Abstract
Germinomas presenting with a pituitary stalk lesion and panhypopituitarism are rare in children, and their definite diagnosis is challenging. An invasive diagnostic approach, such as a transsphenoidal biopsy, is often required prior to establishing a treatment regimen. A 13-year-old female presented with 1 year of secondary amenorrhea, fatigue, and progressive thirst with polyuria. Laboratory work-up revealed panhypopituitarism (central hypothyroidism, hypogonadotropic hypogonadism, adrenal insufficiency and central diabetes insipidus). α-Fetoprotein and β-human chorionic gonadotropin were not elevated in serum nor in cerebrospinal fluid. The magnetic resonance imaging (MRI) of the pituitary region showed an enhancing infundibular lesion, extending into the hypothalamus, and infiltrating the pituitary gland. A transsphenoidal biopsy of the infundibular lesion confirmed the diagnosis of germinoma (germ-cell tumor). After appropriate hormone replacement therapy, chemotherapy and low-dose radiation therapy, the patient achieved complete resolution of the pituitary stalk lesion on the MRI.
Keywords: germ-cell tumor, germinoma, pituitary stalk lesion
Introduction
Pituitary stalk lesions are rare and their definite diagnosis is challenging as different etiologies present in a clinically similar manner. Radiographic characteristics are unable to reliably differentiate germinomas from other central nervous system tumors. While tumor markers such as α-fetoprotein (AFP) and β-human chorionic gonadotropin (hCG) may be helpful, they are frequently not elevated.
We report a teenage girl with germinoma presenting with panhypopituitarism (central hypothyroidism, hypogonadotropic hypogonadism, adrenal insufficiency and central diabetes insipidus). A relatively new medical treatment combined with low-dose radiation therapy has been successful in eliminating the tumor so far.
Case report
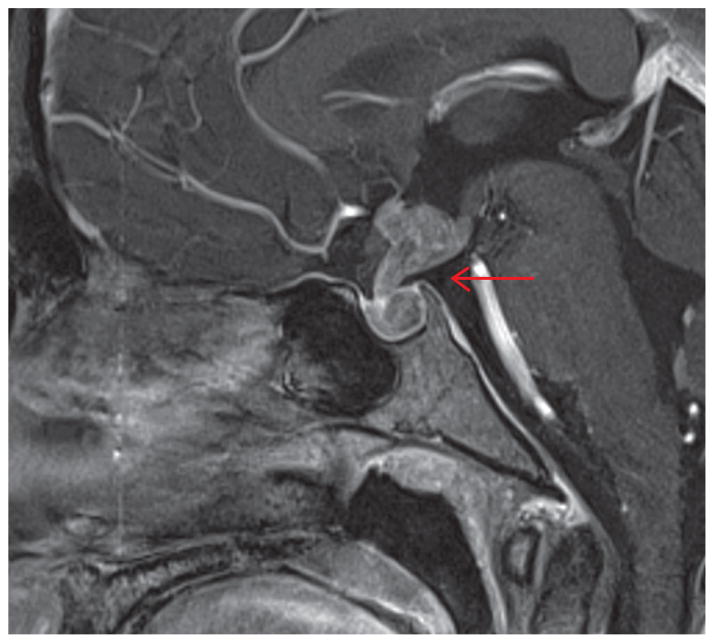
A 13-year-old female presented with 1 year of secondary amenorrhea, fatigue, and progressive thirst with polyuria. Her past medical history was unremarkable. Menarche was at the age of 10 with subsequent regular menses. Her maternal grandmother had metastatic lung cancer. A review of her history showed cessation of growth within the past year. Physical examination was significant for normal stature and weight, pale color, and Tanner IV breasts and pubic hair. There were no visual field defects and no galactorrhea. Laboratory work-up revealed: elevated serum sodium level, luteinizing hormone (LH) <0.1 IU/L (normal range 1–77 IU/L), follicular-stimulating hormone (FSH) <1 IU/L (normal range 1–21 IU/L), estradiol <18.4 pmol/L (normal range 45.1–183.6 pmol/mL), thyroid-stimulating hormone (TSH) < 0.01 μg/mL (normal range 0.4–4.0 μg/mL), and adrenocorticotropic hormone (ACTH) <1.1 pmol/L (normal range 0–10.1 pmol/L) and cortisol <27.6 nmol/L (normal range 138.0–689.8 nmol/L). Serum prolactin was 2278 pmol (normal range 87.0–1087.0 pmol) confirmed upon dilution. α-Fetoprotein (AFP) and β-human chorionic gonadotropin (β-hCG) were not elevated in serum nor in cerebrospinal fluid (CSF). The history and appropriate testing excluded common infectious causes. The magnetic resonance imaging (MRI) of the pituitary region showed an enhancing infundibular lesion, extending into the hypothalamus, compressing the anterior third ventricle, and infiltrating the pituitary gland (Figure 1).
Figure 1.
Magnetic resonance imaging (MRI) of the pituitary region.
Clinical challenge
The key feature in this patient is the presence of, what appears to be, panhypopituitarism because of secondary amenorrhea due to hypogonadotrophic hypogonadism, along with hypothyroidism, adrenal insufficiency, and diabetes insipidus. These findings point toward the involvement of the entire pituitary gland or the infundibulum (i.e., pituitary stalk). MRI of the pituitary region confirmed our suspicions.
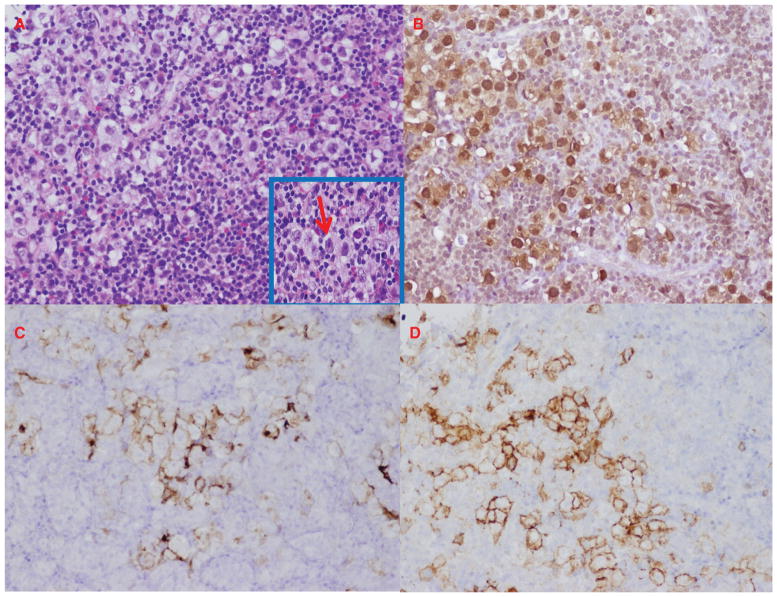
It is imperative to establish a definitive diagnosis of a lesion prior to initiating any treatment. Because of the rapid onset of the symptoms a clinician should suspect a rapidly advancing process that may require invasive diagnostic approaches. As common infectious causes were ruled out and tumor markers (AFP, hCG) were non-contributory, a transsphenoidal biopsy of the infundibular lesion was the best next step in the management of the patient. The pathology revealed clusters of large cells with vesicular nuclei and prominent pink nucleoli dispersed in a rich background of lymphohistiocytic infiltrate with eosinophils (Figure 2A). The tumor cells were positive for markers OCT-4, C-KIT, placental alkaline phosphatase (PLAP), consistent with a diagnosis of germ-cell tumor (Figure 2B–D).
Figure 2.
Transsphenoidal pituitary stalk biopsy. (A) Hematoxylin and eosin stain. Neoplastic cells with clear cytoplasm, vesicular nuclei and prominent nucleoli in a background of lymphoid infiltrates [magnification × 20 (inset magnification, × 60)]. (B) OCT-4 stain (magnification, × 20). (C) PLAP stain (magnification, × 20). (D) C-KIT stain (magnification, × 20).
Discussion
Secondary amenorrhea associated with central diabetes insipidus (CDI) requires prompt investigation and treatment as delay can be life-threatening. CDI is commonly seen at the time of presentation in pituitary stalk lesions, such as germinoma (1). Its presence may reflect rapid growth of the lesion. Causes of CDI in children were previously reviewed (2). Cases can be divided into congenital and acquired sources, with the latter being more common. The differential diagnosis includes tuberculosis, neurosarcoidosis, amyloidosis, primary tumors including germinoma (3), pinealoma, glioma or craniopharingeoma (4, 5), Langerhans cell histiocystosis (6), or metastatic disease.
Germinomas are relatively rare in children, accounting for < 3% of primary pediatric brain tumors, and are commonly associated with endocrine abnormalities (7). In some cases, elevation of tumor markers, such as AFP and β-hCG in serum or CSF can be seen, but it is not always there. In this case, all tumor markers in serum and CSF were not elevated.
Diagnosis should be established histologically, especially if tumor markers in serum and CSF are non-contributory (8). Histological diagnosis is fundamental in establishing a treatment regimen (7). A transsphenoidal biopsy approach is usually safe, particularly in centers with robust experience
Prognosis and treatment
Pure germ-cell tumors, generally associated with negative tumor markers, carry a favorable prognosis, with a 5-year survival rate > 90% with appropriate treatment (9). These tumors are usually responsive to radiation therapy, and in most cases, radiotherapy alone may be sufficient for complete response. However, cranial radio-therapy is linked to undesirable late effects (neurocognitive defects, headaches) (3). Neoadjuvant chemotherapy plus radiotherapy was another viable option for the patient, and allowed for decreased doses of radiotherapy and lower risk of late effects. Kretschmar et al. reported promising results from their clinical trial, evaluating pre-radiation chemotherapy with response-based radiation therapy in children (10).
Patient outcome
The patient of the vignette was appropriately treated with hormone replacement therapy for diabetes insipidus, adrenal insufficiency and hypothyroidism. After four cycles of chemotherapy (carboplatin plus etoposide) and low-dose radiation therapy, she achieved complete resolution of the pituitary stalk lesion on the MRI.
Recommendations
Transspenoidal biopsy of a pituitary stalk lesion is necessary to establish histological diagnosis, which is fundamental to selecting the treatment regimen.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development, protocol 1997-CH-0076 (ClinicalTrials.gov Identifier: NCT00001595). These organizations had no further role in the collection, analysis and interpretation of data; in the writing of the report, and in the decision to submit the paper for publication. The principal investigator had full access to all the data in the case and takes responsibility for the integrity of the data and the accuracy of the data interpretation.
We thank our oncology colleagues at the NIH for treating the case patient. We thank Diane Cooper, MSLS, NIH Library for providing assistance in writing this manuscript. We thank Maya Nadison, MHS who assisted with proofreading the manuscript.
Footnotes
Disclosures: The authors have nothing to disclose.
Contributor Information
Mihail Zilbermint, Program on Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Mary S. Ramnitz, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Maya B. Lodish, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Christina Kanaka-Gantenbein, First Department of Pediatrics, Athens University Medical School, “Aghia Sophia” Children’s Hospital, Athens, Greece.
Antonis Kattamis, First Department of Pediatrics, Athens University Medical School, “Aghia Sophia” Children’s Hospital, Athens, Greece.
Charalampos Lyssikatos, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Nicholas J. Patronas, Department of Diagnostic Radiology, Warren Grant Magnuson Clinical Center, National Institutes of Health, Bethesda, MD, USA
Martha M. Quezado, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Constantine A. Stratakis, Email: stratakc@mail.nih.gov, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Building 10/CRC 1-3330, 10 Center Drive, Bethesda, MD 20892, USA, Phone: +1 (301) 594-5984, Fax: +1 (301) 480-6480
References
- 1.Afzal S, Wherrett D, Bartels U, Tabori U, Huang A, et al. Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol. 2010;97:393–9. doi: 10.1007/s11060-009-0033-z. [DOI] [PubMed] [Google Scholar]
- 2.Maghnie M, Cosi G, Genovese E, Manca-Bitti ML, Cohen A, et al. Central diabetes insipidus in children and young adults. New Engl J Med. 2000;343:998–1007. doi: 10.1056/NEJM200010053431403. [DOI] [PubMed] [Google Scholar]
- 3.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–55. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. Oncologist. 2000;5:312–20. [PubMed] [Google Scholar]
- 5.Nielsen EH, Jorgensen JO, Bjerre P, Andersen M, Andersen C, et al. Acute presentation of craniopharyngioma in children and adults in a Danish national cohort. Pituitary. 2012;12:1–8. doi: 10.1007/s11102-012-0451-3. [DOI] [PubMed] [Google Scholar]
- 6.Marchand I, Barkaoui MA, Garel C, Polak M, Donadieu J Writing Committee. Central diabetes insipidus as the inaugural manifestation of Langerhans cell histiocytosis: natural history and medical evaluation of 26 children and adolescents. J Clin Endocrinol Metab. 2011;96:E1352–60. doi: 10.1210/jc.2011-0513. [DOI] [PubMed] [Google Scholar]
- 7.Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13:690–9. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 8.Crawford JR, Santi MR, Vezina G, Myseros JS, Keating RF, et al. CNS germ cell tumor (CNSGCT) of childhood: presentation and delayed diagnosis. Neurology. 2007;68:1668–73. doi: 10.1212/01.wnl.0000261908.36803.ac. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama H, Shirato H, Kakuto Y, Inakoshi H, Nishio M, et al. Pathologically-proven intracranial germinoma treated with radiation therapy. Radiother Oncol. 1998;47:201–5. doi: 10.1016/s0167-8140(98)00017-6. [DOI] [PubMed] [Google Scholar]
- 10.Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, et al. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;48:285–91. doi: 10.1002/pbc.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]