking cystathionine-γ-lyase (CSE) and pharmacological inhibition of CSE and cystathionine-β-synthase (CBS) exhibited a reduced carotid sinus nerve activity in response to hypoxia compared to that of wild type mice (Li et al., 2010; Peng et al., 2010), suggesting that H2S may be an endogenous hypoxic signal.
As suggested earlier, perhaps the O2 sensing is so fundamental to the survival of the animal that several sensors and signals have evolved to maintain the normal range of arterial O2 tension (Kemp, 2006; Prabhakar, 2006). Each O2 sensor may be working within a limited range of hypoxia. Thus, distinctly different O2 sensors and signals may be operating at mild, moderate, and severe levels of hypoxia to achieve an optimal output by the glomus cells at different levels of O2. The transduction of the hypoxia signal by multiple proteins (such as mitochondrial hemeproteins and O2-sensitive K+ channels) and their interactions have been referred earlier as the “chemosome hypothesis” (Prabhakar, 2006). The sensitivity of some or all of these O2 sensors to hypoxia may be low just after birth and increase over the several weeks of the postnatal period, as the concentrations of cellular signals generated from O2 sensors increase to elicit a stronger inhibition of the K+ channel current.
4. Voltage-gated K+ channels (KV channels)
4.1. Properties of KV channels in glomus cells
KV channels are characterized by their activation upon depolarization, and therefore are primarily involved in cell repolarization. If the KV channel is a fast inactivating channel, it helps in shaping the discharge pattern such that the action potential frequency is usually increased. If the KV channel is a non-inactivating or slowly inactivating channel, it helps to terminate an action potential. KV current has been identified from glomus cells from different species (rat, rabbit, mouse and cat), as determined by the sensitivity to TEA and 4-AP. In rat and mouse glomus cells, the large outward K+ current is mildly inhibited by charybdotoxin, very weakly inhibited by 4-AP, but strongly reduced by TEA (Lopez-Lopez et al., 1997; Perez-Garcia et al., 2004), suggesting that a large portion of the outward K+ current is due to opening of non-inactivating KV channels and BK. Thus, the KV current in glomus cells provides a strong repolarizing force that brings the Em of the cell to the resting level following an excitation induced by acute hypoxia.
Does the level of expression of KV channels in glomus cells change over the first few weeks after birth? This question has not yet been clearly answered. In one study, the outward K+ current recorded from glomus cells from 5 week-old rats was found to be ~2 fold larger than that from 4-day-old rats (Hatton et al., 1997). This increase in outward K+ current with development may be necessary to counter the strong depolarizing effect of hypoxia in glomus cells of adult rat. Furthermore, hypoxia inhibited the outward K+ current to a greater extent in glomus cells from the adult rat than that from the newborn, suggesting that the sensitivity of the outward K+ current to hypoxia increased during the early postnatal period. Because the outward K+ current consists of KV, BK and TASK currents that are O2-sensitive, it is possible that the sensitivity of all three K+ channels to hypoxia was increased in glomus cells of adult animals, resulting in greater inhibition of the outward K+ current and stronger depolarization. Clearly, the relative contributions by BK, KV and TASK to the outward K+ current need to be defined more clearly in normoxia and hypoxia in glomus cells from newborn and older animals.
How would an increase in KV channel sensitivity to hypoxia contribute to the postnatal maturation of O2 sensing by glomus cells? In rat and mouse, KV channels may not be active at rest because the activation threshold for KV channels is approximately −40 mV that is significantly more positive than the typical resting Em of isolated glomus cells (−50 mV to −60 mV). Therefore, KV channels are unlikely to be involved in the initiation of hypoxia-induced depolarization in glomus cells of both newborn and older animals. In mildly depolarized glomus cells, KV channels may exhibit some basal activity and thus participate in keeping the cell from further depolarization. Most likely, a strong inhibition of KV channels by hypoxia in glomus cells from adult animals would be associated with a more pronounced and prolonged depolarization. By contrast, a weak inhibition of KV channels by hypoxia in glomus cells from the newborn would still leave a large repolarizing force that reduces the magnitude and duration of hypoxia-induced depolarization. In addition to changes in the O2 sensitivity of the KV current itself, factors that modify the sensitivity of the KV channel to hypoxia could contribute to the postnatal maturation of O2 sensing. For example, in rabbit glomus cells, receptor agonists such as angiotensin II and endothelin sensitized the KV channel to hypoxia (Li and Schultz, 2006). Therefore, a difference in the efficiency of the agonist-receptor signaling in glomus cells between newborn and older animals could modulate the postnatal maturation of O2 sensing by glomus cells in vivo.
4.2. Inhibition of KV current by hypoxia
The O2 sensors and signals that inhibit the KV current in glomus cells are not clearly known. In the pulmonary vascular smooth muscle, hypoxia and mitochondrial electron transport inhibitors both reduced the KV current, suggesting that mitochondria hemeproteins may be the O2 sensor in this tissue (Mauban et al., 2005). The effect of drugs that inhibit the mitochondrial function on the KV current in glomus cells has not been reported. Interestingly, the degree of inhibition of the KV current by hypoxia and mitochondrial electron transport inhibitors was found to depend on the localization of mitochondria in pulmonary smooth muscle cells (Firth et al., 2009). Mitochondria located close to the plasma membrane were more effective in KV current inhibition. This makes sense if signals from mitochondria need to move rapidly to the plasma membrane to regulate the KV channel activity. Such a mechanism would be interesting to examine in glomus cells for inhibition of not only KV channels, but also other O2 sensitive K+ channels. If differences in the localization of mitochondria in glomus cells of newborn and adult animals do exist, this can partly account for the postnatal development of O2 sensing by glomus cells.
In outside-out patches from rabbit glomus cells, hypoxia inhibited the open probability of the voltage-gated K+ channel by ~50% (Ganfornina and Lopez-Barneo, 1991). In HEK cells expressing the cloned KV4.2/Kvβ1.2, hypoxia produced a partial inhibition in excised macropatches, and the KVβ 1.2 subunit was responsible for this effect (Perez-Garcia et al., 1999). These findings support the idea that the hypoxia-induced inhibition of KV current occurs at the level of the membrane. Therefore, the O2 sensor probably lies within the plasma membrane or the channel itself. If so, this would preclude the involvement of mitochondria in hypoxia-induced inhibition of KV current, as mitochondria are not expected to be isolated together with membrane patches. The question on the role of mitochondrial hemeproteins as the O2 sensor for the KV channel may be resolved by testing the effect of mitochondrial electron transport inhibitors and uncouplers of oxidative phosphorylation on the KV channel function in excised and cell-attached patches.
The signals and mechanisms that mediate the hypoxic inhibition of a K+ channel are probably different in non-chemoreceptor cells (i.e., transfected cells) and glomus cells and, therefore, the mechanisms observed in HEK cells may not apply to glomus cells. Nevertheless, the involvement of KVβ subunits as potential modifiers of O2 sensing by glomus cells during the early postnatal period deserves to be tested. A number of interacting proteins such as KCHIPs (K+ channel interacting proteins) and KCHAPs (K+ channel associated proteins) may also modulate KV channels (Lopez-Lopez and Perez-Garcia, 2007), and affect the sensitivity of KV channels to hypoxia. If so, age-dependent changes in the expression levels of these interacting proteins could contribute to the postnatal maturation of O2 sensing by glomus cells.
4.3 HERG (Kv11.1)
HERG (human ether-a-go-go-related channel) is a voltage-dependent K+ channel that has unique biophysical properties. HERG activates upon depolarization but inactivates rapidly, and recovers from inactivation upon repolarization. In rabbit glomus cells, the measurement of steady-state activation suggests that HERG is active near the resting Em, and thus is likely to contribute to the resting K+ conductance (Overholt et al., 2000). Dofetilide, an inhibitor of HERG, depolarized rabbit glomus cells from −48 mV to −35 mV and elevated intracellular [Ca2+], whereas 10 mM TEA showed no effect. These findings suggest that HERG serves as a major background K+ conductance in rabbit glomus cells. The lack of effect of TEA on the resting Em suggests that KV and BK channels were not active at rest in rabbit glomus cells. In a different study, however, 4-AP was found to depolarize rabbit glomus cells by ~10 mV, suggesting that KV channels may also serve as background K+ channels in this species (Perez-Garcia et al., 2000). The different findings are difficult to resolve at this time.
HERG is also expressed in rat glomus cells, as judged by the inhibition of the outward K+ current by E-4301, a specific blocker of HERG (Kim et al., 2005). In our own studies in cell-attached patches from rat glomus cells, opening of a small conductance K+ channel (~12-pS) with long openings was observed. As the single channel conductance of cloned HERG expressed in Cos-7 cells is ~12-pS and the channel shows similar long open kinetics, the 12-pS channel in rat glomus cells may very well be HERG (see Fig. 1). Based on single channel recordings, the contribution of the 12-pS channel compared to that of TASK was small in rat glomus cells.
Interestingly, the HERG current as determined from the E4301-sensitive current density was ~2-fold larger in glomus cells isolated from newborn rats than that from 11–16 day-old rats (Kim et al., 2005). E4301 produced a small depolarization (2.2–2.7 mV) of glomus cells from both age groups, indicating that the contribution of HERG to the background K+ conductance is relatively small regardless of age. However, E4301 produced a small but significant increase in the hypoxia-induced depolarization and rise in [Ca2+] in glomus cells from newborn but not from 11–16 day old rats (Kim et al., 2005). Thus, HERG is probably not significantly involved in the initiation of glomus cell excitation by hypoxia, but helps to slightly reduce the magnitude of excitation during hypoxia in the newborn but not in older rats. Therefore, the reduction of HERG expression over the ~two weeks of the early postnatal period may contribute slightly to the postnatal maturation of glomus cell response to hypoxia in the rat. It would be interesting to study the developmental changes in the functional expression of HERG in rabbit glomus cells that express HERG whose inhibition causes moderate depolarization (Overholt et al., 2000).
5. Ca2+-activated K+ channel (KCa channel, BK, maxi-K)
5.1. Properties of BK in glomus cells
The functional expression of BK in glomus cells in rabbit, rat and mouse is well established. The large single channel conductance (~200–250 pS) of BK allows it to be easily recognized in single channel recording experiments when the channels are active. The defining property of BK is its sensitivity to cell membrane potential and intracellular [Ca2+], but many other factors have been shown to modulate the BK activity. These include the redox state of the cell, reactive oxygen species, free fatty acids, phosphatidylinositol-4,5-bisphosphate (PIP2), carbon monoxide (CO), heme, kinases and phosphatases, arachidonic acid and its metabolites, membrane stretch, pH and O2 tension (Hou et al., 2009). Therefore, the BK activity observed in excised patches or whole-cells dialyzed with a pipette solution probably does not reflect the true BK activity in undisturbed intact cells. Any change in the levels of these factors during the early postnatal period would affect the BK activity and alter the excitability of glomus cells. In rat glomus cells under the whole-cell configuration, ~20–50% of the outward voltage-dependent K+ current was provided by BK, as judged by the effect of charybdotoxin that specifically blocks BK (Lopez-Lopez et al., 1997). Blocking voltage-dependent Ca2+ channels with Cd2+ or removing extracellular Ca2+ also reduced the outward K+ current to a similar degree, indicating that BK is expressed in the cell membrane (Peers and Wyatt, 2007).
5.2. Inhibition of BK by hypoxia
Studies show that hypoxia inhibits BK in glomus cells as well as cloned BK (Slo1) expressed in mammalian cell lines. In some studies, inhibition of BK by hypoxia was observed in excised patches, suggesting that soluble cytosolic molecules were not required for inhibition (Lewis et al., 2002; Riesco-Fagundo et al., 2001). Other studies have reported that BK became more sensitive to hypoxia only when the cytoplasm was present (Wyatt and Peers, 1995). Interestingly, BK in rabbit glomus cells was reported to be insensitive to hypoxia (Ganfornina and Lopez-Barneo, 1991), but this could be because excised patches were used. In support of the involvement of a cytosolic signal, BK in rabbit glomus cells was inhibited by hypoxia in cell-attached patches (Delpiano and Hescheler, 1989). Therefore, it is quite conceivable that hypoxia inhibits BK both directly and indirectly.
In a recent study using inside-out patches, hypoxia was found to inhibit only the splice variant of BKα-subunit that possesses a cysteine-rich, stress-regulated exon (STREX) within the intracellular C-terminus of BK (McCartney et al., 2005). The inhibition by hypoxia was independent of the redox state or CO. However, glomus cells from 10-day old rats expressed a BK variant with no STREX insert (Ross et al., 2011), questioning the role of the STREX region for hypoxia sensitivity in glomus cells. It is possible that the levels of expression of BK variants change during the early postnatal period and modify the response to hypoxia. In mice, hypoxia inhibited the outward K+ current from glomus cells of undissociated carotid body more in the DBA/2J strain than the A/J strain, and this was associated with a higher expression of BK channel α and β2 subunits in the DBA/2J strain than in the A/J strain (Otsubo et al., 2011). Mild hypoxia reduced the K+ current only in the DBA/2J strain. The inhibition of the K+ current by mild hypoxia was abolished in the presence of iberiotoxin, supporting the idea that BK was the primary target of hypoxia in these mice. This is somewhat surprising because KV and TASK channels are also sensitive to hypoxia. Perhaps, the expression of KV and TASK channels are low in the DBA/2J strain such that the outward K+ current is mostly from BK in these mice. It would be important to re-assess the differences in the hypoxic sensitivities of these K+ channels in the two mice strains, and compare the findings with the hypoxic sensitivity of K+ channels in rat glomus cells.
Does the expression level of BK and the sensitivity to hypoxia change during the 2–3 weeks after birth? One study addressed these questions in rat glomus cells and found that the magnitude of the BK current increased during development (Hatton et al., 1997). However, a specific inhibitor of BK was not used to isolate the BK current. The high Mg2+/low Ca2+ solution used to block BK would also block other K+ channels such as TASK. Hypoxia-induced inhibition of the outward K+ current increased from ~10% in glomus cells from 4-day old to ~30% from 10-day old to adult rats, showing a developmental increase in the sensitivity of the outward K+ current to hypoxia. A part of the increase in sensitivity to hypoxia may involve BK, but this needs to be tested further using specific blockers of BK.
At present, there is controversy as to whether BK is active or closed near the resting Em (see Section 5.3 for further discussion). If BK were active at rest, an increase in the sensitivity of BK to hypoxia would lead to an increased inhibition of BK and depolarization. If BK were not open at rest, BK would not be involved in the initiation of hypoxia-induced depolarization. However, BK is likely to become active as the depolarization progresses and intracellular [Ca2+] rises during hypoxia, and modulate the magnitude of glomus cell excitation, provided that hypoxia does not inhibit BK completely. A recent study showed that charybdotoxin had no effect on the afferent nerve activity in both glomus cells from newborn and 3 week old rats in response to ~8% O2 (Donnelly et al., 2011), suggesting that BK did not become active in response to moderate hypoxia and that BK is unlikely to be involved in the postnatal maturation of O2 sensing by glomus cells. However, BK may become active during severe hypoxia when [Ca2+] rises and the cell depolarizes strongly, and limit the degree of hypoxia-induced excitation. Evidence for such a role has been presented recently (Donnelly et al., 2011; Gomez-Nino et al., 2009). A better understanding of the changes in BK function in glomus cells over 2–3 weeks of the postnatal period in normoxia and under varying degrees of hypoxia would be necessary to clearly assess the role of BK in the initiation of hypoxia-induced excitation as well as the postnatal maturation of O2 sensing.
Regardless of whether BK is active or closed in resting glomus cells, several mechanisms have been proposed for hypoxia-induced inhibition of BK. One mechanism involves HO-2 that may be closely associated with BK in the plasma membrane (Williams et al., 2004). The proposed idea is that HO-2 generates CO that keeps BK active in normoxia and that hypoxia reduces CO formation resulting in reduction of BK activity, leading to cell depolarization. Therefore, it is plausible that [CO] and HO-2 activity are lower in glomus cells from newborn than those from older animals to account for the reduced hypoxia-induced depolarization in the newborn compared to that in older animals. Although CO itself is known to activate BK , further studies are needed to show that the basally generated CO is sufficient to activate BK and that the hypoxia-induced reduction of CO generation by specific inhibition of HO-2 reduces the BK current in intact glomus cells, but not in excised patches. The role of HO-2 in O2 sensing therefore remains unresolved at present, and stronger evidence is needed to support the HO-2/CO pathway.
The role of reactive oxygen species as hypoxia-induced signals that modulate ion channels has been closely scrutinized for many years but remains unresolved. H2O2, the reactive oxygen species most likely to be in the cytoplasm inhibited hSlo1 (cloned human BK) when applied to the cytoplasmic face of inside-out patches in HEK cells (Tang et al., 2004). Thus, the BK activity would be reduced if hypoxia were to increase the concentration of ROS in the cell. However, it is not clear that acute hypoxia generates enough [ROS] to inhibit BK in vivo. A good test for the role of ROS would be to apply H2O2 directly to inside-out patches of glomus cells showing active BK. The results from such an experiment show that H2O2 (1–32 mM) does not cause inhibition of BK in rat glomus cells during ~2 minutes of perfusion (Kim and Papreck, 2011). Superoxide radical generated by adding xanthine and xanthine oxidase also failed to inhibit BK in rat glomus cells. Therefore, a short exposure to ROS does not seem to affect BK in glomus cells, and thus ROS are unlikely to be involved in the acute phase of the hypoxia-induced excitation of glomus cells, and in the postnatal development of O2 sensing by glomus cells.
Another proposed pathway for the hypoxic inhibition of BK involves AMP kinase (AMPK), an enzyme believed to serve as a metabolic sensor that responds to changes in [AMP]/[ATP] ratio in the cell (Mihaylova and Shaw, 2012). In rat glomus cells, AICAR (aminoimidazole carboxamide ribonucleotide), an activator of AMPK, inhibited the outward K+ current sensitive to iberiotoxin, indicating that AMPK reduced the BK current (Wyatt et al., 2007). AICAR also caused cell depolarization, elevation of intracellular [Ca2+] and increased the carotid sinus nerve activity, supporting the AMPK hypothesis. The BK isoform expressed in rat glomus cells was found to be a STREX-free variant, and this lack of STREX region was important for AMPK-induced inhibition of BK (Ross et al., 2011). Blockade of some of these effects by Compound C, an inhibitor of AMPK, and phosphorylation of BKα subunit by AMPK provided additional proof that AMPK inhibits BK. The ability of Compound C to fully block the hypoxia-induced elevation of intracellular [Ca2+] suggests that the AMPK-induced inhibition of BK is the primary signaling pathway in the hypoxia-induced excitation of rat glomus cells. In AMPKα2 knockout mice, the hypoxia-induced increase in ventilation frequency was attenuated (Evans et al., 2009), further supporting the role of AMPK as an O2 sensor. Corroboration of the hypoxia-AMPK-BK signaling pathway using glomus cells from the AMPK knockout mouse should further strengthen the AMPK hypothesis. If the reduction of [ATP] and the increase in the [AMP]/[ATP] ratio are small in response to hypoxia in glomus cells from newborn compared to that from older animals, AMPK signaling could very well contribute to the postnatal maturation of glomus cell response to hypoxia. This could involve all O2-sensitive K+ channels that are phosphorylated by AMPK.
Recent studies suggest that hydrogen sulfide (H2S) may be involved in the hypoxia-induced inhibition of BK and excitation of glomus cells (Li et al., 2010; Olson, 2011). H2S is constitutively generated in many cell types including glomus cells via CSE and CBS, and degraded to hydrogen sulfite in mitochondria in normoxia (Kamoun, 2004). Thus, intracellular [H2S] is suspected to be in the low nanomolar range in normoxia. Studies suggest that hypoxia reduces the mitochondrial metabolism of H2S, thus elevating [H2S] in the cytoplasm. Could H2S generated by hypoxia also serve as a signal that inhibits BK to excite glomus cells? Like hypoxia, NaHS (a H2S donor) strongly increased the secretory response of glomus cells. The hypoxia-induced secretory response was diminished in mice with deletion of CSE or when a CSE inhibitor (DL-propargylglycine) was used (Peng et al., 2010). Direct application of NaHS to glomus cells inhibited BK in glomus cells (Li et al., 2010; Telezhkin et al., 2010). The mechanism of BK inhibition by H2S was not related to the effect of CO and did not involve altered Ca2+ sensitivity via the Ca2+ binding domain. As H2S inhibited BK in inside-out patches, a direct effect on BK seems likely, possibly involving sulfhydration. If these effects of H2S are valid, a reduced generation of H2S and/or a reduced sensitivity of K+ channel to H2S in glomus cells from newborn compared to that from older animals could partly account for the postnatal maturation of O2 sensing by glomus cells.
Evidence against the involvement of H2S as a hypoxic signal has also been presented (Fitzgerald et al., 2011; Haouzi et al., 2011). Evidence against the role of H2S is that the [H2S] required to inhibit BK is relatively high (~200 µM) and such a high [H2S] would strongly inhibit mitochondrial cytochrome oxidase and be lethal to the animal (Cooper and Brown, 2008). Also, such a high [H2S] may not be produced by hypoxia, as H2S is rapidly degraded in the blood. It is interesting to note that H2S has been shown to increase the BK activity in rat pituitary tumor cells via reduction of sulfhydryl groups (Sitdikova et al., 2009), an effect opposite of that observed in glomus cells. Further studies to clearly define the role of H2S as a hypoxic signal are necessary before one can study the involvement of this gas molecule in the postnatal maturation of O2 sensing by glomus cells.
5.3. Is BK active at the resting Em?
The role of BK in the hypoxia-induced excitation of glomus cells and in the postnatal maturation glomus cell response to hypoxia depends on how active BK is at rest, how sensitive it is to hypoxia and whether changes occur over several weeks of the postnatal period. Many aspects of these issues remain unknown or unresolved at this time. For BK to be involved in the hypoxia-induced depolarization, it has to be open at some level of activity near the resting Em. Charybdotoxin failed to elicit an increase in [Ca2+], suggesting that BK was not active enough to produce a shift in the resting Em (Buckler, 1997). Similar lack of BK activity was suggested from studies using TEA and charybdotoxin that showed small or no effect on catecholamine secretion and chemosensory discharge (Gomez-Nino et al., 2009; Osanai et al., 1997). In other studies, however, charybdotoxin elicited depolarization and stimulated catecholamine release from glomus cells, suggesting that BK was active at rest (Pardal et al., 2000; Wyatt and Peers, 1995). The strikingly different findings on the contribution of BK to the resting K+ conductance are difficult to resolve. Could it be due to the heterogeneous nature of isolated glomus cells and different metabolic states of glomus cells at the time of study?
Different membrane potential responses to hypoxia of isolated and clustered glomus cells have been observed (Pang and Eyzaguirre, 1992). Even in isolated glomus cells, the degree of depolarization and elevation of [Ca2+]i in response to hypoxia can be highly variable among cells, due to either the true intrinsic variability or the cell isolation technique that affects cell viability and metabolism. For example, the resting Em of glomus cells used in one study was −43 mV and this was shifted to −32 mV by charybdotoxin (Wyatt and Peers, 1995), and hypoxia (12–20 mmHg pO2) produced a similar change in Em. In the study using carotid body slices, in which TEA and charybdotoxin increased the secretory response of glomus cells (Pardal et al., 2000), it is plausible that the glomus cells in slices were partially depolarized such that BK was active at rest. This would cause charybdotoxin to cause further depolarization and elicit a secretory response. In studies in which the glomus cells were not affected by TEA or charybdotoxin, the resting Em was close to −60 mV (Buckler, 1999). Thus, at moderately depolarized potentials, BK could be basally active, such that the cells respond with depolarization when a BK blocker is applied. Because BK is a high conductance channel, even a low level of activity may have a significant effect on the resting Em and cause the secretory response. In our own studies, the resting Em of isolated single glomus cells was close to −60 mV, and that may explain the lack of BK activity in cell-attached patches of glomus cells in their normal resting state (Kim et al., 2009a). Although there is no clear evidence suggesting that the resting Em of glomus cells in situ and after isolation are different, it would make sense to carefully consider the effect of the resting Em when studying the role of BK in hypoxia-induced excitation of glomus cells in both newborn and older animals.
6. TASK-1 and TASK-3 channels (K2P3.1 and K2P9.1)
6.1. TASK channels in glomus cells
Studies prior to ~1996 have not considered the role of background K+ channels in hypoxia-induced excitation of glomus cells, mainly because of poor characterization and unknown molecular identity of these channels. The low sensitivity of background K+ channels to various inhibitors of K+ channels (TEA, 4-AP, Ba2+) also contributed to the difficulty in studying these channels. In some experiments, the leak current was subtracted before recording the whole-cell current, thus removing the contribution by background K+ channels. As the molecular identity of background K+ channels such as TASK-1 and TASK-3 began to be defined and their sensitivity to various biological factors characterized, it became clear that such background K+ channels provided important functions in various cell types. Indeed, the hypoxia-sensitive current in rat glomus cells was found to exhibit properties of a background K+ current (Buckler, 1997).
Test of the idea that TASK encodes the background K+ current in these cells showed that TASK was functionally well expressed in rat glomus cells, active at rest and inhibited by hypoxia (Buckler et al., 2000). The effect of hypoxia on TASK activity occurred very rapidly (~2–3 seconds). These findings led to the hypothesis that hypoxia inhibits TASK thus exciting the glomus cells in the rat. The effect of a selective inhibitor of TASK on membrane potential and [Ca2+] in glomus cells is yet to be tested to confirm the role of TASK in hypoxia-induced depolarization. Because TASK is highly active at rest, the prediction is that a selective inhibition of TASK would be sufficient to excite glomus cells and mimic the effect of hypoxia. Earlier studies showed that the pO2-[Ca2+] plot followed a hyperbolic relationship (Buckler and Vaughan-Jones, 1994b; Wasicko et al., 1999). The pO2-TASK activity relationship also followed a similar hyperbolic relationship (Kim et al., 2011b), supporting the idea that the inhibition of TASK is an important part of the O2 sensing mechanism in rat glomus cells.
The TASK channel in glomus cells from 0–3 week old rats consists of TASK-1 homomer, TASK-3 homomer and TASK-1/3 heterodimer (Kim et al., 2009a). TASK-1 and TASK-3 show single channel conductance levels of ~15-pS and ~36-pS, respectively, in an external solution that contains 150 mM K+ and 2 mM divalent cation (Kim et al., 2009a). TASK-1 and TASK-3 can form a heterodimer that has a single channel conductance nearly identical to that of the homomeric TASK-3 under similar ionic conditions (Kang et al., 2004). The majority (~70%) of TASK in glomus cells between the ages of 0–18 days was determined to be TASK-1/3 heteromer, based on experiments using ruthenium red and different extracellular divalent cation concentrations. The single channel conductance of TASK-1 was insensitive to changes in external divalent cation concentration whereas TASK-3 was highly sensitive due to negatively charged amino acids at the M1-P1 region. TASK-like channels in glomus cells showed a sensitivity to external divalent concentration that was intermediate between those of TASK-1 and TASK-3, similar to the cloned heteromeric TASK-1/3 expressed in mammalian cells (Kang et al., 2004). At high divalent cation concentrations (at and above 4 mM), the difference in single channel conductance between TASK-1 and TASK-3 became minimal, and TASK-1/3 could not be distinguished from TASK-1. Thus, it is important to use a recording solution containing less than 2 mM divalent cations when studying the TASK channels.
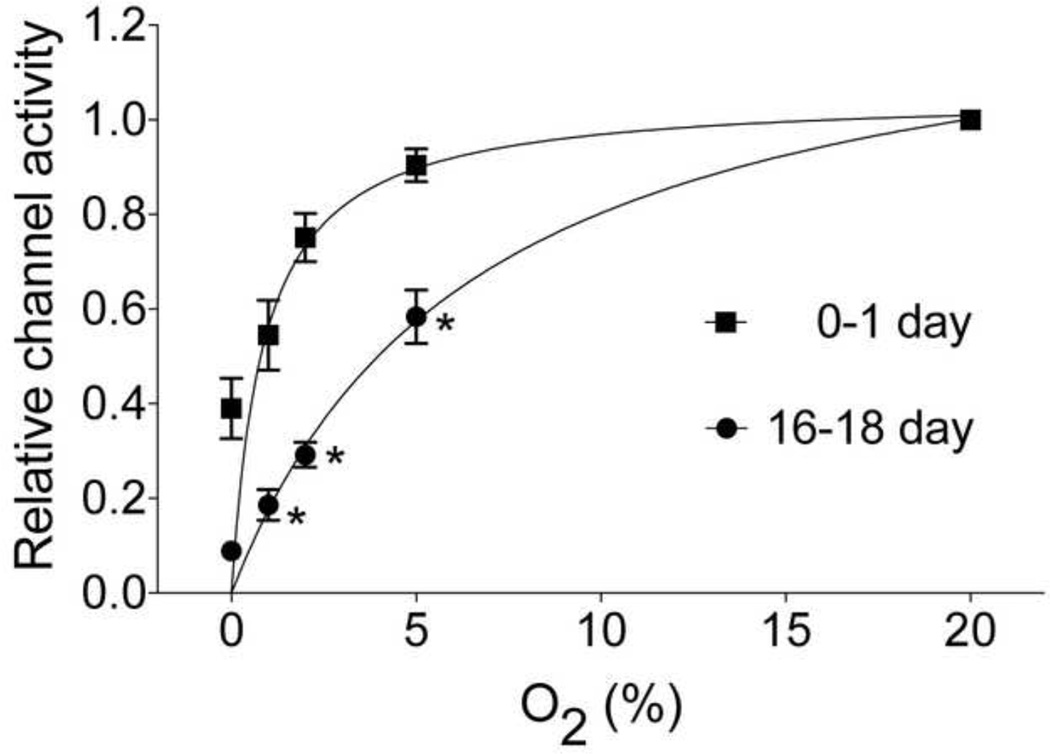
When these unique features of TASK were considered, single channel recordings from cell-attached patches of rat glomus cells showed that TASK was the most active K+ channel at rest both in the newborn (0–1 day old) and 2–3 week old rats (Kim et al., 2011b). TASK activities in glomus cells isolated from newborn and 16–18 day old rats were also similar, in agreement with earlier whole-cell recordings near the resting Em (Wasicko et al., 2006). Test of hypoxia showed that TASK in glomus cells from newborn rats was much less sensitive to hypoxia than TASK from 16–18 day old rats (K1/2 at 1.6% vs. 5.3% O2); 5% O2 inhibited TASK in glomus cells by ~50% in 16–18 day old rats but only ~10% in the 0–1 day old rats (Kim et al., 2011b). The age-dependent difference in the sensitivity of TASK was observed with mild, moderate and severe hypoxia (Fig. 3). The increased sensitivity of TASK to hypoxia was closely associated with an increased level of depolarization under identical experimental conditions, in support of the role of TASK as an O2-sensitive ion channel that mediates the hypoxia-induced excitation of glomus cells. Thus, the increased sensitivity of TASK to hypoxia is clearly a major factor for the postnatal maturation of O2 sensing by rat glomus cells. What causes the sensitivity of TASK to hypoxia to increase over the 2–3 weeks after birth is difficult to study at this time, because the mechanism of inhibition of TASK by hypoxia itself is still not well defined (see Section 6.2).
Figure 3.
Age-dependent changes in hypoxia-induced inhibition of TASK in rat glomus cells. Cell-attached patches were formed and pipette potential set at 0 mV. Pipette solution contained (mM) 140 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3) and the bath perfusion solution contained (mM) 117 NaCl, 23 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 glucose (pH 7.3). Cells were perfused with normoxic and hypoxic solutions. The O2 levels at which half maximal inhibition of TASK was observed was 1.6% O2 for 0–1 day old and 5.3% O2 for 16–18-day old rats. The data points (for TASK activity) were fitted to an exponential function (Y = Yo +(plateau-Yo)(1−exp(−kx)), where x=O2 level, Yo is the Y value when x = zero, plateau is the Y value at maximum x and k is the rate constant). (Reproduced from Kim et al., Respir. Physiol. Neurobiol. 2011
So far, an age-dependent increase in the sensitivity of TASK to hypoxia has been reported only in rat glomus cells. Mouse (C57BL/6 strain) glomus cells also express TASK-like current (Ortega-Saenz et al., 2010), but the sensitivity of TASK to hypoxia has not yet been tested directly. Whole-cell currents near the resting Em are ~10 pA in both rat and mouse glomus cells, suggesting that the density of TASK in rat and mouse may be similar. Mice lacking TASK-1 or both TASK-1 and TASK-3, but not TASK-3, was found to exhibit reduced ventilatory responses to hypoxia (10% O2 in inspired air) and moderate normoxic hypercapnia (3–6% CO2 in inspired air), suggesting that TASK-1 is involved in the hypoxic response (Trapp et al., 2008). However, in another study, TASK-3, but not TASK-1, was found to contribute to the background K+ current in mice (Ortega-Saenz et al., 2010). Clearly, additional studies are necessary to define the expression of different isoforms of TASK and how much they contribute to the outward K+ current in glomus cells of wild type mice. A direct cell-attached patch recording of single channel openings in mouse glomus cells should help to determine the level of expression of TASK-1, TASK-3 and TASK-1/3 heteromer in this species. Whether an age-dependent increase in TASK sensitivity to hypoxia is also observed in mouse glomus cells remains to be tested.
In glomus cells from adult rabbit, the outward K+ current was nearly fully blocked by TEA, suggesting that TASK may be absent in this species (Urena et al., 1989). In glomus cells from rabbit embryos, however, TEA only partially reduced the outward K+ current (Hescheler et al., 1989), suggesting that TEA-insensitive K+ channels such as TASK could be expressed in the newborn and decrease with age. Authors in many early studies did not explicitly state whether a leak subtraction was performed while recording whole-cell currents. Therefore, it is quite possible that TASK was functionally expressed in glomus cells of non-rodent animals but was removed by leak subtraction protocol that eliminated the contribution background channels. Again, single channel recordings in cell-attached patches should help to show the presence or absence of TASK in glomus cells of non-rodent species.
Recent studies show that TASK-1, but not TASK-3, is expressed in the human carotid body, as judged by mRNA and protein expression (Fagerlund et al., 2010) and microarray studies (Mkrtcian et al., 2012), suggesting that TASK-1, not TASK-1/3 heteromer, may be the background K2P channel in human glomus cells. The significance of expression of TASK-1 vs. TASK-3 or TASK-1/3 is not obvious, as they all behave as background K+ channels. Subtle differences in pH sensitivity have been observed. For example, TASK-1 is slightly more sensitive to acid between the pH 6.5 and 7.5 range than TASK-1/3 and TASK-3 (Kang et al., 2004), suggesting that the acid sensitivity of human glomus cells may be slightly higher than that of rodent glomus cells. Additional differences in physiological properties among TASK-1, TASK-3 and TASK1/3 are likely to exist, as functionally different tissues express specific TASK isoforms. For example, cardiac myocytes express only TASK-1 whereas cerebellar granule neurons express both TASK-1 and TASK-3. It will be important to confirm the expression of TASK-1 in human glomus cells by single channel recording, test its sensitivity to acid and hypoxia, and determine how much TASK-1 contributes to the outward K+ current in glomus cells from newborn and adult humans.
In addition to the sensitivity to hypoxia, TASK is modulated by extracellular pH, anesthetics, and agonists that act on Gq-coupled receptors. These properties of TASK are highly relevant for glomus cell function because acid and hypercapnia both excite glomus cells. Presumably, TASK helps to regulate cell excitability in response to changes in blood pH and CO2 tension. Volatile anesthetics such as halothane activate TASK (Patel et al., 1999), and thus may stabilize the resting Em of glomus cells. Therefore, volatile anesthetics may reduce the depolarizing effect of hypoxia in glomus cells. Other properties of TASKs are their sensitivity to receptor agonists that are coupled to Gq (Mathie, 2007), and this could be important for transmitter-induced auto-regulation of glomus cell function, as Gq-coupled muscarinic and purinergic receptors, among others, are expressed in glomus cells. Glomus cells secrete transmitters basally and during hypoxia (Donnelly, 2000), and this could affect the receptor-Gq signaling pathway and the excitability of the cells by modulating TASK activity. Therefore, the increase in O2-sensitivity of TASK during the early postnatal period may involve changes in the receptor-mediated modulation of TASK.
6.2. Inhibition of TASK by hypoxia
To understand how TASK becomes more sensitive to hypoxia during the early postnatal period, the identity of cellular O2 sensors and signals for TASK must be known. One proposed mechanism for hypoxia-induced inhibition of TASK involves a reduction of intracellular ATP production by mitochondria. Single channel recordings showed that TASK is active in cell-attached patches formed on isolated rat glomus cells. When inside-out patches were formed, TASK activity quickly decreased within seconds to less than 20% of the control level. TASK activity returned to the original level when 4–5 mM ATP was applied to the cytosolic side of the membrane (Williams et al., 2004). The rundown of channel activity upon forming inside-out patches and recovery of activity with ATP are not unique to TASK, and has been attributed to the loss and synthesis of membrane PIP2 (Gamper and Shapiro, 2007). The decrease in TASK activity that occurs following patch excision in glomus cells may be due to a similar mechanism, i.e., rapid loss of membrane PIP2 as a result of ATP depletion that limits the formation of PIP2 in the face of continued lipid phosphatase activity. In support of the modulation of TASK by ATP/PIP2, our preliminary test showed that a direct application of 10 µM PIP2 to inside-out patches of rat glomus cells re-activated the TASK channel (unpublished observation, D. Kim).
The effect of depletion of ATP on TASK activity has not been tested in intact glomus cells, as this is very difficult to do. Could hypoxia-induced decrease in intracellular [ATP] be small in glomus cells from newborn but large in cells from older animals? Such an effect could explain part of the postnatal increase in the sensitivity of glomus cells to hypoxia. The activity of enzymes involved in the generation of PIP2 could be low in glomus cells just after birth and increase gradually over time. It seems unlikely that any structural changes in TASK occur during the 2–3 weeks after birth to modify the effect of ATP/PIP2, as no TASK variants with different biophysical properties have been found and the biophysical properties of TASK do not appear to change with development (Kim et al., 2011b).
One interesting question relates to the speed by which hypoxia decreases intracellular [ATP] and reduces TASK activity in glomus cells. Because hypoxia is able to inhibit TASK within ~3 seconds, one must ask whether hypoxia can reduce [ATP] in such a short time period. Fluorescent studies measuring free [Mg2+] in the cell to reflect changes in [ATP] suggest that hypoxia may be able to reduce intracellular [ATP] reasonably rapidly (Varas et al., 2007). Other studies have questioned the role of ATP depletion as a mechanism underlying the hypoxia-induced excitation of glomus cells, as hypoxia inhibited the outward K+ current regardless of whether ATP was added to the pipette or not (Lopez-Barneo et al., 1988). In these studies, however, the outward K+ current inhibited by hypoxia was probably KV /BK current, as the leak current from TASK may have been subtracted. Overall, the ATP hypothesis remains a viable one, but needs to be supported by more direct measurements of changes in intracellular [ATP] in response to hypoxia, and by assessing the effect of [ATP] reduction on TASK in intact cells rather than in excised patches. Once the role of ATP is settled, one can then test the hypothesis that the hypoxia-induced reduction in [ATP] is small in the glomus cells from the newborn and increases during several weeks of the postnatal period.
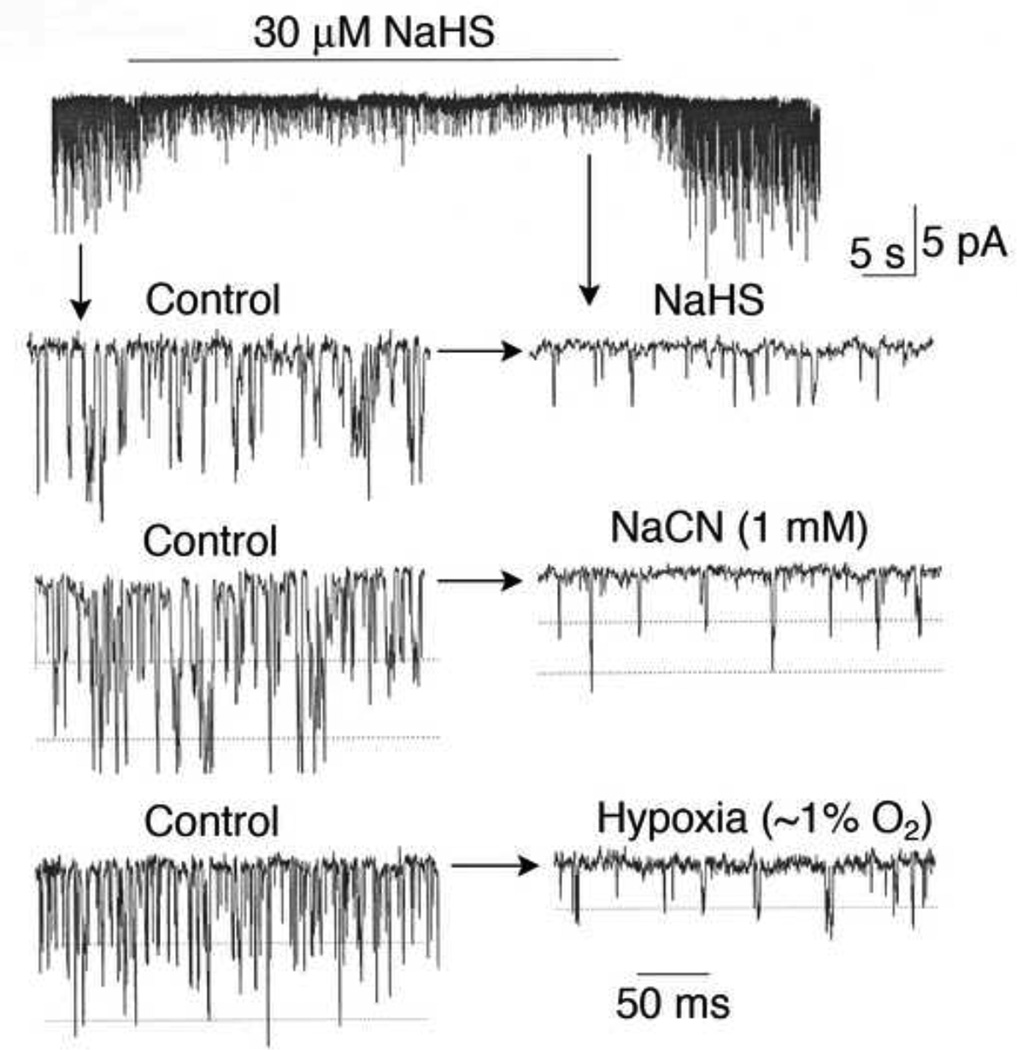
For TASK, mitochondria are very likely the O2 sensor, as mitochondrial electron transport inhibitors (cyanide, rotenone) and uncoupler of oxidative phosphorylation (FCCP) inhibit TASK activity (see Fig. 4). Therefore, it is possible that the sensitivity of the mitochondrial O2 sensor to hypoxia is low in glomus cells from newborn and increases over the 2–3 week period, due to changes in mitochondrial function. Perhaps the enzymes within the electron transport complex in glomus cells are less sensitive to hypoxia in newborn than older animals. It is also possible that the distance between mitochondria and the plasma membrane (where TASK is located) decreases during glomus cell maturation such that the signal from mitochondria is coupled to TASK inhibition more efficiently. Study of the differences in the mitochondrial biochemistry of electron transport, oxidative phosphorylation and oxygen consumption in glomus cells from newborn and older animal should reveal valuable information on the mechanism of postnatal maturation of O2 sensing by glomus cells.
Figure 4.
Inhibition of TASK by hypoxia, sodium cyanide and hydrogen sulfide. Cell-attached patches were formed and pipette potential set at 0 mV. Pipette solution contained (mM) 140 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3) and the bath perfusion solution contained (mM) 117 NaCl, 23 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 glucose (pH 7.3). Patches containing TASK were perfused with normoxic solution for ~2 min and then perfused for ~1–2 min with solution containing 30 µM NaHS, 1 mM NaCN or with solution bubbled with 0%O2/5%CO2. All three treatments produced ~80% inhibition of TASK activity, and this was associated with the reduction of single channel amplitude produced by cell depolarization from ~−60 mV to ~− 30 mV.
Another potential mitochondrial signal is AMPK, an enzyme that is stimulated by metabolic stress and hypoxia as a result of increased [AMP]/[ATP] ratio. There is evidence suggesting that AMPK may be involved in hypoxia-induced inhibition of TASK. For example, AICAR (an activator of AMPK) was found to inhibit a background K+ current in rat glomus cells (Wyatt et al., 2007). Additional evidence showing that hypoxia increases the [AMP]/[ATP] ratio, stimulates the AMPK activity and phosphorylates TASK at a specific residue in the cytoplasmic domain of TASK should strongly support the role of AMPK in the hypoxia-induced inhibition of TASK. Such studies must be done using glomus cells rather than mammalian cell lines, as TASK expressed in cloned cell lines has a very low sensitivity to hypoxia (Lee et al., 2006). Similarly, TASK-3 and TASK-1/3 expressed in cell lines (Cos-7, HeLa) showed no sensitivity to hypoxia (unpublished data; D. Kim). It would be interesting to test whether the AMPK-induced inhibition of TASK is weak in glomus cells from newborn and becomes stronger during the early postnatal period. It is possible that both a reduction of [ATP] and an increase in AMPK activity are involved in hypoxia-induced inhibition of TASK.
In addition to AMPK, ROS generated from NADPH oxidase or mitochondria could be involved in hypoxia-induced inhibition of TASK. In a recent preliminary study, direct application of H2O2 (1–30 mM) and xanthine/xanthine oxidase mixture (that generates superoxide radical) to inside-out patches of rat glomus cells failed to inhibit TASK (Kim and Papreck, 2011). In fact, high concentrations of H2O2 (>30 mM) stimulated TASK activity. ROS are therefore unlikely to be the signals that mediate the hypoxia-induced inhibition of TASK, and unlikely to be involved in the postnatal increase in TASK sensitivity to hypoxia.
As discussed in Section 5.2, H2S has been suggested as a signal for hypoxia-induced increase in transmitter secretion from glomus cells. In mice, the inhibition of CSE and/or CBS impaired the carotid sinus nerve and ventilatory responses to hypoxia (Li et al., 2010; Peng et al., 2010). In support of this finding, H2S was found to inhibit the outward K+ current, consistent with the excitatory effect of H2S on glomus cells isolated from mice (Li et al., 2010). Because the outward K+ current from rat and mouse glomus cells includes TASK, H2S may inhibit TASK and depolarize glomus cells. Indeed, a recent study showed that H2S inhibited TASK expressed in rat glomus cells (Buckler, 2012). Similar test in our laboratory also showed that NaHS is a strong inhibitor of TASK in intact glomus cells (see Fig. 4). Because H2S is a potent inhibitor of cytochrome oxidases in mitochondria, and mitochondrial electron transport inhibitors such as cyanide and rotenone inhibit TASK (Buckler, 2007), H2S could be inhibiting the mitochondrial electron transport to eventually inhibit TASK. Indeed, the changes in glomus cell Em, intracellular [Ca2+], [Mg2+] and [NADH] produced by NaHS were similar to those produced by cyanide, suggesting that H2S excites glomus cells via inhibition of mitochondrial oxidative phosphorylation (Buckler, 2012).
Regardless of the mechanism of action of H2S-induced excitation of glomus cells, a change in the rate of production and degradation of H2S would be expected to affect the basal [H2S] and affect TASK activity. For example, H2S generation could be high in glomus cells from the adult animal compared to that in the newborn, and this could partially contribute to the postnatal increase in TASK sensitivity to hypoxia. Although the H2S hypothesis is attractive, much more evidence is needed to show that H2S is an endogenous signal that mediates the hypoxia-induced inhibition of TASK and excitation of glomus cells. To show that H2S is involved in hypoxia-induced inhibition of TASK, it would be important to test the effect of specific blockers of all enzymes that synthesize H2S (CBS, CSE and mitochondrial 3-mercaptopyruvate sulfurtransferase) on TASK activity in response to hypoxia.
6.3. Role of TREK in the postnatal development of glomus cell response to hypoxia
In addition to TASK, rat glomus cells express TREK-1 and TREK-2. TREKs are high conductance (50–200 pS) K2P channels, and are characterized by their sensitivity to pH, mechanical stress, heat, unsaturated free fatty acids and receptor agonists that bind to Gq- and Gs-coupled receptors (Kim, 2003; Simkin et al., 2008). TREKs were found to be inactive in cell-attached patches of glomus cells and become active only when stimulated with acid (pH 6.0 solution), unsaturated free fatty acids and membrane stretch. Interestingly, the percentage of patches that showed opening of TREKs in response to these stimuli was ~30% in glomus cells from 0–1 day old and ~0% in 16–18 day old, showing that the expression of TREKs decreases during the early postnatal development (Kim et al., 2009b), somewhat analogous to the decrease in HERG current during the same period. As TREKs are normally in the closed state, they are probably not involved in the postnatal maturation of glomus cell response to hypoxia.
It is possible that TREKs are involved in other mechanisms to alter the excitability of glomus cells in response to hypoxia. For example, TREKs are associated with actin cytoskeletons and affect cytoskeletal remodeling during cell growth (Lauritzen et al., 2005). Cytoskeletal architecture could affect the function of O2 sensors and O2-sensitive ion channels that transduce the hypoxic signal to cell excitation. One important point to note is that, although TREKs are in the closed state in isolated glomus cells, they could be active in vivo, if the cells are exposed to an environment that contains appropriate stimuli such as neurotransmitters and lipids. TREK-1 knockout mice showed reduced response to volatile anesthetics and brain ischemia, exhibited an antidepressant phenotype and were more sensitive to pain (Honore, 2007), suggesting that TREKs are likely to be active in vivo. At present, the recording of TREK activity from glomus cells in carotid body slices has not been reported, and there are no highly specific inhibitors of TREKs. Therefore, it is difficult to assess the role of TREKs in the postnatal development of O2 sensing by glomus cells, despite the interesting finding that the expression level decreases during this critical period.
7. Inwardly rectifying K+ channels (Kir)
7.1. Kir4.1 and Kir5.1
Rat glomus cells express Kir4.1 and Kir5.1 channels, as judged by immunohistochemical experiments (Yamamoto et al., 2008). The single channel conductance of Kir4.1 is 22–27 pS, and Kir5.1 does not form a functional channel. Kir4.1 and Kir5.1 can form heteromers with a single channel conductance of 50–60 pS, and the heteromer is sensitive to changes in intracellular pH. When expressed in mammalian cell lines, Kir4.1 and Kir4.1/5.1 exhibit long lasting openings with brief closures. Because of their pH sensitivity and expression in central chemosensitive regions in the brainstem, the heteromeric Kir4.1/5.1 has been suggested to be a potential target for hypercapnia. However, mice with deletion of Kir5.1 showed no effect on central and peripheral chemosensitivity (Trapp et al., 2011). Although mRNA and proteins of these K+ channel subunits can be detected in glomus cells, no single channel currents with kinetics similar to those of Kir4.1 or Kir4.1/5.1 have yet been reported in glomus cells isolated from newborn or adult rats. The most likely explanation is that the expression levels of these inwardly rectifying K+ channels are very low in rat glomus cells. Therefore, Kir4.1/5.1 probably do not contribute significantly to the background K+ current and are unlikely to be involved in the postnatal development of O2 sensing by glomus cells.
7.2. ATP-sensitive K+ channel (KATP channel; Kir6/SUR)
A functional KATP channel is made up of four Kir6 and four SUR subunits. The biophysical and pharmacological properties of the KATP channel depend on subunit composition, and different tissues express specific isoforms. In rat glomus cells, KATP channel is present, as judged by the detection of mRNA and protein expression of Kir and SUR subunits, and opening of 72-pS channel with properties indistinguishable from those of cardiac and pancreatic KATP channels (Kim et al., 2011a). Expression of Kir6.1 has also been shown in glomus cells from cat (Fitzgerald et al., 2011). Based on single channel kinetics, the ATP-sensitive K+ channel in rat glomus cells is most likely a heteromer of Kir6.2 and SUR subunits. The KATP channel was not active in cell-attached patches, probably due to inhibition by intracellular ATP. In inside-out patches with no ATP in the bath solution, the KATP channel became transiently active, presumably due to relief from inhibition of the KATP channel by ATP. When active, the KATP channel could be blocked by glybenclamide, an inhibitor of the KATP channel, and stimulated by low level of ATP (0.1 mM) that keeps the channel phosphorylated. These properties of the KATP channel in glomus cells are similar to those of KATP channel in cardiac and neuronal tissues. Interestingly, acute hypoxia or glucose deprivation failed to activate the KATP channel, suggesting that the KATP channel is not involved in hypoxia-induced excitation of glomus cells. This is in keeping with the finding that glybenclamide or glucose deprivation had no effect on hypoxia-induced elevation of intracellular [Ca2+] (Kim et al., 2011a). As the KATP channel was not open in glomus cells from newborn and 16-day old rats, it would also not be involved in the postnatal development of glomus cell response to hypoxia. In general, the density of the KATP channel expression in rat glomus cells was low compared to those of BK and TASK, further questioning the role of this KATP channel in cell excitability under normal physiological conditions.
8. Concluding remarks
Studies have shown that the increase in the sensitivity of glomus cells to hypoxia during the early postnatal period largely occurs at an early step involving K+ current inhibition. Thus, an understanding of the mechanism of postnatal maturation of glomus cell response to hypoxia requires the knowledge of all functional K+ channels expressed in glomus cells from different ages, and the effect of hypoxia on their function. In this article, an attempt was made to summarize and discuss what is known about the functions of various K+ channels expressed in glomus cells from different species, their response to hypoxia and the underlying mechanisms involved, and their role in the postnatal development of glomus cell response to hypoxia. At present, there are more questions than answers with respect to the role of different K+ channels in hypoxia-induced excitation of glomus cells. Therefore, it is still too early to make any conclusive statements about how much Kv, BK and TASK channels contribute to the process and what the underlying mechanisms are. Given this uncertainty, identifying the mechanisms for the postnatal maturation of O2 sensing by glomus cells remains a challenging task.
Studies so far suggest that, in the rat, TASK has an important role in hypoxia-induced excitation of glomus cells as well as in the postnatal maturation of O2 sensing by glomus cells, and that mitochondrial hemeproteins may be involved. Much more studies are needed to understand the role of all K+ channels in these processes, and following questions may be useful to consider for future studies: (1) Which K+ channels are inhibited by hypoxia in intact glomus cells and how sensitive are they to different levels of hypoxia? (2) What are the O2 sensors and the associated signals for each type of O2-sensitive K+ channel? (3) Is the postnatal increase in the sensitivity of the K+ current to hypoxia also observed in glomus cells from non-rodent mammals, and which K+ channels are involved? (4) Is there a species- and age-dependent difference in the expression and function of BK and how sensitive is BK to different levels of hypoxia? (5) How do mitochondrial electron transport inhibitors and uncouplers of oxidative phosphorylation inhibit the activity of TASK and possibly other K+ channels, and how is the signaling process altered during maturation of glomus cells? (6) Are ion channels other than K+ channels involved in O2 sensing, and does the O2 sensitivity of such channels change during the early postnatal period? Answers to these questions are fundamental for further understanding of O2 sensing and the process of the postnatal development of glomus cell response to hypoxia. As has been in the past, the experimental results obtained in different laboratories even using the same species may not always be supportive of each other, perhaps due to differences in preparations used, methodology and animal strains. However, the resolution of controversial issues may reveal important information and insight that are helpful for future experiments.
Acknowledgments
The author thanks Drs. Insook Kim, David F. Donnelly and John L. Carroll who have been invaluable in the study of carotid body glomus cells, and have made possible the writing of this article. The author also thanks Dr. Charles E. McCormack for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Adachi T, Ishikawa K, Hida W, Matsumoto H, Masuda T, Date F, Ogawa K, Takeda K, Furuyama K, Zhang Y, Kitamuro T, Ogawa H, Maruyama Y, Shibahara S. Hypoxemia and blunted hypoxic ventilatory responses in mice lacking heme oxygenase-2. Biochem Biophys Res Commun. 2004;320:514–522. doi: 10.1016/j.bbrc.2004.05.195. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 1999;276:L875–L884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498(Pt 3):649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. Background leak K+-currents and oxygen sensing in carotid body type 1 cells. Respir Physiol. 1999;115:179–187. doi: 10.1016/s0034-5687(99)00015-8. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012 doi: 10.1007/s00424-012-1089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994a;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994b;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter E, Peers C. A standing Na+ conductance in rat carotid body type I cells. Neuroreport. 2001;12:1421–1425. doi: 10.1097/00001756-200105250-00025. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Kim I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir Physiol Neurobiol. 2005;149:201–215. doi: 10.1016/j.resp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- Delpiano MA, Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989;249:195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Developmental aspects of oxygen sensing by the carotid body. J Appl Physiol. 2000;88:2296–2301. doi: 10.1152/jappl.2000.88.6.2296. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Development of carotid body/petrosal ganglion response to hypoxia. Respir Physiol Neurobiol. 2005;149:191–199. doi: 10.1016/j.resp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Kim I, Carle C, Carroll JL. Perinatal hyperoxia for 14 days increases nerve conduction time and the acute unitary response to hypoxia of rat carotid body chemoreceptors. J Appl Physiol. 2005;99:114–119. doi: 10.1152/japplphysiol.01009.2004. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Kim I, Yang D, Carroll JL. Role of MaxiK-type calcium dependent K+ channels in rat carotid body hypoxia transduction during postnatal development. Respir Physiol Neurobiol. 2011;177:1–8. doi: 10.1016/j.resp.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, Barr BL, Rafferty JN, Ogunbayo O. Ion channel regulation by AMPK: the route of hypoxia-response coupling in thecarotid body and pulmonary artery. Ann N Y Acad Sci. 2009;1177:89–100. doi: 10.1111/j.1749-6632.2009.05041.x. [DOI] [PubMed] [Google Scholar]
- Fagerlund MJ, Kahlin J, Ebberyd A, Schulte G, Mkrtchian S, Eriksson LI. The human carotid body: expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiology. 2010;113:1270–1279. doi: 10.1097/ALN.0b013e3181fac061. [DOI] [PubMed] [Google Scholar]
- Firth AL, Gordienko DV, Yuill KH, Smirnov SV. Cellular localization of mitochondria contributes to Kv channel-mediated regulation of cellular excitability in pulmonary but not mesenteric circulation. Am J Physiol Lung Cell Mol Physiol. 2009;296:L347–L360. doi: 10.1152/ajplung.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Chang I, Kostuk E, Kiihl S. The impact of hydrogen sulfide (H(2)S) on neurotransmitter release from the cat carotid body. Respir Physiol Neurobiol. 2011;176:80–89. doi: 10.1016/j.resp.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Lopez-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991;88:2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Lopez-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J Gen Physiol. 1992;100:401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nino A, Obeso A, Baranda JA, Santo-Domingo J, Lopez-Lopez JR, Gonzalez C. MaxiK potassium channels in the function of chemoreceptor cells of the rat carotid body. Am J Physiol Cell Physiol. 2009;297:C715–C722. doi: 10.1152/ajpcell.00507.2008. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Bell H, Philmon M. Hydrogen sulfide oxidation and the arterial chemoreflex: effect of methemoglobin. Respir Physiol Neurobiol. 2011;177:273–283. doi: 10.1016/j.resp.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Carpenter E, Pepper DR, Kumar P, Peers C. Developmental changes in isolated rat type I carotid body cell K+ currents and their modulation by hypoxia. J Physiol. 1997;501(Pt 1):49–58. doi: 10.1111/j.1469-7793.1997.049bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Sanders K, Sundar K, Hoidal J, Fidone S. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am J Physiol Cell Physiol. 2002;282:C27–C33. doi: 10.1152/ajpcell.2002.282.1.C27. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Delpiano MA, Acker H, Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989;486:79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24:26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp PJ. Detecting acute changes in oxygen: will the real sensor please stand up? Exp Physiol. 2006;91:829–834. doi: 10.1113/expphysiol.2006.034587. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol. 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Fatty acid-sensitive two-pore domain K(+) channels. Trends Pharmacol Sci. 2003;24:648–654. doi: 10.1016/j.tips.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009a;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim I, Papreck JR, Donnelly DF, Carroll JL. Characterization of an ATP-sensitive K(+) channel in rat carotid body glomus cells. Respir Physiol Neurobiol. 2011a;177:247–255. doi: 10.1016/j.resp.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Papreck JR. Modulation of TASK by reactive oxygen species. FASEB J. 2011;LB474:19. [Google Scholar]
- Kim D, Papreck JR, Kim I, Donnelly DF, Carroll JL. Changes in oxygen sensitivity of TASK in carotid body glomus cells during early postnatal development. Respir Physiol Neurobiol. 2011b;177:228–235. doi: 10.1016/j.resp.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Boyle KM, Carroll JL. Postnatal development of E-4031-sensitive potassium current in rat carotid chemoreceptor cells. J Appl Physiol. 2005;98:1469–1477. doi: 10.1152/japplphysiol.01254.2003. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim D, Cavanaugh EJ, Carroll JL. TASK and TREK channels in rat carotid body chemoreceptor cells during postnatal development. FASEB J. 2009b;1002:3. [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Chemin J, Honore E, Jodar M, Guy N, Lazdunski M, Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Lewis A, Peers C, Ashford ML, Kemp PJ. Hypoxia inhibits human recombinant large conductance, Ca(2+)-activated K(+) (maxi-K) channels by a mechanism which is membrane delimited and Ca(2+) sensitive. J Physiol. 2002;540:771–780. doi: 10.1113/jphysiol.2001.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II. J Physiol. 2006;575:215–227. doi: 10.1113/jphysiol.2006.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez J, Gonzalez C, Urena J, Lopez-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Gonzalez C, Perez-Garcia MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997;499(Pt 2):429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Perez-Garcia MT. Oxygen sensitive Kv channels in the carotid body. Respir Physiol Neurobiol. 2007;157:65–74. doi: 10.1016/j.resp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol. 2005;98:415–420. doi: 10.1152/japplphysiol.00732.2004. [DOI] [PubMed] [Google Scholar]
- McCartney CE, McClafferty H, Huibant JM, Rowan EG, Shipston MJ, Rowe IC. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel alpha-subunits. Proc Natl Acad Sci U S A. 2005;102:17870–17876. doi: 10.1073/pnas.0505270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2012;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtcian S, Kahlin J, Ebberyd A, Gonzalez C, Sanchez D, Balbir A, Kostuk EW, Shirahata M, Jonsson Fagerlund M, Eriksson LI. The human carotid body transcriptome with focus on oxygen sensing and inflammation - a comparative analysis. J Physiol. 2012 doi: 10.1113/jphysiol.2012.231084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR. Hydrogen sulfide is an oxygen sensor in the carotid body. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Saenz P, Pascual A, Gomez-Diaz R, Lopez-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. J Gen Physiol. 2006;128:405–411. doi: 10.1085/jgp.200609591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai S, Buerk DG, Mokashi A, Chugh DK, Lahiri S. Cat carotid body chemosensory discharge (in vitro) is insensitive to charybdotoxin. Brain Res. 1997;747:324–327. doi: 10.1016/s0006-8993(96)01313-3. [DOI] [PubMed] [Google Scholar]
- Otsubo T, Kostuk EW, Balbir A, Fujii K, Shirahata M. Differential Expression of Large-Conductance Ca-Activated K Channels in the Carotid Body between DBA/2J and A/J Strains of Mice. Front Cell Neurosci. 2011;5:19. doi: 10.3389/fncel.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt JL, Ficker E, Yang T, Shams H, Bright GR, Prabhakar NR. HERG-Like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body. J Neurophysiol. 2000;83:1150–1157. doi: 10.1152/jn.2000.83.3.1150. [DOI] [PubMed] [Google Scholar]
- Pang L, Eyzaguirre C. Different effects of hypoxia on the membrane potential and input resistance of isolated and clustered carotid body glomus cells. Brain Res. 1992;575:167–173. doi: 10.1016/0006-8993(92)90440-k. [DOI] [PubMed] [Google Scholar]
- Pardal R, Ludewig U, Garcia-Hirschfeld J, Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97:2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol. 2007;157:75–82. doi: 10.1016/j.resp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci U S A. 2011;108:3065–3070. doi: 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia MT, Lopez-Lopez JR, Gonzalez C. Kvbeta1.2 subunit coexpression in HEK293 cells confers O2 sensitivity to kv4.2 but not to Shaker channels. J Gen Physiol. 1999;113:897–907. doi: 10.1085/jgp.113.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia MT, Lopez-Lopez JR, Riesco AM, Hoppe UC, Marban E, Gonzalez C, Johns DC. Viral gene transfer of dominant-negative Kv4 construct suppresses an O2-sensitive K+ current in chemoreceptor cells. J Neurosci. 2000;20:5689–5695. doi: 10.1523/JNEUROSCI.20-15-05689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piruat JI, Pintado CO, Ortega-Saenz P, Roche M, Lopez-Barneo J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol Cell Biol. 2004;24:10933–10940. doi: 10.1128/MCB.24.24.10933-10940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O(2) modulates large-conductance Ca(2+)-dependent K(+) channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res. 2001;89:430–436. doi: 10.1161/hh1701.095632. [DOI] [PubMed] [Google Scholar]
- Ross FA, Rafferty JN, Dallas ML, Ogunbayo O, Ikematsu N, McClafferty H, Tian L, Widmer H, Rowe IC, Wyatt CN, Shipston MJ, Peers C, Hardie DG, Evans AM. Selective expression in carotid body type I cells of a single splice variant of the large conductance calcium- and voltage-activated potassium channel confers regulation by AMP-activated protein kinase. J Biol Chem. 2011;286:11929–11936. doi: 10.1074/jbc.M110.189779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M, Sham JS. Roles of ion channels in carotid body chemotransmission of acute hypoxia. Jpn J Physiol. 1999;49:213–228. doi: 10.2170/jjphysiol.49.213. [DOI] [PubMed] [Google Scholar]
- Simkin D, Cavanaugh EJ, Kim D. Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J Physiol. 2008;586:5651–5663. doi: 10.1113/jphysiol.2008.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch. 2009;459:389–397. doi: 10.1007/s00424-009-0737-0. [DOI] [PubMed] [Google Scholar]
- Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- Telezhkin V, Brazier SP, Cayzac SH, Wilkinson WJ, Riccardi D, Kemp PJ. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir Physiol Neurobiol. 2010;172:169–178. doi: 10.1016/j.resp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ, Gourine AV. Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16) Exp Physiol. 2011;96:451–459. doi: 10.1113/expphysiol.2010.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena J, Lopez-Lopez J, Gonzalez C, Lopez-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas R, Wyatt CN, Buckler KJ. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J Physiol. 2007;583:521–536. doi: 10.1113/jphysiol.2007.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasicko MJ, Breitwieser GE, Kim I, Carroll JL. Postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2006;154:356–371. doi: 10.1016/j.resp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Wasicko MJ, Sterni LM, Bamford OS, Montrose MH, Carroll JL. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J Physiol. 1999;514(Pt 2):493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem. 2007;282:8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca(2+)-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995;483(Pt 3):559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Ishikawa R, Omoe K, Taniguchi K. Expression of inwardly rectifying K+ channels in the carotid body of rat. Histol Histopathol. 2008;23:799–806. doi: 10.14670/HH-23.799. [DOI] [PubMed] [Google Scholar]