Abstract
BACKGROUND
Corticotropin-independent Cushing’s syndrome is caused by tumors or hyperplasia of the adrenal cortex. The molecular pathogenesis of cortisol-producing adrenal adenomas is not well understood.
METHODS
We performed exome sequencing of tumor-tissue specimens from 10 patients with cortisol-producing adrenal adenomas and evaluated recurrent mutations in candidate genes in an additional 171 patients with adrenocortical tumors. We also performed genomewide copy-number analysis in 35 patients with cortisol-secreting bilateral adrenal hyperplasias. We studied the effects of these genetic defects both clinically and in vitro.
RESULTS
Exome sequencing revealed somatic mutations in PRKACA, which encodes the catalytic subunit of cyclic AMP–dependent protein kinase (protein kinase A [PKA]), in 8 of 10 adenomas (c.617A→C in 7 and c.595_596insCAC in 1). Overall, PRKACA somatic mutations were identified in 22 of 59 unilateral adenomas (37%) from patients with overt Cushing’s syndrome; these mutations were not detectable in 40 patients with subclinical hypercortisolism or in 82 patients with other adrenal tumors. Among 35 patients with cortisol-producing hyperplasias, 5 (including 2 first-degree relatives) carried a germline copy-number gain (duplication) of the genomic region on chromosome 19 that includes PRKACA. In vitro studies showed impaired inhibition of both PKA catalytic subunit mutants by the PKA regulatory subunit, whereas cells from patients with germline chromosomal gains showed increased protein levels of the PKA catalytic subunit; in both instances, basal PKA activity was increased.
CONCLUSIONS
Genetic alterations of the catalytic subunit of PKA were found to be associated with human disease. Germline duplications of this gene resulted in bilateral adrenal hyperplasias, whereas somatic PRKACA mutations resulted in unilateral cortisol-producing adrenal adenomas. (Funded by the European Commission Seventh Framework Program and others.)
Endogenous hypercortisolism, referred to as Cushing’s syndrome, is associated with substantial morbidity and mortality.1 When Cushing’s syndrome is severe, patients have catabolic symptoms such as muscle weakness, skin fragility, osteoporosis, and severe metabolic sequelae.2 Hypersecretion of cortisol can be driven by an excess of pituitary or ectopic corticotropin or can be due to adrenocortical tumors or hyperplasias with corticotropin-independent cortisol production. Adrenal adenomas are common, with a prevalence of at least 3% among persons older than 50 years of age.3 Whereas only a subset of these tumors is associated with overt Cushing’s syndrome, some degree of cortisol excess is present, depending on the diagnostic criteria applied,4 in up to 47% of patients with adrenal adenomas and is associated with a range of phenotypes, from hypertension to the metabolic syndrome and osteoporosis.5
The molecular pathogenesis of cortisol-producing adrenal adenomas is not well understood. Whereas somatic mutations in the gene encoding beta-catenin (CTNNB1) have been found primarily in nonsecreting adrenocortical adenomas,6 there is some evidence that increased endocrine activity may be linked to aberrant cyclic AMP (cAMP) signaling.7,8 For instance, ectopic expression of G-protein–coupled receptors for neuroendocrine hormones or neurotransmitters that mediate their effects through cAMP has been implicated in syndromes such as food-dependent hypercortisolism and related conditions9,10 that are caused by cortisol-producing adenomas or bilateral hyperplasias of the adrenal cortex. Moreover, somatic mutations in the gene encoding the α subunit of the stimulatory G protein (GNAS1) cause adenomas or hyperplasias leading to Cushing’s syndrome in patients with McCune–Albright syndrome11 or macronodular hyperplasia.12,13 Finally, mutations in the genes encoding the cAMP-degrading phosphodiesterase 11A (PDE11A)14 and phosphodiesterase 8B (PDE8B)15 and in the gene encoding the regulatory subunit of the cAMP-dependent protein kinase (protein kinase A [PKA]) (PRKAR1A)16,17 have been identified in patients with Cushing’s syndrome due to primary pigmented nodular adrenocortical disease and in a small number of cortisol-producing adrenal adenomas. These genetic alterations, however, explain only a small fraction of cases. The observation that a subset of adrenal adenomas is characterized by abnormal PKA activity, despite the absence of mutations in these candidate genes,18 suggests yet unknown alterations in the cAMP–PKA signaling cascade in these tumors.
METHODS
STUDY PATIENTS AND DNA EXTRACTION
Patients were recruited at three centers that participate in the European Network for the Study of Adrenal Tumors and at the U.S. National Institutes of Health. We evaluated 139 patients with adrenal adenoma, 42 patients with adrenocortical carcinoma, and 35 patients with corticotropin-independent bilateral adrenal hyperplasia who did not have germline mutations in PRKAR1A, PDE11A, or PDE8B or somatic GNAS mutations (33 with micronodular hyperplasia [31 with primary pigmented nodular adrenocortical disease and 2 with isolated micronodular adrenocortical disease] and 2 with macronodular hyperplasia) (Fig. S1 and Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). In all cases, the diagnosis was histologically confirmed after surgical resection. All the patients gave written informed consent, and the study was approved by the ethics committee at each participating institution.
The diagnosis of corticotropin-independent Cushing’s syndrome was based on a combination of biochemical hallmarks of hypercortisolism — elevated urinary excretion of free cortisol, increased late-night salivary or serum cortisol levels, suppressed plasma corticotropin levels (<10 pg per milliliter [<2.2 pmol per liter]), and nonsuppressible serum cortisol levels (>5 μg per deciliter [>138 nmol per liter]) after the administration of 1 mg of dexamethasone — as well as on the presence of catabolic signs of hypercortisolism. Patients were classified as having overt Cushing’s syndrome if they had at least three abnormal biochemical test results or if they had typical catabolic features (i.e., muscle weakness, skin fragility, and osteoporosis) plus at least two abnormal biochemical test results. Patients were considered to have endocrine-inactive adrenal lesions if they had normal biochemical test results and no catabolic signs. All patients who had no catabolic signs but had at least one abnormal result in the abovementioned tests were classified as having subclinical Cushing’s syndrome.
DNA was extracted as described previously19–21 from unilateral adrenocortical tumors in 181 patients and from corresponding normal tissue in 26 of these patients. Furthermore, germline DNA was available for all 35 patients with bilateral hyperplasia, and DNA from adrenal-tissue samples obtained during surgery was available for 10 of these 35 patients.
EXOME AND PRKACA SEQUENCING
Exomes were enriched in solution and indexed with the use of the SureSelect XT Human All Exon 50Mb kit, version 4 (Agilent Technologies). Sequencing was performed as paired-end reads of 100 bp on HiSeq2000 systems (Illumina). Pools of 12 indexed libraries were sequenced on four lanes. Image analysis and base calling were performed with the use of Real-Time Analysis software (Illumina). Methods of variant detection and PRKACA sequencing are described in the Supplementary Appendix.
COMPARATIVE GENOMIC HYBRIDIZATION
Array-based comparative genomic hybridization analysis was performed with the use of commercial arrays (Agilent Technologies), according to the manufacturer’s instructions and as described previously.22 Technical details are provided in the Supplementary Appendix.
IN SILICO ANALYSIS OF HUMAN MUTATIONS
Structural images were prepared with the use of PyMOL software (www.pymol.org). The structure of the mouse full-length tetrameric RIIβ(2):Cα(2) holoenzyme23 (Protein Data Bank entry 3TNP) was used to display the structures of the PKA catalytic subunit (Cα) and regulatory subunit (RIIβ).
DNA CONSTRUCTS AND SITE-DIRECTED MUTAGENESIS
Plasmids encoding nonmutant human RIIβ or Cα subunits were purchased from OriGene Technologies. The PRKACA-containing plasmid was used for site-directed mutagenesis, with the c.617A→C and the c.595_596insCAC mutation introduced with the use of the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies), according to the manufacturer’s protocol. The mutation was confirmed by means of sequencing.
QUANTIFICATION OF PKA ACTIVITY IN INTACT CELLS
Human embryonic kidney 293 cells were transfected with the AKAR4-NES (a protein activity reporter 4 with a nuclear export signal) sensor24 so that PKA activity could be monitored by means of fluorescence resonance energy transfer (FRET) imaging. Transfection and FRET imaging were performed as described previously.25 Equimolar concentrations of a cell-permeable pair of synergistic cAMP analogues were used to activate PKA II.26
QUANTIFICATION OF PRKACA PROTEIN AND PKA ENZYMATIC ACTIVITY
Whole-cell or tissue lysates were studied for PKA Cα subunit expression by means of Western blotting with the use of a specific antibody (sc-903, Santa Cruz Biotechnology). COS-7 cells were transfected with the use of the X-tremeGENE HP DNA Transfection Reagent (Roche) and 500 ng of plasmid DNA per well for 24 hours. For transfections including both PKA Cα (nonmutant or Leu206Arg variant) and RIIβ subunits, a molar ratio of 1:8 was used. In lysed cells from the transfection experiments or patient-derived cells, PKA activity was determined by means of an enzymatic assay (Enzo Life Sciences).
GENE-EXPRESSION MICROARRAY ANALYSIS AND REAL-TIME POLYMERASE-CHAIN-REACTION (PCR) ANALYSIS
An earlier microarray analysis of 22 adenomas27 was expanded to include 39 adenomas in the current study (see Table S1 in the Supplementary Appendix). For quantification of PRKACA expression, real-time quantitative PCR analysis was used. Details of the microarray experiments and real-time PCR analysis are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
Data were compared between two groups with the use of the Mann–Whitney U test and among three groups with the use of the Kruskal–Wallis test. All comparisons were two-sided, and P values of less than 0.05 were considered to indicate statistical significance. The analyses were performed with the use of SPSS software, version 20 (IBM).
RESULTS
SOMATIC PRKACA MUTATIONS AND GERMLINE DUPLICATIONS IN CORTISOL-PRODUCING LESIONS
Exome sequencing was performed in samples from 10 patients with unilateral cortisol-producing adenomas and overt Cushing’s syndrome (Table S2 in the Supplementary Appendix) and revealed a low number of somatic mutations per adenoma (median, 5; range, 1 to 14) (Table S3 in the Supplementary Appendix). Within this small set of genetic alterations, somatic variants in PRKACA, encoding the PKA Cα subunit, were found in 8 of 10 tumors (c.617A→C, p.Leu206Arg in 7 and c.595_596insCAC, Leu199_Cys200insTrp in 1). The affected amino acids were highly conserved across a variety of species (Fig. S2 in the Supplementary Appendix).
On the basis of these initial results, PRKACA was sequenced in 129 additional adenomas (including 89 cortisol-producing, 20 aldosterone-producing, and 20 inactive adenomas). In 33 of these samples, the entire coding sequence was investigated, and in the remaining 96 samples, sequencing of hot-spot regions was performed. The Leu206Arg variant was identified in 14 of these 129 adenomas, and all 14 patients with this variant also had overt Cushing’s syndrome, according to the predefined criteria. Whole-exome and targeted sequencing indicated that both the nonmutated and mutated alleles were present in tumor tissue, consistent with a heterozygous state of PRKACA mutations (Fig. 1A and 1B, and Fig. S3 in the Supplementary Appendix). In the affected patients, there were no PRKACA mutations in DNA derived from leukocytes (19 patients) or fat tissue (1 patient) or in adjacent normal adrenal tissue (6 patients).
Figure 1. Identification of Somatic PRKACA Mutation and Germline Genetic Duplications.
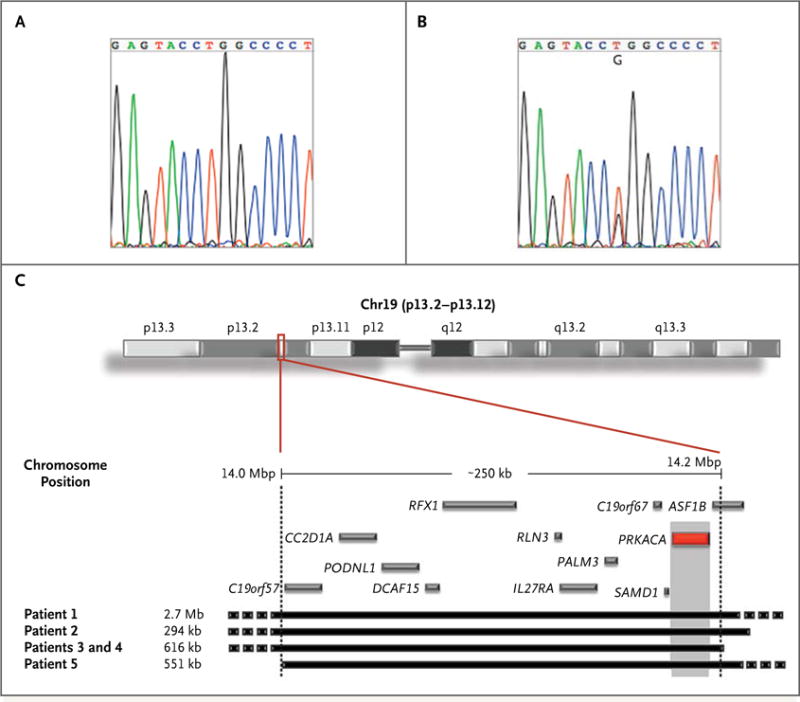
Panel A shows a sequence chromatogram of normal adrenal tissue, and Panel B a chromatogram of a cortisol-producing adrenal adenoma. A somatic mutation in PRKACA (c.617A→C) was identified in the cortisol-producing adenoma, resulting in a Leu206Arg substitution that is not present in the adjacent normal tissue. Panel C shows an ideogram of chromosome 19 (top) with the genes included in the p13.2–p13.12 band (GRCh37/h19). The black blocks (bottom) represent the size and position of the duplications. The gray blocks represent the genes included only in the shared region of duplication. The red block represents PRKACA.
Comparative genomic hybridization of samples from 35 patients with cortisol-secreting bilateral adrenal hyperplasias and overt Cushing’s syndrome identified 5 patients (4 kindreds) with copy-number gains (duplications) of the genomic region on chromosome 19p that includes PRKACA (Fig. 1C, and Table S4 in the Supplementary Appendix). In one case, the defect was inherited: a mother and son, both carriers of the same PRKACA duplication, were affected by bilateral macronodular hyperplasia. In another case, that of a 3-year-old boy with Cushing’s syndrome due to bilateral micronodular hyperplasia, the defect was de novo, because neither parent carried the PRKACA duplication. No amplification of PRKACA was found in 24 cortisol-producing adrenal adenomas analyzed by means of single-nucleotide polymorphism array profiling.28
No PRKACA mutations were detected in 1600 in-house exomes or in the 1000 Genomes Project data set (version 0.0.14). Although PRKACA duplications are reported in public databases of copy-number variants in at least six instances (occurring in patients referred for genetic testing because of developmental delay), no PRKACA whole-gene duplications are included in the Database of Genomic Variants, which is based on the general population. Moreover, no PRKACA duplications were found in 2000 persons with intellectual disability, congenital malformations, or both in an internal database.
PRKACA MUTATIONS AND REGULATION OF CATALYTIC SUBUNITS BY REGULATORY SUBUNITS
Analysis of the mouse full-length tetrameric RIIβ(2):Cα(2) holoenzyme structure23 revealed that this mutation is located in the highly conserved core of the interaction between the regulatory (RIIβ) and catalytic (Cα) subunits of PKA — a finding that supports a functional relevance of the Leu206Arg variant. Leu206 is part of the active-site cleft of the catalytic subunit to which the inhibitory sequence of the regulatory subunit binds, mimicking a substrate for the catalytic subunit. This interaction keeps the catalytic subunit inactive in the absence of cAMP. Exchanging Leu206 with the bulky and positively charged amino acid Arg in silico yields steric hindrance between the side chain of the mutated Arg206 in the Cα subunit and Val115 and Tyr228 in the RIIβ subunit (Fig. 2A and 2B).29
Figure 2. Functional Characterization of PRKACA Variants.
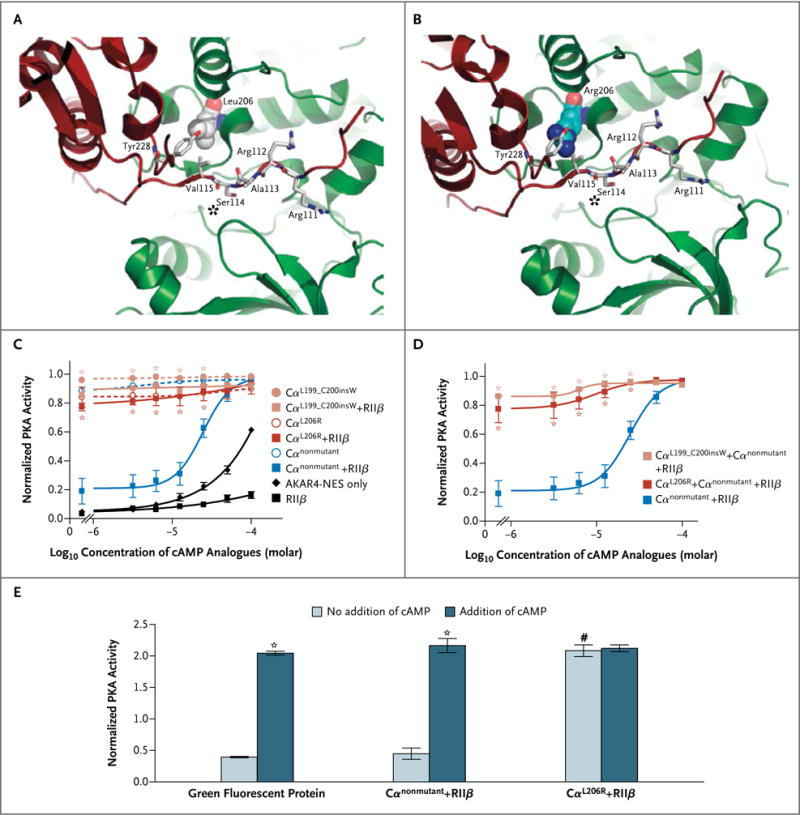
Panel A shows the structure derived from the protein kinase A (PKA) tetramer, with the nonmutant catalytic subunit (Cα) depicted in green and the regulatory subunit (RIIβ) depicted in red. A zoomed view into the region of Leu206 in the Cα subunit is shown. Leu206 is depicted as a space-filling representation; the two residues in close proximity (Val115 and Tyr228) and additional residues from the inhibitory site (Arg111–Ser114, marked with an asterisk) of the regulatory subunit are depicted as sticks. Panel B shows the same region of the PKA tetramer, with Leu206 in the Cα subunit replaced by Arg206, also depicted as a space-filling representation. Panel C shows PKA activity of nonmutant and mutant PKA Cα subunits transfected in human embryonic kidney 293 cells, as determined by means of fluorescence resonance energy transfer (FRET) assay with a PKA reporter (for details, see Fig. S4 in the Supplementary Appendix). The results indicate that the mutant variants are constitutively active. Asterisks indicate P<0.05 for the comparison with Cαnonmutant + RIIβ. AKAR4-NES denotes a protein activity reporter 4 with a nuclear export signal. Panel D shows that high constitutive PKA activity was maintained when either mutant was cotransfected with an equal amount of non-mutant Cα subunit. Asterisks indicate P<0.05 for the comparison with Cαnonmutant + RIIβ. The data in Panels C and D were compared by means of a two-way analysis of variance followed by Bonferroni’s test. Panel E shows the quantification of enzymatic PKA activity; COS-7 cells were transfected with Cα (nonmutant or mutant) and RIIβ, with or without the addition of cyclic AMP (cAMP). Asterisks indicate P<0.05 for the comparison between samples with and those without the addition of cAMP. The hatch mark indicates P<0.05 for the comparison between samples transfected with nonmutant Cα subunit and those transfected with mutant Cα subunit without the addition of cAMP. In Panels C, D, and E, the I bars represent the standard error.
The functional consequences of the two detected variants (Leu206Arg and Leu199_Cys200insTrp) were investigated in intact cells by means of FRET microscopy with the use of a sensor for PKA activity (AKAR4-NES).24 The PKA activity in cells transfected only with either nonmutant Cα or the variants was high and was not further stimulated by cAMP analogues, indicating preservation of the catalytic activity in the mutants (Fig. 2C, and Fig. S4A and S4C in the Supplementary Appendix). However, after cotransfection with excess nonmutant RIIβ, basal PKA activity was decreased in cells transfected with nonmutant Cα and became responsive to cAMP analogues, whereas PKA activity in the cells transfected with the mutants remained high and was not responsive to cAMP analogues (Fig. 2C, and Fig. S4B and S4D in the Supplementary Appendix). This finding indicates that the mutations made the catalytic subunit resistant to the physiologic suppression by the regulatory subunit. The lack of suppression remained when an equal amount of nonmutant Cα was cotransfected, indicating a dominant effect of the mutations (Fig. 2D).
Similarly, transfection of the mutant Cα Leu206Arg variant caused a profound increase in PKA activity under basal conditions so that PKA activity was in the same range as that in cells transfected with the nonmutant Cα on cAMP stimulation, and this activity was not suppressed by cotransfection with RIIβ, indicating again a lack of suppression of the activity of the mutant catalytic subunit by the regulatory subunit (Fig. 2E). Consistent with these findings, basal PKA activity in tumor tissue was found to be higher in adenoma samples with PRKACA mutations than in those without such mutations (Fig. S5 in the Supplementary Appendix). Taken together, these data indicate that the Leu206Arg mutant protein is constitutively active and is not suppressed by the regulatory subunit.
ASSOCIATION OF GERMLINE DUPLICATION OF PRKACA WITH INCREASED PROTEIN LEVELS AND PKA ACTIVITY
As compared with tumor-tissue samples from patients without any known genetic defects, tumor-tissue samples from patients with duplications of PRKACA had higher PKA Cα messenger RNA and protein levels (Fig. S6A and S6B, respectively, in the Supplementary Appendix). Immunohistochemical experiments confirmed higher expression of the PKA Cα subunit in adrenal tissue from these patients (Fig. S6C in the Supplementary Appendix). The higher expression of the PKA Cα subunit was associated with higher basal and cAMP-stimulated PKA activity (Fig. S6D in the Supplementary Appendix).
CLINICAL PHENOTYPES AND PRKACA MUTATION STATUS
Whereas 22 of 59 patients (37%) with overt Cushing’s syndrome due to a unilateral adenoma harbored a PRKACA mutation, this alteration was not present in any adenoma associated with subclinical Cushing’s syndrome (40 patients), nor was it present in inactive adrenal adenomas (20 patients), aldosterone-producing adrenal adenomas (20 patients), or adrenocortical carcinomas (42 patients) or in adrenal tissue (10 patients) or lymphocytic DNA (35 patients) from patients with corticotropin-independent adrenal hyperplasia (35 patients). Furthermore, in the group of patients with overt Cushing’s syndrome, the presence of PRKACA mutations was associated with a more severe phenotype (Table 1). Accordingly, expression levels of a variety of steroidogenic enzymes in mutant adenoma tissues were higher in the presence of a PRKACA mutation (Table S5 in the Supplementary Appendix).
Table 1.
Clinical Characteristics of Patients with Adrenal Adenomas in Relation to PRKACA Mutational Status.*
| Characteristic | Endocrine-Inactive Adenoma (N = 20) |
Subclinical Cushing’s Syndrome (N = 40) |
Overt Cushing’s Syndrome (N = 59) |
P Value | |||
|---|---|---|---|---|---|---|---|
| Total | No PRKACA Mutation | PRKACA Mutation | Comparison of Endocrine-Inactive Adenoma, Subclinical Cushing’s Syndrome, and Overt Cushing’s Syndrome† | Comparison of Overt Cushing’s Syndrome with and without PRKACA Mutation‡ | |||
| Age at diagnosis (yr) | |||||||
|
| |||||||
| Median | 49.5 | 54.0 | 41.0 | 41.0 | 41.0 | <0.001 | 0.37 |
|
| |||||||
| Interquartile range | 39.3–59.0 | 48.3–66.0 | 34.0–49.0 | 35.0–50.0 | 33.0–47.3 | ||
|
| |||||||
| Adenoma size (mm)§ | |||||||
|
| |||||||
| Median | 43.0 | 40.0 | 30.0 | 30.0 | 30.0 | <0.001 | 0.86 |
|
| |||||||
| Interquartile range | 33.0–50.0 | 30.0–50.0 | 30.0–36.0 | 29.5–40.0 | 30.0–35.5 | ||
|
| |||||||
| Corticotropin (pg/ml)¶ | |||||||
|
| |||||||
| Median | 14.0 | 6.0 | 2.5 | 2.3 | 2.5 | <0.001 | 0.69 |
|
| |||||||
| Interquartile range | 11.0–18.0 | 5.0–12.9 | 1.0–5.0 | 1.0–5.0 | 1.0–6.0 | ||
|
| |||||||
| Serum cortisol after 1 mg of dexamethasone (μg/dl)‖ | |||||||
|
| |||||||
| Median | 1.2 | 2.9 | 15.9 | 15.0 | 22.0 | <0.001 | 0.005 |
|
| |||||||
| Interquartile range | 1.0–1.7 | 2.3–7.5 | 13.3–23.0 | 9.4–19.5 | 16.5–27.7 | ||
|
| |||||||
| Urinary cortisol ÷ ULN** | |||||||
|
| |||||||
| Median | 0.62 | 0.46 | 3.67 | 3.14 | 5.33 | <0.001 | 0.03 |
|
| |||||||
| Interquartile range | 0.38–0.72 | 0.26–0.99 | 1.88–6.01 | 1.53–5.23 | 2.46–9.08 | ||
|
| |||||||
| Midnight cortisol ÷ ULN†† | |||||||
|
| |||||||
| Median | 0.6 | 1.6 | 3.9 | 3.6 | 4.3 | <0.001 | 0.02 |
|
| |||||||
| Interquartile range | 0.4–1.0 | 0.8–2.6 | 2.8–4.6 | 2.3–4.5 | 3.9–5.9 | ||
For reference ranges of the endocrine characteristics, see the Methods section in the Supplementary Appendix. ULN denotes upper limit of the normal range.
Values were compared with the use of the Kruskal–Wallis test.
Values were compared with the use of the Mann–Whitney U test.
Data were not available for 1 patient with endocrine-inactive adenoma, 2 patients with subclinical Cushing’s syndrome, and 4 patients with overt Cushing’s syndrome (3 with no PRKACA mutation and 1 with PRKACA mutation).
Data were not available for 9 patients with endocrine-inactive adenoma, 9 patients with subclinical Cushing’s syndrome, and 12 patients with overt Cushing’s syndrome (9 with no PRKACA mutation and 3 with PRKACA mutation). To convert values for corticotropin to picomoles per liter, multiply by 0.22.
Data were not available for 12 patients with endocrine-inactive adenoma, 11 patients with subclinical Cushing’s syndrome, and 22 patients with overt Cushing’s syndrome (14 with no PRKACA mutation and 8 with PRKACA mutation). To convert values for serum cortisol to nanomoles per liter, multiply by 27.6.
Values are based on 24-hour urine samples. Data were not available for 3 patients with endocrine-inactive adenoma, 11 patients with subclinical Cushing’s syndrome, and 6 patients with overt Cushing’s syndrome (3 with no PRKACA mutation and 3 with PRKACA mutation).
Values are based on midnight salivary or serum cortisol levels. Data were not available for 12 patients with endocrine-inactive adenoma, 21 patients with subclinical Cushing’s syndrome, and 23 patients with overt Cushing’s syndrome (15 with no PRKACA mutation and 8 with PRKACA mutation).
There were no obvious phenotypic differences between patients with germline PRKACA duplications and those without duplications, although the number of patients with duplications was small. The affected mother and son had mild Cushing’s syndrome of insidious onset, caused by bilateral macronodular hyperplasia manifesting in the third and fourth decade of life. The three young boys presenting with Cushing’s syndrome due to bilateral hyperplasia (two with micronodular disease and one with macronodular disease) (Table S4 in the Supplementary Appendix) had severe disease similar to that of patients with somatic PRKACA mutations. One patient with PRKACA duplication had a paradoxical increase in cortisol secretion after administration of dexamethasone,30 whereas the other four patients did not undergo a long-term dexamethasone suppression test (Liddle’s test) before surgery.
DISCUSSION
Despite evidence that enhanced cAMP signaling is the culprit in many benign adrenal lesions leading to Cushing’s syndrome,8,18 the search for tumorigenic mutations in adrenal adenomas with the use of a candidate-gene approach has revealed only very rare mutations in a distinct subgroup of patients.12,13,17,31 The current study suggests that more than one third of cortisol-producing adenomas associated with overt Cushing’s syndrome have unique somatic mutations in PRKACA (which encodes the main catalytic subunit of PKA), resulting in constitutive PKA activation. Although the mutation is present only in tumor cells in these patients, germline duplication of PRKACA was identified in a group of patients with bilateral adrenal hyperplasias. Consistent with the hypothesis that the resulting increased PKA activity is responsible for adrenocortical tumor formation, patients with somatic defects had single adenomas, whereas patients with germline duplications presented with bilateral adrenal hyperplasias. All the patients with PRKACA defects, whether germline or somatic, had overt Cushing’s syndrome, and none of the patients with subclinical Cushing’s syndrome or other adrenal tumors had genetic PRKACA alterations.
Thus, our findings provide evidence that PRKACA activation leads to marked excess of cortisol, as one would expect from constitutive activation of the enzymes that mediate corticotropin-dependent effects on adrenal steroidogenesis. The findings also indicate that subclinical Cushing’s syndrome is not an early form of overt disease but a pathophysiologically distinct entity. Because PRKACA mediates most of the effects of inactivating PRKAR1A mutations32 and because mutations of PRKAR1A are associated with a variety of tumors in humans and mice,33,34 we would speculate that somatic PRKACA defects might also play a role in other forms of endocrine and nonendocrine tumors.
PKA, a cAMP-dependent serine–threonine kinase, is perhaps the best characterized protein kinase and provides a clear example of allosteric regulation.35 In its inactive state, it is a tetrameric holoenzyme consisting of a dimer of two regulatory and two catalytic subunits; under physiologic conditions, PKA activity is induced by G-protein–coupled receptors through increased levels of cAMP.23,35 On binding of cAMP, the regulatory subunits dissociate from the catalytic subunits, allowing the enzyme to become active.35 Although some randomly introduced mutations in PRKACA have been shown to result in unopposed catalytic activation in vitro,36 such alterations have not been linked to human disease. The only naturally found gain-of-function PRKACA mutations are those described in the Cos1(A1) drosophila mutant.37 The two PRKACA mutants identified in the current study alter the structure of the catalytic subunit at a site that is essential for interaction with the regulatory subunit, thus maintaining high activity of the catalytic subunit in the absence of cAMP.23,35 The critical position of the Leu206Arg mutation at the core of the interaction between the catalytic subunit and the inhibitory site of the regulatory subunit, combined with the steric hindrance involving Val115 and Tyr228 in the regulatory subunit, may explain the high grade of specificity of this particular mutation.
In conclusion, the current study links genetic variants of the main catalytic subunit of PKA with both hyperplasias and adenomas of the adrenal cortex leading to corticotropin-independent Cushing’s syndrome. These observations are consistent with the known role of the cAMP signaling pathway in adrenal lesions that have been associated with Cushing’s syndrome.
Supplementary Material
Acknowledgments
Supported by a grant (FP7/2007-2013) from the European Commission Seventh Framework Program under grant agreement 259735 (to Drs. Beuschlein, Fassnacht, Bertherat, and Allolio) and, in part, by grants from the Wilhelm Sander-Stiftung (2012.095.1, to Dr. Allolio), the Else Kröner-Fresenius-Stiftung (2012_A103, to Dr. Reincke), Bundesministerium für Bildung und Forschung (BMBF 01EO1004-D2, to Drs. Fassnacht and Allolio), the COMETE Network (Programme Hospitalier de Recherche Clinique grant AOM95201), the Institut National du Cancer Recherche Translationelle 2009-RT-02, INSERM (with Dr. Assié a recipient of a Contrat d’Interface), the Conny-Maeva Charitable Foundation, the European Research Council (Advanced Grant TOPAS, to Dr. Lohse), the Deutsche Forschungsgemeinschaft (DFG) (Rudolf Virchow Center and DFG Research Center for Experimental Biomedicine FZT82, to Drs. Kisker and Lohse, and DFG grant CA1014/1-1, to Dr. Calebiro), the Fondazione Telethon 2010 (GGP10121, to Dr. Zuffardi), and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
We thank the staff at the tumor bank of Cochin Hospital (in particular, Prof. Benoît Terris) for help with sample collection; Drs. Anelia Horvath (now at George Washington University), Isaac Levy (now at Toronto Children’s Hospital, Toronto), Kiran Nadela (now at Otsuka America Pharmaceutical), and Maria Nesterova (NICHD) for work on earlier phases of this project; Jin Zhang (Johns Hopkins University School of Medicine) for providing the AKAR4-NES sensor; JoAnn C. Kelly, Zunyan Dai, Eric D. Smith, Marc A. Sanidad, and Philip Mowrey (Quest Diagnostics Nichols Institute) for technical assistance with the fluorescence in situ hybridization analysis; Marco Fichera for help with the Database of Genomic Variants; Drs. Glenn Braunstein (Cedars–Sinai Medical Center, Los Angeles), Louise Izatt (Guy’s Hospital, London), and James Gardner (Pediatric Endocrinology Clinic, Our Lady of the Lake Physician Group, Baton Rouge, LA) for referral of patients who were then found to have PRKACA defects; and Prof. Wiebke Arlt (University of Birmingham) for helpful comments on an earlier version of the manuscript.
APPENDIX
The authors’ affiliations are as follows: Medizinische Klinik und Poliklinik IV, Ludwig-Maximilians-Universität München (F.B., M.F., A.O., S.S., K.S., M.R.), Institute of Human Genetics, Technische Universität München (T.M., T.M.S.), and Deutsches Zentrum für Herz-Kreislauf-Forschung partner site, Munich Heart Alliance (T.M., T.M.S.), Munich, the Department of Medicine I, Endocrine and Diabetes Unit, University Hospital (M.F., S.S., B.A.), Comprehensive Heart Failure Center (M.F., M.J.L., B.A.), Institute of Pharmacology and Toxicology (D.C., U.Z., M.J.L.), Rudolf Virchow Center and Deutsche Forschungsgemeinschaft Research Center for Experimental Biomedicine (D.C., C.K., M.J.L.), and Comprehensive Cancer Center Mainfranken (C.L.R.), University of Würzburg, Würzburg, and the Institute of Human Genetics, Helmholtz Zentrum München, Neuherberg (T.W., A.S., T.S., S.D., T.M., T.M.S.) — all in Germany; INSERM Unité 1016, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8104, Institut Cochin, Faculté de Médecine Paris Descartes, Université Paris Des cartes, Sorbonne Paris Cité (G.A., O.B., D.V., M.R.-R., J.B.), and the Department of Endocrinology, Referral Center for Rare Adrenal Diseases, Assistance Publique–Hôpitaux de Paris, Hôpital Cochin (G.A., O.B., J.B.), Paris; Section on Endocrinology and Genetics, Program on Developmental Endocrinology and Genetics, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (C.A.S., F.R.F., E.S., P.S.); and Dipartimento di Medicina Molecolare, University of Pavia (A.F., O.Z.), and Biotechnology Research Laboratory, Foundation Istituto di Ricovoro e Cura a Carattere Scientifico, Policlinico San Matteo (A.V.), Pavia, Italy.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Lindholm J, Juul S, Jørgensen JO, et al. Incidence and late prognosis of Cushing’s syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86:117–23. doi: 10.1210/jcem.86.1.7093. [DOI] [PubMed] [Google Scholar]
- 2.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367:1605–17. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 3.Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”) Ann Intern Med. 2003;138:424–9. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 4.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–40. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 5.Terzolo M, Pia A, Reimondo G. Subclinical Cushing’s syndrome: definition and management. Clin Endocrinol (Oxf) 2012;76:12–8. doi: 10.1111/j.1365-2265.2011.04253.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet S, Gaujoux S, Launay P, et al. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96(2):E419–E426. doi: 10.1210/jc.2010-1885. [DOI] [PubMed] [Google Scholar]
- 7.Lacroix A, Bourdeau I, Lampron A, Mazzuco TL, Tremblay J, Hamet P. Aberrant G-protein coupled receptor expression in relation to adrenocortical overfunction. Clin Endocrinol (Oxf) 2010;73:1–15. doi: 10.1111/j.1365-2265.2009.03689.x. [DOI] [PubMed] [Google Scholar]
- 8.Stratakis CA. cAMP/PKA signaling defects in tumors: genetics and tissue-specific pluripotential cell-derived lesions in human and mouse. Mol Cell Endocrinol. 2013;371:208–20. doi: 10.1016/j.mce.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacroix A, Bolté E, Tremblay J, et al. Gastric inhibitory polypeptide–dependent cortisol hypersecretion — a new cause of Cushing’s syndrome. N Engl J Med. 1992;327:974–80. doi: 10.1056/NEJM199210013271402. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone–dependent Cushing’s syndrome. N Engl J Med. 1999;341:1577–81. doi: 10.1056/NEJM199911183412104. [DOI] [PubMed] [Google Scholar]
- 11.Carney JA, Young WF, Stratakis CA. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol. 2011;35:1311–26. doi: 10.1097/PAS.0b013e31821ec4ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragoso MC, Domenice S, Latronico AC, et al. Cushing’s syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J Clin Endocrinol Metab. 2003;88:2147–51. doi: 10.1210/jc.2002-021362. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao HP, Kirschner LS, Bourdeau I, et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94:2930–7. doi: 10.1210/jc.2009-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath A, Boikos S, Giatzakis C, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 15.Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cyclic AMP–specific phosphodiesterase in adrenal hyperplasia. N Engl J Med. 2008;358:750–2. doi: 10.1056/NEJMc0706182. [DOI] [PubMed] [Google Scholar]
- 16.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 17.Bertherat J, Groussin L, Sandrini F, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63:5308–19. [PubMed] [Google Scholar]
- 18.Bimpaki EI, Nesterova M, Stratakis CA. Abnormalities of cAMP signaling are present in adrenocortical lesions associated with ACTH-independent Cushing syndrome despite the absence of mutations in known genes. Eur J Endocrinol. 2009;161:153–61. doi: 10.1530/EJE-09-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–4. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 20.Ronchi CL, Leich E, Sbiera S, et al. Single nucleotide polymorphism microarray analysis in cortisol-secreting adrenocortical adenomas identifies new candidate genes and pathways. Neoplasia. 2012;14:206–18. doi: 10.1593/neo.111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent-Dejean C, Cazabat L, Groussin L, et al. Identification of a clinically homogenous subgroup of benign cortisol-secreting adrenocortical tumors characterized by alterations of the protein kinase A (PKA) subunits and high PKA activity. Eur J Endocrinol. 2008;158:829–39. doi: 10.1530/EJE-07-0819. [DOI] [PubMed] [Google Scholar]
- 22.Bonaglia MC, Giorda R, Beri S, et al. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet. 2011;7(7):e1002173. doi: 10.1371/journal.pgen.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Smith-Nguyen EV, Keshwani MM, Deal MS, Kornev AP, Taylor SS. Structure and allostery of the PKA RIIβ tetrameric holoenzyme. Science. 2012;335:712–6. doi: 10.1126/science.1213979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst KJ, Allen MD, Zhang J. Spatiotemporally regulated protein kinase A activity is a critical regulator of growth factor-stimulated extracellular signal-regulated kinase signaling in PC12 cells. Mol Cell Biol. 2011;31:4063–75. doi: 10.1128/MCB.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calebiro D, Nikolaev VO, Gagliani MC, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7(8):e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calebiro D, de Filippis T, Lucchi S, et al. Selective modulation of protein kinase A I and II reveals distinct roles in thyroid cell gene expression and growth. Mol Endocrinol. 2006;20:3196–211. doi: 10.1210/me.2005-0493. [DOI] [PubMed] [Google Scholar]
- 27.Wilmot Roussel H, Vezzosi D, Rizk-Rabin M, et al. Identification of gene expression profiles associated with cortisol secretion in adrenocortical adenomas. J Clin Endocrinol Metab. 2013;98(6):E1109–E1121. doi: 10.1210/jc.2012-4237. [DOI] [PubMed] [Google Scholar]
- 28.Ronchi CL, Sbiera S, Leich E, et al. Single nucleotide polymorphism array profiling of adrenocortical tumors — evidence for an adenoma carcinoma sequence? PLoS One. 2013;8(9):e73959. doi: 10.1371/journal.pone.0073959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol. 2012;13:646–58. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louiset E, Stratakis CA, Perraudin V, et al. The paradoxical increase in cortisol secretion induced by dexamethasone in primary pigmented nodular adrenocortical disease involves a glucocorticoid receptor-mediated effect of dexamethasone on protein kinase A catalytic subunits. J Clin Endocrinol Metab. 2009;94:2406–13. doi: 10.1210/jc.2009-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi H, Usui T, Fukata J, Yoshimasa T, Oki Y, Nakao K. Mutation analysis of Gsalpha, adrenocorticotropin receptor and p53 genes in Japanese patients with adrenocortical neoplasms: including a case of Gsalpha mutation. Endocr J. 2000;47:461–6. doi: 10.1507/endocrj.47.461. [DOI] [PubMed] [Google Scholar]
- 32.Meoli E, Bossis I, Cazabat L, et al. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 2008;68:3133–41. doi: 10.1158/0008-5472.CAN-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvath A, Bertherat J, Groussin L, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31:369–79. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirschner LS, Kusewitt DF, Matyakhina L, et al. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–14. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 35.Das R, Esposito V, Abu-Abed M, Anand GS, Taylor SS, Melacini G. cAMP activation of PKA defines an ancient signaling mechanism. Proc Natl Acad Sci U S A. 2007;104:93–8. doi: 10.1073/pnas.0609033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orellana SA, McKnight GS. Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc Natl Acad Sci U S A. 1992;89:4726–30. doi: 10.1073/pnas.89.10.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier LS, Suyama K, Anderson JH, Scott MP. Drosophila Costal1 mutations are alleles of protein kinase A that modulate hedgehog signaling. Genetics. 2004;167:783–96. doi: 10.1534/genetics.103.024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


