Abstract
Background
Neotropical primates are important sylvatic hosts of Trypanosoma cruzi, the etiological agent of Chagas disease. Infection is often subclinical, but severe disease has been described in both free-ranging and captive primates. Panstrongylus megistus, a major T. cruzi vector, was found infesting a small-primate unit at Brasília zoo (ZooB), Brazil. ZooB lies close to a gallery-forest patch where T. cruzi circulates naturally. Here, we combine parasitological and molecular methods to investigate a focus of T. cruzi infection involving triatomine bugs and Neotropical primates at a zoo located in the Brazilian Savannah.
Methods
We assessed T. cruzi infection in vectors using optical microscopy (n = 34) and nested PCR (n = 50). We used quantitative PCR (qPCR) to examine blood samples from 26 primates and necropsy samples from two primates that died during the study. We determined parasite lineages in five vectors and two primates by comparing glucose-6-phosphate isomerase (G6pi) gene sequences.
Results
Trypanosoma cruzi was found in 44 vectors and 17 primates (six genera and eight species); one Mico chrysoleucus and one Saguinus niger had high parasitaemias. Trypanosoma cruzi DNA was detected in three primates born to qPCR-negative mothers at ZooB and in the two dead specimens. One Callithrix geoffroyi became qPCR-positive over a two-year follow-up. All G6pi sequences matched T. cruzi lineage TcI.
Conclusions
Our findings strongly suggest vector-borne T. cruzi transmission within a small-primate unit at ZooB – with vectors, and perhaps also parasites, presumably coming from nearby gallery forest. Periodic checks for vectors and parasites would help eliminate T. cruzi transmission foci in captive-animal facilities. This should be of special importance for captive-breeding programs involving endangered mammals, and would reduce the risk of accidental T. cruzi transmission to keepers and veterinarians.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1334-7) contains supplementary material, which is available to authorized users.
Keywords: Trypanosoma cruzi, Neotropical primates, Panstrongylus megistus, Brasília, Zoo
Background
About one third of all protozoan parasite species detected in non-human primates can also infect humans; Trypanosoma cruzi, the etiological agent of Chagas disease, is among the most epidemiologically relevant ones [1–3], [see also http://www.mammalparasites.org]. Chagas disease is endemic throughout Latin America, where about six million people are infected with T. cruzi [4–6]. Trypanosoma cruzi, a parasite of mammals, is transmitted primarily through the faeces of blood-sucking triatomine bugs; less often, infection can be acquired congenitally, through blood transfusion or organ or bone marrow transplantation, by consuming contaminated food or beverages, or accidentally in the laboratory [4, 5]. Seven highly diverse T. cruzi lineages circulate among mammals (at least eight orders and over 50 genera) and triatomines (over 140 species) in all continental American countries except Canada [3, 7, 8].
Carlos Chagas was the first to describe experimental (Callithrix spp.) and natural (Saimiri sciureus) T. cruzi infections in primates [9, 10]. Since then, the infection has been recorded in free-ranging individuals of 12 genera and over 30 species in all four Neotropical primate families – tamarins, marmosets, pygmy marmosets, squirrel monkeys and capuchins (Cebidae); titis, sakis and uakaris (Pitheciidae); night monkeys (Aotidae); and spider and howler monkeys (Atelidae) [3], [see also www.mammalparasites.org]. Lion tamarins (Leontopithecus spp.) can sustain long-lasting infections, often with high parasitaemias but with a relatively mild clinical picture, in the Brazilian Atlantic forest [3, 11–14].
Trypanosoma cruzi infections have also been reported in captive Neotropical primates. In the USA, T. cruzi was found in Saimiri boliviensis imported from Latin America [15]. Trypanosoma cruzi was detected in captive, wild-born Callithrix penicillata, Cebuella pygmaea, Saguinus imperator, and S. fuscicollis kept at the Brazilian National Primate Centre in Pará state [16]. Anti-T. cruzi antibodies were detected in 40 out of 198 captive primates (Cacajao, Callicebus, Callithrix, Cebus, Chiropotes, Leontopithecus, and Saguinus) from the Primatology Centre of Rio de Janeiro, Brazil, where transmission mediated by Panstrongylus megistus was suspected [17]. Captive Old World primates, including lemurs, macaques, baboons, and chimpanzees, can also become naturally infected with T. cruzi and may develop severe Chagas disease [18–22].
Infection of captive primates with T. cruzi is relevant in several respects. First, T. cruzi can kill valuable specimens including those belonging to endangered species; second, infection of laboratory primates can distort the results of animal-based research aimed at other ends; third, infected individuals in translocation-reintroduction programs can contribute to the spread of the parasite among free-ranging populations; finally, and importantly, infection can result in accidental transmission of the parasite to primate keepers, handlers, or veterinarians. Here, we combine parasitological and molecular methods to investigate a focus of T. cruzi infection involving triatomine bugs and Neotropical primates at a zoo located in the Brazilian Cerrado, where T. cruzi circulates extensively among wildlife and native vectors.
Methods
Ethics statement
This study was approved by the institutional review board of the Institute of Biological Sciences, University of Brasília, Brazil (CEUA-UnB No. 155506/2013).
Study site
Brasília, Brazil’s capital city, lies within the Cerrado eco-region, a mosaic of savannahs, dry forests/shrubs, and gallery forests originally covering most of central Brazil. Enzootic T. cruzi cycles are common in the Cerrado [3]. Brasília zoo (ZooB; 15°51’00”S, 47°56’20”W) spans ~140 hectares; to the south and south-west, it is adjacent to a protected, and hence relatively well-preserved, gallery-forest patch (~490 hectares) where T. cruzi infection has been recorded in Didelphis albiventris [23]. The small-primate unit at ZooB has four lodgings, each with a wire-mesh–fenced outdoor area and a masonry room with a wooden-box shelter. In 2012, ZooB keepers detected a triatomine bug colony in the small-primate unit.
Trypanosoma cruzi in triatomine bugs
Triatomines collected in the ZooB small-primate unit were identified after Lent & Wygodzinsky [24]. Trypanosoma cruzi infection was first investigated by optical microscopy (OM) in the bugs that arrived alive to the laboratory (see Table 1); fresh (400x) and Giemsa-stained (1000x) hindgut contents were examined. Next, we used a nested PCR (nPCR) to test for T. cruzi DNA in triatomine intestinal tissue. DNA was extracted using Illustra tissue and cells genomic Prep Mini Spin Kit (GE Healthcare). We first amplified 188 bp from the T. cruzi nuclear repetitive satellite region with primers TCZ1 and TCZ2 [25]; amplicons produced in this PCR were used in a second PCR with primers TCZ3 and TCZ4 [26]; see Additional file 1: Table S1). DNA extracted from a T. cruzi culture (Berenice strain, TcII) was used as a positive control, and MilliQ water and DNA from lab-reared, uninfected triatomines as negative controls. PCR products were resolved in 1.3 % agarose gel, stained with ethidium bromide, and visualised using UV fluorescence.
Table 1.
Trypanosoma cruzi infection among Panstrongylus megistus collected in a captive-primate unit at Brasília zoo, Federal District, Brazil: bug characteristics and results of optical microscopy and nested PCR
| Sex (adults) and stage (nymphs) | Optical microscopy | Nested PCR | ||
|---|---|---|---|---|
| Testeda | Positiveb | Tested | Positive | |
| Female | 12 | 2 | 13 | 11 |
| Male | 7 | 1 | 7 | 7 |
| Nymph II | 0 | - | 4 | 3 |
| Nymph III | 8 | 1 | 18 | 16 |
| Nymph V | 7 | 1 | 8 | 7 |
| Total | 34 | 5 | 50 | 44 |
a:Bugs that arrived dead and dry to the laboratory could not be tested by optical microscopy
b:Bugs with a positive optical microscopy were all also positive by nested PCR
Trypanosoma cruzi in primates
Twenty-six Neotropical primates were investigated, six of which were born at ZooB (see Table 2). Blood samples (1 mL) were drawn once (nine specimens) or on two occasions separated by ~24 months (17 specimens) for PCR-based T. cruzi detection and quantification. Necropsy samples (intestine, heart, and spleen) from one Saguinus niger and one Callithrix penicillata that died during the course of the study were also investigated. DNA was extracted from blood samples using the WizardTM Genomic DNA Purification Kit (Promega), and from necropsy samples using the Mini Spin Plus Kit (Biopur). PCR reactions were first carried out with primers TCZ1 and TCZ2 as described above; the products of this PCR were diluted 1:60 in MilliQ water and 2 μL were used as template for real-time quantitative PCR (qPCR) with Power SYBR© Green chemistry (Applied Biosystems) and primers TCZ3 and TCZ4 [26]; [see Additional file 1: Table S1]. Reactions were run in an ABI 7500 Real-Time PCR System thermocycler (Applied Biosystems) and the results analysed using StepOne v2.3 software (Applied Biosystems). We used MilliQ water and DNA extracted from uninfected mice blood as negative controls. Absolute quantification of parasite DNA was achieved by developing a standard curve with DNA extracted from a Berenice strain T. cruzi culture (108 parasites/mL) and serially diluted ten-fold to between 105 and 10−2 parasite. The standard curve relates qPCR threshold cycle values and known log-scale DNA concentrations [27, 28]; in our case, theoretical amplification efficiency was ~91 % (slope = −3.6) and the standard curve coefficient of determination was R2 = 0.99.
Table 2.
Trypanosoma cruzi infection among captive primates kept at Brasília zoo, Federal District, Brazil: primate characteristics and quantitative real-time PCR (qPCR) results
| Number | Species | Origin | State | Year of birth or arrival at ZooB | qPCRa | |
|---|---|---|---|---|---|---|
| First | Second | |||||
| 1 | Alouatta seniculus | IBAMA | AC | 2010 | 0.000 | ND |
| 2 | Aotus nigriceps b | Born at ZooB | DF | 2010 | 0.003 | 0.001 |
| 3 | Aotus nigriceps b | Born at ZooB | DF | 2012 | 1.051 | 0.002 |
| 4 | Aotus nigriceps | IBAMA | AC | 2006 | 0.000 | 0.000 |
| 5 | Aotus nigriceps b | Born at ZooB | DF | 2009 | 0.000 | 0.000 |
| 6 | Aotus nigriceps | ND | ND | 2007 | 0.000 | 0.000 |
| 7 | Callicebus cupreus | AIPU | MA | 2006 | 0.000 | 0.000 |
| 8 | Callithrix geoffroyi | Jequitinhonha | MG | 2010 | 0.000 | 0.012 |
| 9 | Callithrix penicillata | IBAMA | DF | 2012 | 0.039 | ND |
| 10 | Callithrix penicillata | IBAMA | DF | 2008 | 0.025 | 0.001 |
| 11 | Callithrix penicillata | IBAMA | ND | 2008 | 0.029 | Dead |
| 12 | Callithrix penicillata | IBAMA | DF | 2012 | ND | 0.105 |
| 13 | Callithrix penicillata | IBAMA | DF | 2012 | 0.000 | 0.000 |
| 14 | Leontopithecus chrysomelas | AIPU | MA | 2006 | 0.016 | ND |
| 15 | Leontopithecus chrysomelas | ND | ND | 2007 | 0.000 | 0.000 |
| 16 | Leontopithecus chrysomelas | Born at ZooB | DF | 1999 | 0.006 | ND |
| 17 | Leontopithecus chrysomelas c | Born at ZooB | DF | 2008 | 0.214 | ND |
| 18 | Leontopithecus chrysomelas d | Born at ZooB | DF | 2013 | ND | 0.007 |
| 19 | Leontopithecus rosalia | AIPU | MA | 2010 | 0.000 | 0.000 |
| 20 | Mico chrysoleucus | IBAMA | AM | 2008 | 0.025 | 17.000 |
| 21 | Mico argentatus e | AIPU | MA | 2007 | 0.010 | 0.006 |
| 22 | Pithecia irrorata | BH zoo | MG | 2012 | 0.073 | 0.003 |
| 23 | Saguinus imperator | AIPU | MA | 2008 | 0.000 | 0.000 |
| 24 | Saguinus niger | AIPU | MA | 2007 | 0.003 | 0.018 |
| 25 | Saguinus niger | ND | PA | 2008 | 0.042 | 0.001 |
| 26 | Saguinus niger | AIPU | MA | 2006 | 4.000 | Dead |
a:Parasite equivalents/100 ng DNA; First and Second qPCRs were carried out ~24 months apart. b:Born to qPCR-negative mothers (# 4 and 6). c:Born to qPCR-positive mother (# 16). d:Born to qPCR-negative mother (#15). e:Died in 2015. IBAMA Instituto Brasileiro do Meio Ambiente e Recursos Renováveis, ZooB Brasília zoo, AIPU Ararajuba do Ipê primate unit; BH, Belo Horizonte
Brazilian states: AC Acre, AM Amazonas, DF Distrito Federal, MA Maranhão, MG Minas Gerais, PA Pará. ND no data/not done
Identification of Trypanosoma cruzi lineages
A fragment of the single-copy nuclear glucose-6-phosphate isomerase (G6pi) gene was PCR-amplified as described in Brenière et al. [29] (see Additional file 1: Table S1), with positive and negative controls as described above for nPCR. Amplicons were analysed in 1.0 % agarose gel, stained with ethidium bromide, and visualised by UV fluorescence. PCR products were purified with the Illustra GFX PCR DNA & Gel Band Purification Kit (GE Healthcare) and submitted to Sanger sequencing. Sequences were edited using Geneious software (Biomatters) and compared with sequences deposited in GenBank using the BLASTn algorithm (http:blast.ncbi.nlm.nih.gov).
Results
We collected 20 adult bugs and 30 nymphs at the ZooB small-primate unit (Table 1, Fig. 1); all were identified as Panstrongylus megistus. Thirty-four of those triatomines arrived alive to the laboratory and were examined by OM; five (14.7 %) were found infected with T. cruzi (Table 1, Fig. 1). nPCR was positive in the five OM-positive bugs, in 24 OM-negative specimens, and in 15 bugs not examined by OM; thus, overall nPCR positivity was 88 % (Table 1).
Fig. 1.
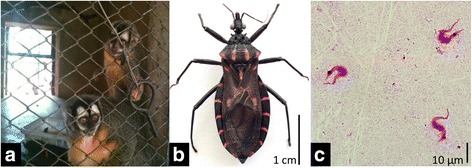
Trypanosoma cruzi infection among Panstrongylus megistus collected in a captive-primate unit at Brasília zoo, Federal District, Brazil. a Captive-primate unit where P. megistus specimens were collected. b Adult specimen of P. megistus. c Trypomastigotes detected in P. megistus feces after Giemsa staining
Trypanosoma cruzi DNA was detected in 17 out of 26 (65.4 %) primates tested by qPCR (Table 2). Qualitative results from blood samples taken ~24 months apart were consistent in 16 out of 17 specimens tested twice; the exception was one Callithrix geoffroyi that became qPCR-positive over the course of the study (Table 2). Absolute DNA quantification suggested high parasitaemias in one Mico chrysoleucus (17 parasite equivalents/100 ng DNA) and one Saguinus niger (4 parasite equivalents/100 ng DNA) (Table 2). Three individuals with T. cruzi DNA-positive blood samples died during the study (Table 2); necropsy samples from two of them were submitted to qPCR, which detected small amounts of T. cruzi DNA (<1 parasite equivalents/100 ng DNA) in the spleen of one C. penicillata and in the heart, spleen, and intestine of the highly parasitaemic S. niger mentioned above. Five out of six primates born at ZooB tested positive for T. cruzi DNA by qPCR, including three individuals born to qPCR-negative mothers (Table 2).
G6pi sequences from five P. megistus, one S. niger, and one M. chrysoleucus were all 99-100 % identical to that of T. cruzi strain OPS21cl11 (TcI lineage, GenBank accession number AY484472.1; see Broutin et al. [30]).
Discussion
We have presented a detailed description of a T. cruzi infection focus in triatomine bugs and captive Neotropical primates at Brasília zoo in central Brazil. Highly-sensitive molecular assays detected T. cruzi nuclear DNA in most of the vectors (88 %) and primates (65.4 %) we tested. Infection was identified in primates of six genera and eight species, including the endangered Leontopithecus chrysomelas, the vulnerable Saguinus niger, and species with unknown preservation status such as Mico chrysoleucus and Pithecia irrorata (see www.iucnredlist.org). Infection with T. cruzi is harmful to the primates and brings about a non-negligible risk of accidental transmission of the parasite to animal keepers, handlers, and veterinarians.
Three primates born to qPCR-negative mothers at ZooB were infected with the same T. cruzi strain as P. megistus caught in their lodgings. This finding is strongly suggestive of within-cage, P. megistus-mediated parasite transmission. Although we did not test for anti-T. cruzi antibodies through serology, which might have revealed infection in qPCR-negative individuals [3], the high sensitivity of our qPCR [28] and the rarity of vertical transmission among tamarins [14] make us think that vector-borne transmission was likely the source of most primate infections at ZooB. Primates including humans can acquire T. cruzi from triatomines either through direct contact of infected vector faeces with skin or mucosae or by the oral route when bugs carrying the parasite are eaten or contaminate foods or beverages [4, 5, 31]. In addition, that most of the P. megistus nymphs we collected inside primate lodgings tested positive for T. cruzi (Table 1) clearly implies within-cage transmission of the parasite from infected primates to the vectors – triatomine nymphs lack wings and get T. cruzi through infected bloodmeals [24].
Captive-breeding and translocation-reintroduction programs are important for the management (and possibly recovery) of endangered species such as the flagship lion tamarins [32–34]. Preventing or limiting infectious disease spread is one crucial component of such programs [33, 34]. Although T. cruzi occurs naturally across the range of all continental American primate species, and although infection with the parasite seems common in many wild populations, the release of infected specimens can be problematic in at least three relevant ways. First, T. cruzi is a highly diverse parasite [8, 35], so that foreign strains can be introduced into an area where they do not circulate naturally. Second, T. cruzi infection seems to be strongly focal among free-ranging Neotropical primate populations [11, 14]; infected individuals introduced into a low-prevalence site can hence contribute to spreading the parasite. Finally, T. cruzi-infected individuals may have relatively low odds of surviving when released into the wild, which may threaten reintroduction success [36]. By showing how high T. cruzi infection rates can be among captive Neotropical primates, our results underscore the need to carefully test specimens scheduled for release in the context of endangered-species translocation-reintroduction programs [3, 14].
On a more local scale, we found a thriving P. megistus colony within the small-primate unit at ZooB. Infected bugs were found inside primate wooden shelters resembling the hollow-tree vertebrate nests and refuges P. megistus occupies in the wild [37]. Although widely distributed, P. megistus is primarily associated with the humid Brazilian Atlantic forest [38, 39]; in the Cerrado and other seasonally dry eco-regions, it occurs mainly in moister forest patches. The close proximity of ZooB to a preserved gallery-forest patch where T. cruzi (TcI lineage) has been shown to circulate [23] suggests a likely origin for the bugs (and possibly also the parasites) we found. Panstrongylus megistus, one of the most important vectors of human Chagas disease, can also colonise in and around human dwellings [9, 24, 37, 38]. Our findings warn about the possibility of domestic or peridomestic, P. megistus-borne T. cruzi transmission foci in the vicinity of preserved forest patches in Brasília [39] and elsewhere across the Cerrado [37, 38].
Finally, our results highlight the latent risk of accidental T. cruzi transmission from infected primates (and probably other mammals) to zoo workers including keepers and veterinarians. If unaware of the infection status of the animals they handle, these workers may be at risk of acquiring the infection while drawing or manipulating biological samples, during surgical or dental procedures, or even when performing necropsies on fresh carcasses. Although needles are involved in most of the accidents reported, T. cruzi transmission can also occur through intact mucosae or apparently intact skin, and possibly via droplets or aerosols [40]. Zookeepers may undergo additional risks if they have contact with infected triatomines infesting animal facilities.
Conclusions
The findings we have presented strongly suggest vector-borne T. cruzi transmission within a small-primate unit at Brasília zoo. We suspect that the vectors, and possibly also the parasites, originally came from nearby gallery forest – a hypothesis that can be tested with samples from both habitats and high-resolution molecular markers [35]. In practical terms, we suggest that periodic checks for triatomine infestations and T. cruzi infections should become routine practice in captive-animal facilities located near known or suspected vector habitat. Pyrethroid insecticide spraying yields efficient, short-term infestation control; in the long run, the use of bug-refractory animal lodgings – i.e., with fewer potential bug-hiding sites (crevices, cracks…) and easier to inspect and treat – could help prevent or slow re-infestation. Early detection and elimination of T. cruzi transmission foci should be of particular interest for captive-breeding and translocation-reintroduction programs involving endangered mammal species. Together with specific training on Chagas disease and T. cruzi-related biohazards, this would also help reduce the risk of accidental T. cruzi transmission from infected mammals or vectors to captive-animal handlers, keepers, and veterinarians.
Acknowledgements
We thank F Abad-Franch, RV Monteiro, V Martins, VJ de Mendonça, and AJ de Andrade for reviewing the manuscript; the Brasília zoo staff for logistic support; and MB de Castro and the staff of the University of Brasília Veterinary Hospital.
Funding
The study was supported by CNPq and CAPES (grant n ° 1276/11).
Abbreviations
- FD
Federal District
- G6pi
Single-copy nuclear glucose-6-phosphate isomerase
- kDNA
Kinetoplast DNA
- PCR
Polymerase Chain Reaction
- nPCR
Nested Polymerase Chain Reaction
- qPCR
Quantitative real-time PCR
- UV
Ultraviolet
- ZooB
Brasília zoo.
Additional file
PCR conditions and primers used for Trypanosoma cruzi detection. (DOC 69 kb)
Footnotes
Competing interests
None declared.
Authors’ contributions
TTCMS, NN and RGG conceived and designed the study; all authors participated in conducting the study and were involved in the analysis and interpretation of data; TTCMS wrote the article; RGG, NN, LH, CACC and MMH reviewed the manuscript; all authors had full access to all data and read and approved the final manuscript.
Contributor Information
Thaís Tâmara Castro Minuzzi-Souza, Email: thaistam@yahoo.com.br.
Nadjar Nitz, Email: nnitz@unb.br.
Monique Britto Knox, Email: monaknox@gmail.com.
Filipe Reis, Email: filipereisbio@gmail.com.
Luciana Hagström, Email: loubex@hotmail.com.
César A. Cuba Cuba, Email: cesarcuba@hotmail.com
Mariana Machado Hecht, Email: marianahecht@gmail.com.
Rodrigo Gurgel-Gonçalves, Email: gurgelrg@hotmail.com.
References
- 1.Nunn CL, Altizer SM. The Global Mammal Parasite Database: an online resource for infectious disease records in wild primates. Evol Anthropol. 2005;14(1):1–2. doi: 10.1002/evan.20041. [DOI] [Google Scholar]
- 2.Brinkworth JF, Pechenkina K. Primates, pathogens and evolution: an introduction. In: Brinkworth JF, Pechenkina K, editors. Primates, Pathogens, and Evolution. New York: Springer, X + 428p; 2013. pp. 1–14. [Google Scholar]
- 3.Jansen AM, Xavier SC, Roque AL. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015;151:1–15. doi: 10.1016/j.actatropica.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 5.Bern C. Chagas’ Disease. N Engl J Med. 2015;373:456–66. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 6.WHO – World Health Organization Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90:33–43. [PubMed] [Google Scholar]
- 7.Noireau F, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40(2):26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messenger LA, Miles MA, Bern C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev Anti Infect Ther. 2015;13(8):995–1029. doi: 10.1586/14787210.2015.1056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen. n. sp. ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1(2):159–218.
- 10.Chagas C. Sobre a verificação do Trypanosoma cruzi em macacos do Pará (Chrysothrix sciureus) Nota Prévia Sciencia Medica. 1924;2:75–76. [Google Scholar]
- 11.Lisboa CV, Mangia RH, De Lima NRC, Martins A, Dietz J, Baker AJ, et al. Distinct patterns of Trypanosoma cruzi infection in Leontopithecus rosalia in distinct Atlantic coastal rainforest fragments in Rio de Janeiro – Brazil. Parasitology. 2004;129:703–711. doi: 10.1017/S0031182004005918. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro RV, Baldez J, Dietz J, Baker A, Lisboa CV, Jansen AM. Clinical, biochemical, and electrocardiographic aspects of Trypanosoma cruzi infection in free-ranging golden lion tamarins (Leontopithecus rosalia) J Med Primatol. 2006;35(1):48–55. doi: 10.1111/j.1600-0684.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro RV, Dietz JM, Jansen A. The impact of concomitant infections by Trypanosoma cruzi and intestinal helminths on the health of wild golden and golden-headed lion tamarins. ResVet Sci. 2010;89(1):27–35. doi: 10.1016/j.rvsc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Lisboa CV, Monteiro RV, Martins AF, Xavier SC, Lima VS, Jansen AM. Infection with Trypanosoma cruzi TcII and TcI in free-ranging population of lion tamarins (Leontopithecus spp): an 11-year follow-up. Mem Inst Oswaldo Cruz. 2015;110:394–402. doi: 10.1590/0074-02760140400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan JJ, Steurer F, Benavides G, Tarleton RL, Eberhard ML, Landry S. Trypanosomes and microfilariae in feral owl and squirrel monkeys maintained in research colonies. Am J Trop Med Hyg. 1993;49(2):254–59. doi: 10.4269/ajtmh.1993.49.254. [DOI] [PubMed] [Google Scholar]
- 16.Ziccardi M, Lourenço-de-Oliveira R, Lainson R, Brigido MCO, Muniz JAPC. Trypanosomes of non-human primates from the National Centre of Primates, Ananindeua, State of Pará, Brazil. Mem Inst Oswaldo Cruz. 2000;95(2):157–59. doi: 10.1590/S0074-02762000000200004. [DOI] [PubMed] [Google Scholar]
- 17.Lisboa CV, Mangia RH, Rubião E, de Lima NRC, Xavier SCC, Picinatti A, et al. Trypanosoma cruzi transmission in a captive primate unit, Rio de Janeiro, Brazil. Acta Trop. 2004;90(1):97–106. doi: 10.1016/j.actatropica.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Kasa TJ, Lathrop GD, Dupuy HJ, Bonney CH, Toft JD. An endemic focus of Trypanosoma cruzi infection in a subhuman primate research colony. J Am Vet Med Assoc. 1977;171(9):850–54. [PubMed] [Google Scholar]
- 19.Teixeira AR, Nascimento RJ, Sturm NR. Evolution and pathology in Chagas disease – a review. Mem Inst Oswaldo Cruz. 2006;101(5):463–91. doi: 10.1590/S0074-02762006000500001. [DOI] [PubMed] [Google Scholar]
- 20.Hall CA, Polizzi C, Yabsley MJ, Norton TM. Trypanosoma cruzi prevalence and epidemiologic trends in lemurs on St. Catherines Island, Georgia. J Parasitol. 2007;93:93–96. doi: 10.1645/GE-936R.1. [DOI] [PubMed] [Google Scholar]
- 21.Seiler BM, Dick EJ, Jr, Guardado-Mendoza R, VandeBerg JL, Williams JT, Mubiru JN, et al. Spontaneous heart disease in the adult chimpanzee (Pan troglodytes) J Med Primatol. 2009;38(1):51–58. doi: 10.1111/j.1600-0684.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24(4):655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurgel-Gonçalves R, Ramalho ED, Duarte MA, Palma ART, Abad-Franch F, Carranza JC, et al. Enzootic transmission of Trypanosoma cruzi and T. rangeli in the Federal District of Brazil. Rev Inst Med Trop S Paulo. 2004;46:323–30. doi: 10.1590/S0036-46652004000600005. [DOI] [PubMed] [Google Scholar]
- 24.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull Am Mus Nat History. 1979;163:123–520. [Google Scholar]
- 25.Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989;27(7):1477–82. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochs DE, Hnilica VS, Moser DR, Smith JH, Kirchhoff LV. Postmortem diagnosis of autochthonous acute chagasic myocarditis by polymerase chain reaction amplification of a species-specific DNA sequence of Trypanosoma cruzi. Am J Trop Med Hyg. 1996;54(5):526–29. doi: 10.4269/ajtmh.1996.54.526. [DOI] [PubMed] [Google Scholar]
- 27.Rutledge RG, Côté C. Mathematics of quantitative kinetic PCR and the application of standard curves. Nucleic Acids Res. 2003;31(16):e93. doi: 10.1093/nar/gng093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, et al. Accurate Real-Time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl Trop Dis. 2009;3(4):e419. doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenière SF, Aliaga C, Waleckx E, Buitrago R, Salas R, Barnabé C, et al. Genetic characterization of Trypanosoma cruzi DTUs in wild Triatoma infestans from Bolivia: predominance of TcI. PLoS Negl Trop Dis. 2012;6(5):e1650. doi: 10.1371/journal.pntd.0001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broutin H, Tarrieu F, Tibayrenc M, Oury B, Barnabé C. Phylogenetic analysis of the glucose-6-phosphate isomerase gene in Trypanosoma cruzi. Exp Parasitol. 2006;113(1):1–7. doi: 10.1016/j.exppara.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Shikanai-Yasuda MA, Carvalho NB. Oral transmission of Chagas disease. Clin Infect Dis. 2012;54(6):845–52. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 32.Kleiman DG, Mallison JJC. Recovery and management committees for lion tamarins: partnerships in conservation planning and implementation. Conserv Biol. 1998;12:27–38. doi: 10.1046/j.1523-1739.1998.96287.x. [DOI] [Google Scholar]
- 33.Seddon PJ, Armstrong DP, Maloney RF. Developing the science of reintroduction biology. Conserv Biol. 2007;21(2):303–312. doi: 10.1111/j.1523-1739.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong DP, Seddon PJ. Directions in reintroduction biology. Trends Ecol Evol. 2008;23(1):20–25. doi: 10.1016/j.tree.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Messenger LA, Yeo M, Lewis MD, Llewellyn MS, Miles MA. Molecular genotyping of Trypanosoma cruzi for lineage assignment and population genetics. Methods Mol Biol. 2015;1201:297–337. doi: 10.1007/978-1-4939-1438-8_19. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham AA. Disease risks of wildlife translocations. Conserv Biol. 1996;10(2):349–53. doi: 10.1046/j.1523-1739.1996.10020349.x. [DOI] [Google Scholar]
- 37.Patterson JS, Barbosa SE, Feliciangeli MD. On the genus Panstrongylus Berg 1879: evolution, ecology and epidemiological significance. Acta Trop. 2009;110(2-3):187–99. doi: 10.1016/j.actatropica.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Forattini OP. Biogeografia, origem e distribuição da domiciliação de triatomíneos no Brasil. Rev Saude Publica. 1980;14:265–99. doi: 10.1590/s0034-89101980000300002. [DOI] [PubMed] [Google Scholar]
- 39.Maeda MH, Knox MB, Gurgel-Gonçalves R. Occurrence of synanthropic triatomines (Hemiptera: Reduviidae) in the Federal District, Brazil. Rev Soc Bras Med Trop. 2012;45(1):71–76. doi: 10.1590/S0037-86822012000100014. [DOI] [PubMed] [Google Scholar]
- 40.Herwaldt BL. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev. 2001;14(4):659–88. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


