Abstract
Phosphodiesterases (PDEs) are enzymes that regulate the intracellular levels of cyclic adenosine monophosphate and cyclic guanosine monophosphate, and, consequently, exhibit a central role in multiple cellular functions. The pharmacological exploitation of the ability of PDEs to regulate specific pathways has led to the discovery of drugs with selective action against specific PDE isoforms. Considerable attention has been given to the development of selective PDE inhibitors, especially after the therapeutic success of PDE5 inhibitors in the treatment of erectile dysfunction. Several associations between PDE genes and genetic diseases have been described, and more recently PDE11A and PDE8B have been implicated in predisposition to tumor formation. This review focuses on the possible function of PDEs in a variety of tumors, primarily in endocrine glands, both in tumor predisposition and as potential therapeutic targets.
Introduction
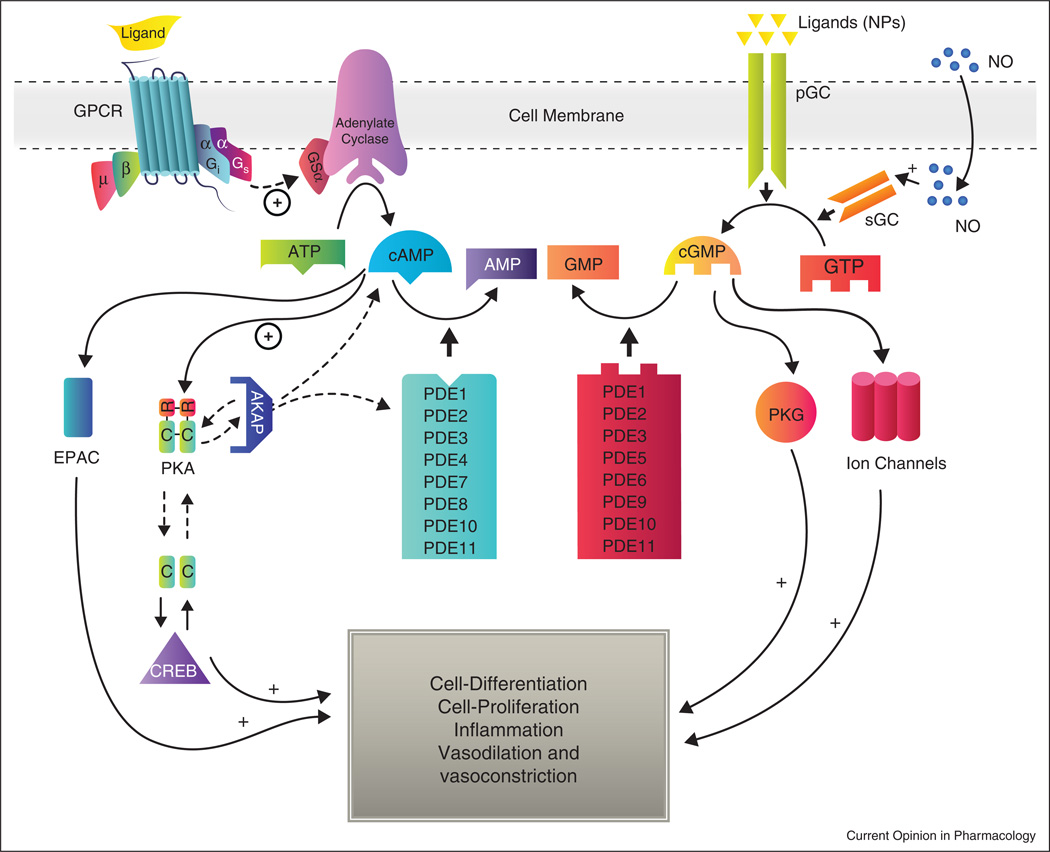
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are important second messengers in signaling, involved in cell proliferation, cell-cycle regulation, and metabolic function. Intracellular cAMP and cGMP levels are controlled both at their production, by activated adenylyl-cyclase and guanylyl-cyclase, which catalyze conversion of ATP and GTP to cAMP and cGMP, respectively, and at their destruction, by cyclic nucleotide phosphodiesterases (PDEs) [1] (Figure 1).
Figure 1.
Summary of cyclic nucleotide signaling pathways: cyclic nucleotides are generated by adenylyl-cyclase and guanylyl-cyclase; the former, activated by G-protein-coupled receptors, and the latter, by molecules such as natriuretic peptide or nitric oxide. In turn, cAMP activates PKA and EPAC. EPAC is involved in the regulation of several cellular processes, including integrin-mediated cell adhesion and cell–cell junction formation [74], exocytosis [75,76,77], and insulin secretion, while PKA is involved in metabolic processes, cell growth, differentiation, and proliferation. cGMP activates PKG which in turn mediates the phosphorylation of proteins involved in apoptosis, inflammation, and other physiologic processes, including smooth muscle contractility [78], the visual transduction cascade, and platelet aggregation. By catalyzing hydrolysis of cAMP and cGMP, PDEs regulate their intracellular concentrations and, consequently, their myriad biological effects.
Phosphodiesterases are enzymes that catalyze the hydrolysis of the 3′ cyclic phosphate bond of cyclic nucleotides. To date, 11 PDE gene families have been identified, based on their amino acid sequences, biochemical properties, and inhibitor profiles. Different PDEs can share the same catalytic function, but may differ in tissue expression and intracellular localization (Table 1) [2].
Table 1.
Summary of human phosphodiesterases: their substrate, tissue expression, subcellular location and inhibitors.
| Family | Substrate | Tissue/cellular expression and function |
Subcellular localization | Commonly used inhibitors |
|---|---|---|---|---|
| PDE1A PDE1B PDE1B1–2 PDE1C1–2 |
cAMP/cGMP | PDE1A: brain and spermatozoa, kidney, liver, pancreas and thyroid gland [79–81]. Heart [82]. Immune cells [83]. Olfactory epithelium [84]. |
Cytosol | Vinpocetine, IC224, SCH51866, 8-MeoM-IBMX. Zaprinast Sildenafil |
| PDE2A1–3 PDE3B |
cAMP/cGMP | Adrenal glomerulosa [85]. Heart muscle [86]. Immune System [87]. Endothelial permeability and proliferation [88]. Brain [39]. Liver [89]. |
Membrane: PDE2A3, and PDE2A2 Cytosol: PDE2A1 |
EHNA, BAY60-7550, PDP, IC933 |
| PDE3A1–3 | cAMP/cGMP | Heart [90]. Adiposyte, oosyte, cardiac and vascular smooth muscle, myocardium, platelet [91]. |
Membrane Cytosol |
Milrinone, Tolafentrine, Cilostazol, Cilostamide, Trequinsin, OPC-33540, Dihydropyridazinone, Lixazinone Zardaverine |
| PDE4A PDE4B PDE4C PDE4D |
cAMP | Heart and small intestine [92]. Immune cells [93]. Brain [94]. |
Membrane | Cilomilast, Rolipram, Ro20-1724, Roflumilast, AWD12281, V11294A, SCH35159, Denbufylline, Arofylline |
| PDE5A1–3 | cGMP | Lung, penis, smooth muscle [15•]. Platelets [95]. Brain [96]. Cardiac muscle [97]. |
Cytosol | Sildenafil, Tadalafil, DA8159, E402, Vardenafil, Zaprinast, DMPPO, Dipyridamole |
| PDE6A PDE6B PDE6C |
cGMP | Photoreceptors [98]. Pineal gland [99]. |
Membrane and Cytosol | Zaprinast, Dypyridamole, Sildenafil, Verdenafil, Tadalafil |
| PDE7A1–2 PDE7B1–3 |
cAMP | Immune cells [100]. Skeletal and cardiac muscle [101]. Brain [102]. |
Cytosol | BRL 50481, IC242, Dipyridamole, BMS-586353, Thiadiazoles |
| PDE8A1–5 PDE8B1–3 |
cAMP | Immune cells [103]. Heart [104]. Ovary and testes [105]. Thyroid gland [31]. Placenta, Brain [106]. Adrenal gland [28•]. |
Cytosol and particulate fractions | Dipyridamole |
| PDE9 A1–6 | cGMP | Kidney, spleen, gut, prostate [107]. Brain (Rat) [108]. |
Cytosol and nucleus | BAY 73–669, SCH51866, Zaprinast |
| PDE10A1–2 | cAMP/cGMP | Brain, testis, thyroid [109]. | Cytosol and particulate fractions | Papaverine, Zaprinast Dipyridamole, PQ-10 |
| PDE11A1–4 | cAMP/cGMP | Testis, pituitary gland, heart. Kidney, liver [57,110]. Prostate, adrenal, colon [58] |
Cytosol | Dipyridamole, Zaprinast |
Elevation of cAMP induces activation of cAMP-dependent protein kinase A (PKA). PKA is a heterotetramer formed by two catalytic subunits (C) and two regulatory subunits (R) [3]. In the absence of cAMP, PKA is inactive. Upon cAMP binding to the R-PKA, the catalytic subunits are released and phosphorylate different targets, including the cAMP response element binding (CREB) protein, a transcription factor that regulates genes involved in metabolism and proliferation [4,5]. Similarly, cGMP activates protein kinase G (PKG) which catalyzes the phosphorylation of downstream proteins involved in several physiologic functions, such as glycogenolysis, ion channel conductance, and apoptosis [6].
Dysregulation of cAMP homeostasis can be linked to tumorigenesis, both directly and indirectly [7]. Some tumor cells overexpress phosphodiesterases and exhibit lower cAMP levels [8], whereas other tumor types have increased cAMP levels as a protective mechanism against malignancy [9]. Thus, understanding the molecular basis of cAMP signaling can provide new insights for improved pharmaceutical targeting of cancer cells [10,11].
PDEs and endocrine glands: tumors and other phenotypes
Alterations in cAMP signaling pathways have been linked to tumorigenesis at different levels. First, activating mutations of the stimulatory G protein of adenylyl-cyclase, which induces high cAMP levels, leads to endocrine and nonendocrine manifestations in McCune Albright syndrome (MAS) [12]. Second, inactivating germline mutations in the alpha regulatory subunit gene of the PKA gene (PRKARIA) lead to the Carney complex (CNC) [13]. CNC is an autosomal dominant disease characterized by skin pigmentary abnormalities, cardiac myxomas, schwannomas, and endocrine tumors, the most frequent being a type of adrenocortical hyperplasia named primary pigmented nodular adrenocortical disease (PPNAD) [14]. PRKAR1A is located on chromosome 17q22–24, and more than a hundred different mutations of this gene have been described [13,15•,16–19].
Altered cAMP signaling, somatic PRKAR1A mutations, and somatic losses in the 17q22–24 locus have all been reported in adrenocortical adenomas and adrenocortical cancer. Specifically, 17q22–24 losses were found in 23% and 53% of adrenocortical adenomas and adrenocortical cancer samples, respectively. Both cancers and adenomas with 17q losses had higher PKA activity in response to cAMP when compared to similar tumors without 17q losses [20•].
A third link between cAMP and tumorigenesis is through altered PDEs. Inactivating molecular defects in PDEs lead to high cAMP or cGMP levels that in turn generate a continuous activation of the cAMP/PKA cascade. In 2006, our laboratory identified five PDE11A mutations in a group of 16 patients with adrenocortical hyperplasia. Three of these mutations led to premature terminations with truncated proteins, and the other two were missense mutations (R804H and R867G), leading to defective proteins [21••].
Although germline PDE11A truncating-protein mutations are seen in the general population, they are significantly more common among patients with adrenal hyperplasia [22]. Somatic missense mutations are frequently found in adrenocortical tumors: adrenocortical cancer (ACA), adrenocortical adenomas, and corticotrophin (ACTH)-independent macronodular adrenal hyperplasia or AIMAH. In line with the above, higher cAMP levels and lower PDE11A expression were observed in AIMAH and ACA tissues studied by immunohistochemistry [23•]. Interestingly, a higher frequency of PDE11A variants has been found in patients with PRKAR1A mutations, suggesting a contribution of PDE11A to adrenal and testicular tumor formation in CNC [24•]. More recently, PDE11A genetic defects were found to be significantly increased in prostatic cancer patients, compared with healthy controls, suggesting that PDE11A genetic variants may play a role in susceptibility to prostatic cancer, as well [25••].
A second PDE found to be involved in adrenocortical tumor predisposition was PDE8B; its locus on chromosome 5 was the second most highly linked to adrenal hyperplasias in a genome-wide study [21••]. A PDE8B missense mutation (p.H305P) was then described in a young girl with isolated micronodular adrenocortical disease. Functional studies showed high levels of cAMP in HEK293 cells transfected with the mutant gene [26]. Subsequently, additional three novel mutations in PDE8B were described in patients with adrenal tumors [27]. PDE8 is highly expressed in adrenal tissues [28•], and has an important role in steroidogenesis in adrenals, as recently demonstrated [29]. AIMAH and cortisol-producing adenomas specimens were found to have high cAMP levels and, interestingly, decreased PDE activity was shown in cortisol-producing adenomas [30•].
PDE8 is highly expressed in the pituitary gland [31]. A strong association between high TSH levels and polymorphisms in the PDE8B gene was described in a genome-wide association study [32]. The segregation of those polymorphic variants in a family with micronodular adrenal disease, with a PDE8 defect leading to Cushing syndrome was also studied [26]. The analysis revealed separate segregation of an inactivating PDE8B allele from the high TSH-predisposing allele, and showed low TSH levels in individuals who carry an inactivating PDE8B allele [28•].
An association between PDE10A and hypothyroidism was found in a study comprising 1258 individuals from three Alpine villages. In this study, a combination of linkage and association in families with hyperthyrotropinemia pointed to PDE10A and DACT2 as candidate genes. Genome association of the TSH values in a different population set supported the involvement of the PDE10A locus [33].
An association between upregulation of PDEs in a growth hormone (GH)-producing pituitary adenoma carrying a GNAS mutation has been investigated; increased PDE4C and PDE4D expression and activity were discussed as a possible protective mechanism against GNAS-dependent activation of the cAMP pathway [34].
PDE inhibitors and cancer
Vinpocetine, a PDE1 inhibitor, is used for the prevention of cerebrovascular disease and cognitive impairment, and to date no significant side effects or toxicity have been reported. Although the use of PDE1 inhibitors has not been associated with effects on tumorigenesis, in vitro cell studies have suggested a role for PDE1 inhibitors in controlling cell malignancy. For example, inhibition of PDE1B stimulates apoptosis in human leukemia cells [35]. Likewise, PDE1C is overexpressed in human malignant melanoma-associated cells, and growth is inhibited by vinpocetine [1,36,37].
Different PDE2 inhibitors have been experimentally tested but have not been used in humans. PDE2 inhibitors have been mainly tested for effects on endothelial permeability, and to treat learning and memory disorders in animal studies [38,39]. One of them, EHNA (erythro-9-(2-hydroxy-3-nonyl)adenine), has been reported to increase intracellular cAMP levels in human umbilical vein endothelial cells (HUVEC) and inhibit angiogenesis, which can be associated with tumor development and other proliferative pathologies [40].
Cilostazol (Pletal®), a dual inhibitor of PDE3 and adenosine uptake, is used for the treatment of intermittent claudication, due to its anti-aggregant and vasodilator properties [36]. Cilostazol has been tested as a tool for the inhibition of breast cancer metastasis in mice, due to its ability to restrict the aggregability of mouse platelets [41]. Also, cilostazol was reported to block human colon cancer cell motility, and might be effective as an anti-metastasis drug [42].
Rolipram is a PDE4 inhibitor marketed as antidepressant in several countries [43]. Rolipram enhanced the survival of mice bearing xenografts of U87 glioblastoma cells, and augmented the antitumor effect of chemotherapy and radiotherapy [44]. Incubation of a human alveolar epithelial type II cell line with rolipram resulted in inhibition of epithelial-mesenchymal transition (EMT), which is a critical event in the pathogenesis of organ fibrosis and cancer, suggesting that this drug can be used to depress EMT in lung cancer [45]. In CLL cells, rolipram, in a dose-dependent manner, increased intracellular cAMP levels and induced apoptosis [46,47].
Exisulind, a dual inhibitor for PDE4 and PDE5, is a novel drug with proapoptotic properties. In colon cancer cells and in rat bladder tumors; exisulind reduced multiplicity and incidence of the tumorigenic events [48,49]. Zaprinast was the first PDE5 inhibitor used in humans as a mast cell-stabilizer in allergy treatment. Other specific PDE5 inhibitors are sildenafil, vardenafil, and tadalafil which are used for treatment of erectile dysfunction [50]. An interesting connection between PDE5 and melanoma cell invasion has been described by Arozarena et al. [51•]. This study showed that downregulation of PDE5A in melanoma cells led to increased cGMP levels, which in turn caused a mild deceleration in cellular proliferation, but a larger effect on cell contractility. These events culminated in an increased invasion of melanoma cells [51•].
All PDE5 inhibitors weakly inhibit PDE6, which is expressed in rod and cone photoreceptors. This inhibition results in mild and transient visual symptoms that correlate with the inhibitor plasma concentrations [52,53]. The possible effect of therapeutic levels of tadalafil in the physiology of testis and spermatozoids has been a topic of studies that remain inconclusive [54,55,56]. In human tissues that express PDE11A (prostate, pituitary, heart, liver, skeletal muscle testis, bladder, and adrenal gland), no adverse effects related to the use of tadalafil or other PDE5 inhibitors that inhibit PDE11A have been reported to date [21••,55,57–59].
The use of PDE5 inhibitors as possible modulators of cell growth, division, and death has been reported. Sildefanil has been shown to induce an augmented endogenous antitumor immunity in several mouse tumor models [60]. Similar to the aforementioned activity of PDE4 inhibitors, vardenafil and sildenafil can induce apoptosis of chronic lymphocytic leukemia cells in vitro in an induced caspase-dependent mechanism [61].
Another inhibitor of PDE5, sulindac, can inhibit growth and induce apoptosis in human breast tumor cells, through elevation of cGMP and subsequent activation of PKG [62]. Sulindac also induces apoptosis in a non-small cell lung cancer orthotopic lung tumor model via a mechanism involving PDE inhibition — a finding consistent with a cGMP-regulated apoptosis pathway [63]. Furthermore, the use of antisense RNAi that suppresses PDE5 activity in human colon tumor cells inhibited cell growth by inducing cell apoptosis and delaying cell-cycle progression [64].
The effects of sildenafil, tadalafil, and vardenafil were also investigated in human stromal cells involved in bilateral prostatic hypertrophy (BPH). Vardenafil significantly inhibited human stromal cell proliferation in a dose-dependent manner [65]. A possible underlying mechanism involved blocking the degradation of cGMP, thereby augmenting the bioactivity of nitric oxide (NO), which, in turn, inhibited NADPH oxidase (NOX) and contributed to the reduction of superoxide (O2−), a free radical thought to be involved in the genesis of BPH [66]. However, another study showed that inhibition of PDE5 can induce cell proliferation, and enhance new vessel growth and cell migration through activation of MAPKs [67,68]. In addition, an intracellular NO-induced apoptosis mechanism, which was enhanced by Ca2+-dependent NOX activation, was inhibited by downregulation of calcium transport exerted by PDE5 inhibitors [69,70,71].
Regarding the role of PDE7 in cell apoptosis, as previously mentioned for PDE4, the high expression of PDE7B in chronic lymphoid leukemia (CLL) cells, and PDE7 inhibitor-induced apoptosis can imply a new therapeutic target for this entity [72].
A very selective PDE8 inhibitor, PF-04957325 (Pfizer Inc., Groton Laboratories, Groton, CT), has been used in the characterization of T-cell adhesion and proliferation [73]. The association between PDE8B genetic defects and adrenal hyperplasia was described above, and although the use of PF-04957325 is known to potentiate steroidogenesis in Y-1 adrenal cell line and in mouse primary adrenocortical cells, no other reference of effects of PDE8 inhibitors on adrenal hyperplasia or tumorigenesis has been reported [29].
As mentioned, PDE11A defects have been described in different endocrine tumors, and high PDE11A immunoreactivity was detected by immunohistochemistry in renal, prostate, colon, lung, and breast carcinoma tissues [58]. However, PDE11A-specific inhibitors are not available to characterize the role for PDE11A in these tumors.
Conclusions
The continuous interest in PDE research since their discovery goes hand in hand with the development of their inhibitors which are used, first, to biologically characterize PDEs in different tissues and understand their involvement in various physiological and pathological settings, and, second, to selectively target PDEs in the treatment of diseases, avoiding adverse effects. Growing evidence supports a role for cyclic nucleotide signaling pathways in endocrine cell growth and proliferation, and possible tumor development. Additional studies are needed for more conclusive evidence and for the investigation of the role of PDE-modulating drugs in fighting tumor development.
Acknowledgments
This work was supported by the Intramural Research Program (IRP) of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH). The figure was designed by Jeremy Swan and Nichole C. Jonas both from the NICHD IRP Unit on Computer Support Services.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Savai R, Pullamsetti SS, Banat GA, Weissmann N, Ghofrani HA, Grimminger F, Schermuly RT. Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs. 2010;19:117–131. doi: 10.1517/13543780903485642. [DOI] [PubMed] [Google Scholar]
- 2.Makhlouf A, Kshirsagar A, Niederberger C. Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res. 2006;18:501–509. doi: 10.1038/sj.ijir.3901441. [DOI] [PubMed] [Google Scholar]
- 3.Taskén K, Skålhegg BS, Taskén KA, Solberg R, Knutsen HK, Levy FO, Sandberg M, Orstavik S, Larsen T, Johansen AK, et al. Structure, function, and regulation of human cAMP-dependent protein kinases. Adv Second Messenger Phosphoprotein Res. 1997;31:191–204. doi: 10.1016/s1040-7952(97)80019-5. [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–696. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Cheng CY, Saldanha SA, Taylor SS. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell. 2007;130:1032–1043. doi: 10.1016/j.cell.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Lee JH, Park SY, Hong KW, Kim CD, Kim KY, Lee WS. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res. 2006;54:261–267. doi: 10.1016/j.phrs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Murray F, Zahno A, Kanter JR, Chou D, Suda R, Fenlon M, Rassenti L, Cottam H, Kipps TJ, et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:19532–19537. doi: 10.1073/pnas.0806152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengupta R, Sun T, Warrington NM, Rubin JB. Treating brain tumors with PDE4 inhibitors. Trends Pharmacol Sci. 2011;32:337–344. doi: 10.1016/j.tips.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM. Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci. 2005;62:1198–1220. doi: 10.1007/s00018-005-4533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 13.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 14.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 15. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 50-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. This study describes the phenotypic and genotypic from 353 patients who carried a germline PRKAR1A mutation or were diagnosed with Carney complex and/or primary pigmented nodular adrenocortical disease.
- 16.Greene EL, Horvath AD, Nesterova M, Giatzakis C, Bossis I, Stratakis CA. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29:633–639. doi: 10.1002/humu.20688. [DOI] [PubMed] [Google Scholar]
- 17.Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D, Carney JA, Luton JP, et al. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet. 2002;71:1433–1442. doi: 10.1086/344579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libe R, Remmers E, Rene-Corail F, Faucz FR, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31:369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenbuhler A, Stratakis CA. Clinical and molecular genetics of Carney complex. Best Pract Res Clin Endocrinol Metab. 2010;24:389–399. doi: 10.1016/j.beem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20. Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, Papageorgiou T, Bourdeau I, Kirschner LS, Vincent-Dejean C, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63:5308–5319. In this study the authors demonstrate that PRKAR1A-inactivating mutations correlate with activation of PKA in adrenocortical tumors, showing a link between cAMP/PKA signaling pathways and tumorigenesis.
- 21. Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. Here is reported, for the first time, human PDE11A germline inactivating mutations that were associated with adrenocortical hyperplasia, suggesting that dysregulation of cAMP signaling can cause endocrine tumors.
- 22.Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis I, et al. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 2006;66:11571–11575. doi: 10.1158/0008-5472.CAN-06-2914. [DOI] [PubMed] [Google Scholar]
- 23. Libé R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X, Raffin-Sanson ML, et al. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res. 2008;14:4016–4024. doi: 10.1158/1078-0432.CCR-08-0106. This publication describes the link between genetic defects in PDE11A and different adrenocortical tumors, in addition to those previously described in micronodular adrenocortical hyperplasia.
- 24. Libé R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, Ragazzon B, Guillaud-Bataille M, Groussin L, Clauser E, et al. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab. 2011;96:E208–E214. doi: 10.1210/jc.2010-1704. In this article, the role of PDE11A as a possible gene modifier of the phenotype in Carney complex patients was investigated. Interestingly, PDE11A variants were significantly associated with the presence of large-cell calcifying Sertoli cell tumors.
- 25. Faucz FR, Horvath A, Rothenbuhler A, Almeida MQ, Libé R, Raffin-Sanson ML, Bertherat J, Carraro DM, Soares FA, Molina Gde C, et al. Phosphodiesterase 11A (PDE11A) genetic variants may increase susceptibility to prostatic cancer. J Clin Endocrinol Metab. 2011;96:E5–E140. doi: 10.1210/jc.2010-1655. In this study the authors identified PDE11A germline variations and mutations in 50 unrelated patients with prostatic cancer. All missense mutations led to decreased PDE11A activity in vitro and inmmunostaining of the prostatic cancer samples showed decreased PDE11A protein expression.
- 26.Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med. 2008;358:750–752. doi: 10.1056/NEJMc0706182. [DOI] [PubMed] [Google Scholar]
- 27.Rothenbuhler A, Horvath A, Faucz F, Almeida M, Lodish M, Libe R, Nesterova M, Bertherat J, Stratakis CA. Program of the 91st Annual Meeting of the Endocrine Society. Washington, DC: 2009. Three novel mutations in PDE8B, a cAMP phosphodiesterase highly expressed in the adrenal cortex, in a cohort of patients with adrenal tumors. [Google Scholar]
- 28. Horvath A, Giatzakis C, Tsang K, Greene E, Osorio P, Boikos S, Libe R, Patronas Y, Robinson-White A, Remmers E, et al. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet. 2008;16:1245–1253. doi: 10.1038/ejhg.2008.85. In this study a novel PDE8B mutation in a patient with isolated micronodular adrenal hyperplasia (iMAD) was described and characterized. High expression of PDE8B was reported in normal adrenal tissues while downregulation of PDE8B was observed in iMAD tumors tissues from patients without any other genetic defects.
- 29.Tsai LC, Shimizu-Albergine M, Beavo JA. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol. 2011;79:639–648. doi: 10.1124/mol.110.069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bimpaki EI, Nesterova M, Stratakis CA. Abnormalities of cAMP signaling are present in adrenocortical lesions associated with ACTH-independent Cushing syndrome despite the absence of mutations in known genes. Eur J Endocrinol. 2009;161:153–161. doi: 10.1530/EJE-09-0027. This study shows the link between an aberrant cAMP pathway and tumorigenesis in adrenal lesions associated with adrenal independent Cushing syndrome from patients without any other known mutations that lead to this disease.
- 31.Hayashi M, Matsushima K, Ohashi H, Tsunoda H, Murase S, Kawarada Y, Tanaka T. Molecular cloning and characterization of human PDE8B, a novel thyroid-specific isozyme of 3′,5′-cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1998;250:751–756. doi: 10.1006/bbrc.1998.9379. [DOI] [PubMed] [Google Scholar]
- 32.Arnaud-Lopez L, Usala G, Ceresini G, Mitchell BD, Pilia MG, Piras MG, Sestu N, Maschio A, Busonero F, Albai G, et al. Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. Am J Hum Genet. 2008;82:1270–1280. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpato CB, De Grandi A, Gögele M, Taliun D, Fuchsberger C, Facheris MF, Minelli C, Pattaro C, Pramstaller PP, Hicks AA. Linkage and association analysis of hyperthyrotropinaemia in an Alpine population reveal two novel loci on chromosomes 3q28–29 and 6q26–27. J Med Genet. 2011;48:549–556. doi: 10.1136/jmg.2010.088583. [DOI] [PubMed] [Google Scholar]
- 34.Lania A, Persani L, Ballaré E, Mantovani S, Losa M, Spada A. Constitutively active Gs alpha is associated with an increased phosphodiesterase activity in human growth hormone-secreting adenomas. J Clin Endocrinol Metab. 1998;83:1624–1628. doi: 10.1210/jcem.83.5.4814. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Paskind M, Weltzien R, Epstein PM. Expression and regulation of mRNA for distinct isoforms of cAMP-specific PDE-4 in mitogen-stimulated and leukemic human lymphocytes. Cell Biochem Biophys. 1998;28:135–160. doi: 10.1007/BF02737809. [DOI] [PubMed] [Google Scholar]
- 36.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu K, Murata T, Watanabe Y, Sato C, Morita H, Tagawa T. Characterization of phosphodiesterase 1 in human malignant melanoma cell lines. Anticancer Res. 2009;29:1119–1122. [PubMed] [Google Scholar]
- 38.Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–1092. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 39.Domek-Łopacińska K, Strosznajder JB. The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging. Brain Res. 2008;1216:68–77. doi: 10.1016/j.brainres.2008.02.108. [DOI] [PubMed] [Google Scholar]
- 40.Favot L, Keravis T, Holl V, Le Bec A, Lugnier C. VEGF-induced HUVEC migration and proliferation are decreased by PDE2 and PDE4 inhibitors. Thromb Haemost. 2003;90:334–343. doi: 10.1160/TH03-02-0084. [DOI] [PubMed] [Google Scholar]
- 41.Wenzel J, Zeisig R, Fichtner I. Inhibition of metastasis in a murine 4T1 breast cancer model by liposomes preventing tumor cell–platelet interactions. Clin Exp Metastasis. 2010;27:25–34. doi: 10.1007/s10585-009-9299-y. [DOI] [PubMed] [Google Scholar]
- 42.Murata K, Kameyama M, Fukui F, Ohigashi H, Hiratsuka M, Sasaki Y, Kabuto T, Mukai M, Mammoto T, Akedo H, et al. Phosphodiesterase type III inhibitor, cilostazol, inhibits colon cancer cell motility. Clin Exp Metastasis. 1999;17:525–530. doi: 10.1023/a:1006626529536. [DOI] [PubMed] [Google Scholar]
- 43.Bobon D, Breulet M, Gerard-Vandenhove MA, Guiot-Goffioul F, Plomteux G, Sastre-y-Hernández M, Schratzer M, Troisfontaines B, von Frenckell R, Wachtel H. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. doi: 10.1007/BF00381071. [DOI] [PubMed] [Google Scholar]
- 44.Goldhoff P, Warrington NM, Limbrick DD, Jr, Hope A, Woerner BM, Jackson E, Perry A, Piwnica-Worms D, Rubin JB. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin Cancer Res. 2008;14:7717–7725. doi: 10.1158/1078-0432.CCR-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F, Seeger W, Banat GA, Schermuly RT, Pullamsetti SS. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell. 2009;20:4751–4765. doi: 10.1091/mbc.E09-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DH, Lerner A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood. 1998;92:2484–2494. [PubMed] [Google Scholar]
- 47.Lerner A, Kim DH, Lee R. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma. 2000;37:39–51. doi: 10.3109/10428190009057627. Review. [DOI] [PubMed] [Google Scholar]
- 48.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphatephosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 49.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, Fetter JR, Gresh WE, Jr, Klein-Szanto AJ, Farnell DR, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 50.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl. 1):S252–S257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. This study showed that downregulation of PDE5A in melanoma cells led to increased cGMP levels, which in turn caused a mild deceleration in cellular proliferation, but a larger effect on cell contractility. These events culminated in an increased invasion of melanoma cells.
- 52.Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;(Suppl 1):S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- 53.Kerr NM, Danesh-Meyer HV. Phosphodiesterase inhibitors and the eye. Clin Experiment Ophthalmol. 2009;37:514–523. doi: 10.1111/j.1442-9071.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 54.Wayman C, Phillips S, Lunny C, Webb T, Fawcett L, Baxendale R, Burgess G. Phosphodiesterase 11 (PDE11) regulation of spermatozoa physiology. Int J Impot Res. 2005;17:216–223. doi: 10.1038/sj.ijir.3901307. [DOI] [PubMed] [Google Scholar]
- 55.Loughney K, Taylor J, Florio VA. 3′,5′-Cyclic nucleotide phosphodiesterase 11A: localization in human tissues. Int J Impot Res. 2005;17:320–325. doi: 10.1038/sj.ijir.3901317. [DOI] [PubMed] [Google Scholar]
- 56.Francis SH. Phosphodiesterase 11 (PDE11): is it a player in human testicular function? Int J Impot Res. 2005;17:467–468. doi: 10.1038/sj.ijir.3901377. [DOI] [PubMed] [Google Scholar]
- 57.Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA, Phillips SC. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci U S A. 2000;97:3702–3707. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Andrea MR, Qiu Y, Haynes-Johnson D, Bhattacharjee S, Kraft P, Lundeen S. Expression of PDE11A in normal and malignant human tissues. J Histochem Cytochem. 2005;53:895–903. doi: 10.1369/jhc.5A6625.2005. [DOI] [PubMed] [Google Scholar]
- 59.Boikos SA, Horvath A, Heyerdahl S, Stein E, Robinson-White A, Bossis I, Bertherat J, Carney JA, Stratakis CA. Phosphodiesterase 11A expression in the adrenal cortex, primary pigmented nodular adrenocortical disease, and other corticotropin-independent lesions. Horm Metab Res. 2008;40:347–353. doi: 10.1055/s-2008-1076694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, Binet JL, Delic J, Merle-Beral H. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–269. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 62.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, Zhao TL, Piazza G, Klein-Szanto AJ, Pamukcu R, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–488. [PubMed] [Google Scholar]
- 64.Zhu B, Vemavarapu L, Thompson WJ, Strada SJ. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem. 2005;94:336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- 65.Tinel H, Stelte-Ludwig B, Hütter J, Sandner P. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006;98:1259–1263. doi: 10.1111/j.1464-410X.2006.06501.x. [DOI] [PubMed] [Google Scholar]
- 66.Hotston M, Shukla N, Bloor J, Persad R, Jeremy JY. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006;98:1331–1332. doi: 10.1111/j.1464-410X.2006.06628_5.x. [DOI] [PubMed] [Google Scholar]
- 67.Koika V, Zhou Z, Vasileiadis I, Roussos C, Finetti F, Monti M, Morbidelli L, Papapetropoulos A. PKG-I inhibition attenuates vascular endothelial growth factor-stimulated angiogenesis. Vascul Pharmacol. 2010;53:215–222. doi: 10.1016/j.vph.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Pyriochou A, Zhou Z, Koika V, Petrou C, Cordopatis P, Sessa WC, Papapetropoulos A. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J Cell Physiol. 2007;211:197–204. doi: 10.1002/jcp.20929. [DOI] [PubMed] [Google Scholar]
- 69.Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Z, Francis CE, Welch G, Loscalzo J, Ravid K. Reduced glutathione prevents nitric oxide-induced apoptosis in vascular smooth muscle cells. Biochim Biophys Acta. 1997;1359:143–152. doi: 10.1016/s0167-4889(97)00093-1. [DOI] [PubMed] [Google Scholar]
- 71.Fauzc RF, de Alexandre RB, Stratakis CA. Phosphodiesterases: genes and their variants, inhibitors and potential therapeutic applications. Expert Rev Endocrinol Metab. 2011;6:497–499. [Google Scholar]
- 72.Zhang L, Murray F, Zahno A, Kanter JR, Chou D, Suda R, Fenlon M, Rassenti L, Cottam H, Kipps TJ, Insel PA. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:19532–19537. doi: 10.1073/pnas.0806152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vang AG, Ben-Sasson SZ, Dong H, Kream B, DeNinno MP, Claffey MM, Housley W, Clark RB, Epstein PM, Brocke S. PDE8 regulates rapid Teff cell adhesion and proliferation independent of ICER. PLoS One. 2010;5:e12011. doi: 10.1371/journal.pone.0012011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 77.Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 79.Lefièvre L, de Lamirande E, Gagnon C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod. 2002;67:423–430. doi: 10.1095/biolreprod67.2.423. [DOI] [PubMed] [Google Scholar]
- 80.Fidock M, Miller M, Lanfear J. Isolation and differential tissue distribution of two human cDNAs encoding PDE1 splice variants. Cell Signal. 2002;14:53–60. doi: 10.1016/s0898-6568(01)00207-8. [DOI] [PubMed] [Google Scholar]
- 81.Michibata H, Yanaka N, Kanoh Y, Okumura K, Omori K. Human Ca2+/calmodulin-dependent phosphodiesterase PDE1A: novel splice variants, their specific expression, genomic organization, and chromosomal localization. Biochim Biophys Acta. 2001;1517:278–287. doi: 10.1016/s0167-4781(00)00293-1. [DOI] [PubMed] [Google Scholar]
- 82.Loughney K, Martins TJ, Harris EA, Sadhu K, Hicks JB, Sonnenburg WK, Beavo JA, Ferguson K. Isolation and characterization of cDNAs corresponding to two human calcium, calmodulin-regulated, 3′,5′-cyclic nucleotide phosphodiesterases. J Biol Chem. 1996;271:796–806. doi: 10.1074/jbc.271.2.796. [DOI] [PubMed] [Google Scholar]
- 83.Kanda N, Watanabe S. Regulatory roles of adenylate cyclase and cyclic nucleotide phosphodiesterases 1 and 4 in interleukin-13 production by activated human T cells. Biochem Pharmacol. 2001;62:495–507. doi: 10.1016/s0006-2952(01)00688-8. [DOI] [PubMed] [Google Scholar]
- 84.Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K, Beavo JA. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nikolaev VO, Gambaryan S, Engelhardt S, Walter U, Lohse MJ. Real-time monitoring of the PDE2 activity of live cells: hormone-stimulated cAMP hydrolysis is faster than hormone-stimulated cAMP synthesis. J Biol Chem. 2005;280:1716–1719. doi: 10.1074/jbc.C400505200. [DOI] [PubMed] [Google Scholar]
- 86.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, et al. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 87.Bender AT, Ostenson CL, Giordano D, Beavo JA. Differentiation of human monocytes in vitro with granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor produces distinct changes in cGMP phosphodiesterase expression. Cell Signal. 2004;16:365–374. doi: 10.1016/j.cellsig.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 88.Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Bürger A, Hatzelmann A, Tenor H, Schudt C, Krüll M, et al. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood. 2005;105:3569–3576. doi: 10.1182/blood-2004-07-2729. [DOI] [PubMed] [Google Scholar]
- 89.de Oliveira SK, Smolenski A. Phosphodiesterases link the aryl hydrocarbon receptor complex to cyclic nucleotide signaling. Biochem Pharmacol. 2009;77:723–733. doi: 10.1016/j.bcp.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 90.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 2003;64:533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- 91.Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3) J Biol Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 92.Rena G, Begg F, Ross A, MacKenzie C, McPhee I, Campbell L, Huston E, Sullivan M, Houslay MD. Molecular cloning, genomic positioning, promoter identification, and characterization of the novel cyclic amp-specific phosphodiesterase PDE4A10. Mol Pharmacol. 2001;59:996–1011. doi: 10.1124/mol.59.5.996. [DOI] [PubMed] [Google Scholar]
- 93.Wang P, Wu P, Ohleth KM, Egan RW, Billah MM. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56:170–174. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- 94.Bolger G, Michaeli T, Martins T, St John T, Steiner B, Rodgers L, Riggs M, Wigler M, Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dunkern TR, Hatzelmann A. The effect of Sildenafil on human platelet secretory function is controlled by a complex interplay between phosphodiesterases 2, 3 and 5. Cell Signal. 2005;17:331–339. doi: 10.1016/j.cellsig.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 96.Prickaerts J, Sik A, van Staveren WC, Koopmans G, Steinbusch HW, van der Staay FJ, de Vente J, Blokland A. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int. 2004;45:915–928. doi: 10.1016/j.neuint.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 97.Miller CL, Yan C. Targeting cyclic nucleotide phosphodiesterase in the heart: therapeutic implications. J Cardiovasc Transl Res. 2010;3:507–515. doi: 10.1007/s12265-010-9203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: crystal clear. Trends Biochem Sci. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 99.Morin F, Lugnier C, Kameni J, Voisin P. Expression and role of phosphodiesterase 6 in the chicken pineal gland. J Neurochem. 2001;78:88–99. doi: 10.1046/j.1471-4159.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 100.Bloom TJ, Beavo JA. Identification and tissue-specific expression of PDE7 phosphodiesterase splice variants. Proc Natl Acad Sci U S A. 1996;93:14188–14192. doi: 10.1073/pnas.93.24.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han P, Zhu X, Michaeli T. Alternative splicing of the high affinity cAMP specific phosphodiesterase (PDE7A) mRNA in human skeletal muscle and heart. J Biol Chem. 1997;272:16152–16157. doi: 10.1074/jbc.272.26.16152. [DOI] [PubMed] [Google Scholar]
- 102.Sasaki T, Kotera J, Omori K. Transcriptional activation of phosphodiesterase 7B1 by dopamine D1 receptor stimulation through the cyclic AMP/cyclic AMP dependent protein kinase/cyclic AMP-response element binding protein pathway in primary striatal neurons. J Neurochem. 2004;89:474–483. doi: 10.1111/j.1471-4159.2004.02354.x. [DOI] [PubMed] [Google Scholar]
- 103.Glavas NA, Ostenson C, Schaefer JB, Vasta V, Beavo T cell activation upregulates cyclic nucleotide phosphodiesterases 8A1 and 7A3. Proc Natl Acad Sci U S A. 2001;98:6319–6324. doi: 10.1073/pnas.101131098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patrucco E, Albergine MS, Santana LF, Beavo JA. Phosphodiesterase 8A (PDE8A) regulates excitation-contraction coupling in ventricular myocytes. J Mol Cell Cardiol. 2010;49:330–333. doi: 10.1016/j.yjmcc.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 106.Hayashi M, Shimada Y, Nishimura Y, Hama T, Tanaka T. Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem Biophys Res Commun. 2002;297:1253–1258. doi: 10.1016/s0006-291x(02)02371-9. [DOI] [PubMed] [Google Scholar]
- 107.Rentero C, Monfort A, Puigdomenech P. Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem Biophys Res Commun. 2003;301:686–692. doi: 10.1016/s0006-291x(03)00021-4. [DOI] [PubMed] [Google Scholar]
- 108.Andreeva SG, Dikkes P, Epstein PM, Rosenberg PA. Expression of cGMP specific phosphodiesterase 9A mRNA in the rat brain. J Neurosci. 2001;21:9068–9076. doi: 10.1523/JNEUROSCI.21-22-09068.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A) J Biol Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- 110.Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000;275:31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]