Abstract
Background:
Nicotinamide (NA), the active form of vitamin-B3, is hypothesized to have positive effects on the process of wound healing; it has anti-inflammatory, antioxidant, and immunomodulatory properties, as well as an epithelization inducing action.
Objectives:
In the present study, we aimed to determine the effects of topical administration of NA on skin wounds, based on histomorphometrical and pathological criteria.
Materials and Methods:
In this study, 36 male Sprague-Dawley rats (220 ± 20 g each), with 1 cm2 circular full-thickness wounds on their backs were divided into three groups (n = 12): NA group, was treated daily with a Nicotinamide 2% gel , untreated group (control), and base group, which were treated with the vehicle (base) of the gel (carboxymethylcellulose). Skin biopsies were prepared for microscopic analyses. Inflammation, granulation tissue formation, ulceration, epithelization, wound closure rate, fibroblast proliferation, collagen synthesis, and vascularization were studied criteria.
Results:
The results revealed that besides improving the wound healing by its anti-inflammatory, antioxidant, and epithelization inducing effects, NA also improved tissue regeneration through the increment of fibroblast proliferation, collagen synthesis, and vascularization.
Conclusions:
In spite of the few reported side effects, NA can be introduced as an effective agent on the wound healing process, an adjuvant therapy and possibly a treatment by itself. However, its chemical characteristics, as well as possible adverse effects warrants further research.
Keywords: Nicotinamide, Wound, Guided Tissue Regeneration, Pathology
1. Background
One of the main objectives of wound treatment is rapid re-epithelization, along with an aesthetically satisfactory appearance. Recent advances in cellular and molecular biology have greatly promoted our knowledge of the wound healing process and tissue regeneration leading to major improvements in wound care (1). Nicotinamide (NA), also known as niacinamide or nicotinic acid amide, is a water-soluble, active form of vitamin B3. It has been increasingly investigated for variable usage, particularly in the field of dermatology. NA was shown to possess anti-inflammatory, antioxidative properties (2) and regulatory effects on the expression of immunomodulatory proteins (3). It was shown in a study by Myczkowski that using NA infusion , with split skin removal, reduced the wound healing time from 15 - 17 to 7 - 10 days (4). Collins et al. established better healing rate of the wounds following reconstructive plastic surgery, followed by parenteral administration of NA (5). Topical NA also improves the re-epithelization of pemphigus vulgaris skin lesions (6).
2. Objectives
We hypothesized that topical NA could possibly have improving effects on the process of wound healing. Since there are several studies on the aspects of the therapeutic effects of NA, the present study aimed to determine the impact of NA on the wound healing process, by using stereological and histomorphometrical analyses, as well as pathological analysis of full-thickness skin wounds in laboratory rats.
3. Materials and Methods
3.1. Preparation of Nicotinamide Gel
The NA was obtained from the Pharmacology Department of Shiraz University of Medical Sciences, Shiraz, Iran; 2% NA gel was prepared by dissolving 2 g NA in 2 cc sterilized distilled water and transferring the solution to 2% carboxymethylcellulose (CMC) comprising 2 g CMC dissolved in 98 cc distilled water as the gel base (vehicle). For the gel-base treated group, the gel-base was also obtained by dissolving 2 g CMC in 100 cc sterilized distilled water. All of the gels were prepared in sterile conditions before being administered.
3.2. Animals and Excision of Wound Model
The study protocol was approved by the Medical Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran, (Accepted on April 2013, Code: 91-01-61-4854) and the animal care was in accordance with ethical guidelines. In this study, 36 male Sprague-Dawley rats (220 ± 20 g each), aged between 2 - 3 months, were used. Under general anesthesia, a 1 cm2 circular full-thickness wound was created on the posterior surface of the rat’s neck. The rats were randomly divided into three groups (n = 12) namely the group treated with vehicle gel (Base); one group treated with 2% NA gel (NA); and the untreated wounded group (Control), which received no treatment except for cleaning the wound site with sterilized distilled water every day. After 12 days (the day in which at least one of the wounds was completely closed in any group), the animals were euthanized with a high dose of ether and full thickness skin biopsies (with a 1 cm margin) taken from the wound site and fixed in buffered formaldehyde (pH = 7.2) for microscopic study.
3.3. Pathological Study
All the specimens were formalin-fixed with creation of paraffin blocks. Fifteen µm slides were cut from the blocks and stained with Hematoxylin-Eosin (H and E). The slides were inspected by a pathologist who was unaware of the groups. As presented in Table 1, scoring was calculated for acute and chronic inflammation, ulceration, granulation tissue formation, and re-epithelization.
Table 1. Criteria Used for Light Microscopy Analysis.
| Criterion | Score |
|---|---|
| Inflammation | |
| Acute; dominancy of neutrophils among all cells in the field | |
| Chronic; dominancy of chronic inflammatory cells in the field | |
| Ulceration | |
| Absent | (0) |
| Present | (1) |
| Granulation tissue | |
| Absent | (0) |
| Present | (1) |
| Epithelization | |
| Absent | (0) |
| Slight | Covering < 50% of the wound (1) |
| Moderate | Covering > 50% of the wound (2) |
| Full | Covering 100% of the wound (3) |
3.4. Histomorphometrical and Stereological Study
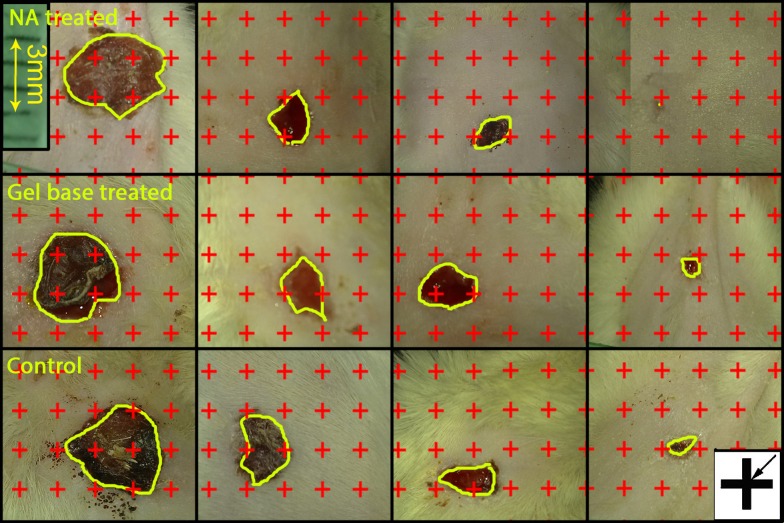
To determine the rate of wound closure, digital photographs were captured from the wound surfaces every four days, with a single lens digital camera. To calibrate the magnification of photographs, a standard ruler was set at the level of the wound site in each photograph, and the wound area (mm2) at each visit was estimated by using a software composed of a point grid, designed at our research center (7), and by using the following Equation:
| (1) |
It was the total points laid on the wound area, a/p, the area surrounded by every four crosses, which was considered as the area per point (mm2, Figure 1) (8, 9). Thereafter, the wound closure rate was calculated as:
Figure 1. Digital photographs were captured from the wound surfaces every other day to measure the wound area. The total number of points within the wound borders was counted. At the corner of this figure, a cross is presented. The right upper corner of the cross is considered as the point (arrow), and it is counted only if the right upper corner hits the wound surface.
| (2) |
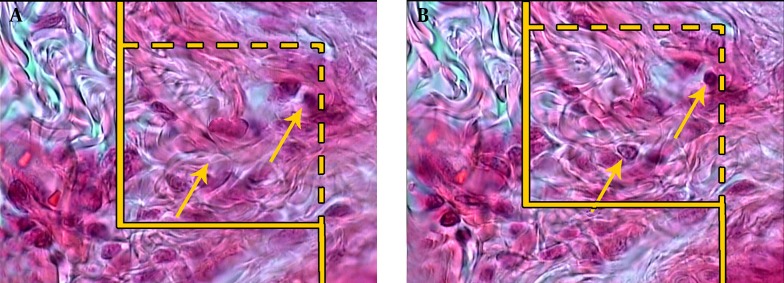
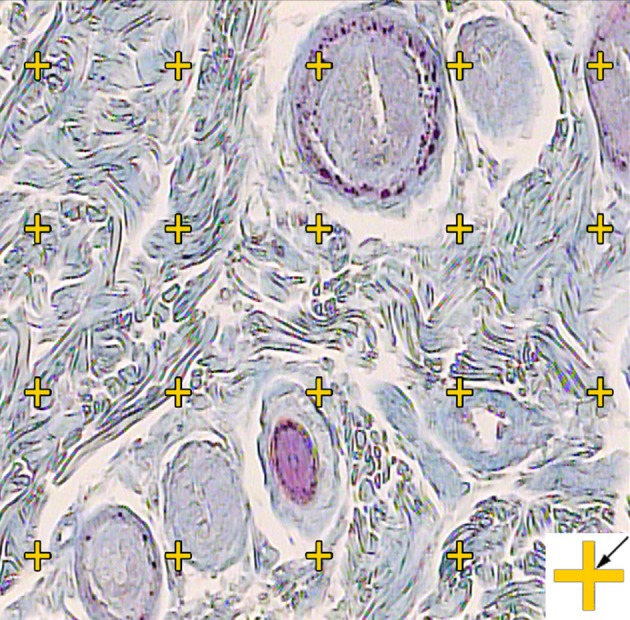
In a systematic random sampling manner (first with a random starting place and the following with equal distances), nine pieces from the skin samples, each of about 1 mm2, were cut and prepared for stereological analysis. The pieces were embedded in a cylindrical paraffin block and the cylindrical blocks were sectioned using orientator methods for generating isotropic uniform random sections. The sections with 5 µm and 15 μm thickness were obtained and stained with Hedenhain’s azan-trichrome. Microscopic analyses of the dermis were done by using a video-microscopy system. Moreover, the volume densities of the collagen bundles were estimated by using the “point counting” method and the following Equation ((8, 9):
| (3) |
Where P (collagen) was the number of the points hitting the collagen bundles, P (dermis) was the number of the points hitting the dermis (not on the collagen bundles), and Vv was the fraction of the unit volume of the dermis that was occupied by the collagen bundles (Figure 2). The length density of the vessels (Lv) was calculated by using the following formula and the 5 µm slides (8, 10):
Figure 2. The volume density (Vv (collagen/dermis)) of the collagen fibers was estimated using a grid of points on the live image of the dermis. The total number of the points hitting the bundles is counted and divided by the total number of the points hitting the reference space (dermis). A cross is presented at the corner of this figure, which is counted only if the right upper corner (arrow) hits the tissue (Hedenhain’s azan stain) (× 450).
| (4) |
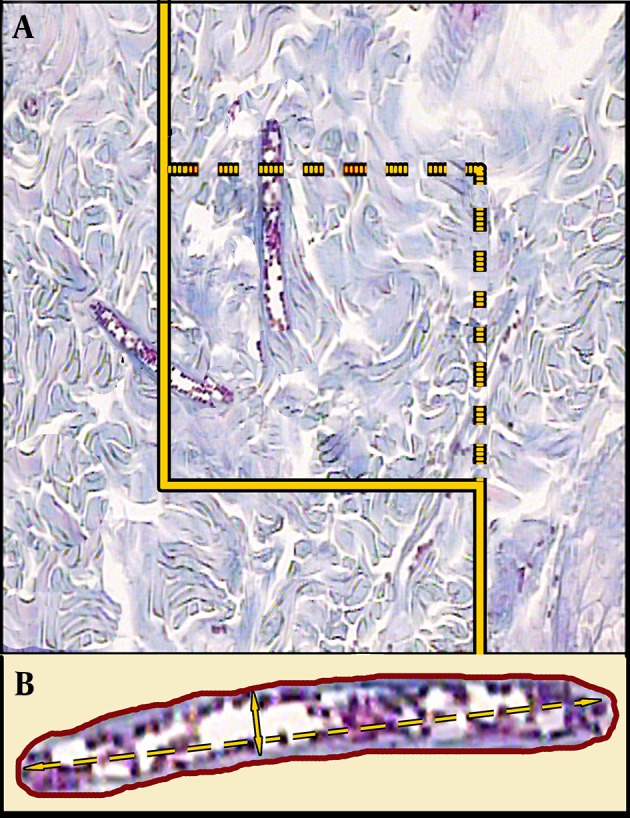
Where “∑Q” was set as the total number of vessel profiles per rat sample, (a/f) was the area of the counting frame, and “∑f” was the total number of the frames counted per animal (Figure 3). The mean diameters of the vessels were calculated by measuring the short axis of a sampled vessel as its diameter. Five µm sections were employed (Figure 3). The numerical density of the fibroblasts was estimated by employing 15 μm sections, the “optical dissector” method, and the following Equation ((9, 10):
Figure 3. A, an unbiased counting frame is randomly laid on the monitor image of the wound dermis at the final magnification of 450 to estimate the vessel’s length density (Lv) and mean diameter. Any vessel which lies in the counting frame or touches the inclusion borders (dotted lines) is selected. The vessels, which touch the exclusion borders (bold continuous lines), are omitted. B, The mean diameter of the vessel is estimated by measuring the short axis of the vessel (short double arrow, Hedenhain’s azan stain).
| (5) |
Where “ΣQ” was the number of the nuclei coming into focus in the dissector height, “ΣA” was the total area of the unbiased counting frame in all microscopic fields, “h” was the height of the dissector, and Nv was the number of the cells per unit volume of the dermis (5 μm here) (Figure 4).
Figure 4. A, An unbiased counting frame laid on the image of the sections to estimate the numerical density (Nv) of the fibroblasts. The nuclei are unclear at the first 5 µm optical section (height of dissector). B, As above, any nucleus which lies in the counting frame or touches the inclusion borders (dotted lines), does not touch the exclusion borders (continuous lines), and comes into the maximal focus within the next traveling 5 µm optical section (height of Dissector) are counted (the two arrows, Hedenhain’s azan stain × 2000).
3.5. Statistical Analysis of the Data
The study data were reported as mean and standard deviation (Mean ± SD). Moreover, one-way analysis of variance (ANOVA) and Tukey’s post hoc test were used to analyze the data. All the statistical analyses were performed using the SPSS statistical software, version 16.0 (SPSS Inc. Chicago, IL, USA). A P ≤ 0.05 was considered as statistically significant.
4. Results
4.1. Pathological Study Results
The pathological investigations on the control group revealed that ulceration, acute inflammation (except for two animals, which had chronic inflammation), granulation tissue formation, and slight epithelization were dominantly present in the specimens. In the base group, acute inflammation was mostly seen in the rats (chronic inflammation in one animal). Ulceration, granulation tissue formation, and slight epithelization (score 1) were also observed, as the dominant state of the base group. However, the NA group had no sign of inflammation on the 12th day, except for one of the animals, with acute inflammation. Full epithelization (score 3) was seen in most of the rats, except for two with score 2 epithelization.
4.2. Area of the Wounds
The mean initial area of the wounds was 106.23 ± 9.51 mm2 (range 97.62 - 114.84 mm2). No significant difference was found among the four groups, regarding the primary wound surface area. However, the rate of wound closure in the NA group (7.92 ± 0.33%; Figure 1) was significantly higher in comparison to the control (4.78 ± 0.81%; P = 0.009) and the base groups (5.68 ± 0.77%; P = 0.021). In addition, the group treated with the cream base revealed an insignificantly slower closure rate, in comparison to the control group.
4.3. Fibroblast Population
The numerical density of the fibroblasts (Nv) in the dermis of the NA group was noticeably higher than that of the control and the base groups. The numerical density of the fibroblasts in the NA-treated group was with 83% higher than in the controls (P = 0.003) and with 76% higher than in the base group (P = 0.004, Table 2).
Table 2. Mean (SD) of the Numerical Density of the Fibroblasts (× 103 per mm3), Volume Densities of the Collagen Bundles (Vv Collagen/Dermis; %) and Vessels (Vv Vessel/Dermis; %), Length Density (mm/mm3) and Mean Diameter (µm) of Vessels in the Dermis of the Wounded Rats Treated With Nicotinamide Gel (NA) , Gel Base (Base) and Untreated Wounded Group (Control).
| Groups | Fibroblasts | Collagen | Vessels | ||
|---|---|---|---|---|---|
| Numerical Density | Volume Density a | Volume Density a | Length Density | Mean Diameter | |
| Control | 173.33 (82.80) | 54 (4) | 3.25 (2.75) | 20.21 (8.10) | 1.87 (0.30) |
| NA | 317.47 (61.47) b | 78 (7) b | 5.30 (3.19) b | 18.91 (4.37) | 3.22 (1.12) b |
| Base | 180.20 (66.55) | 49 (5) | 2.40 (2.79) | 17.16 (7.25) | 1.42 (0.40) |
a Data’s unit is %.
b P < 0.05, NA group vs. control group and base group.
4.4. Volume Density of the Collagen Bundles
The volume density of the collagen bundles in the NA group was significantly higher in comparison to that of the control and the base groups (P < 0.001, Table 2).
4.5. Volume Density, Length Density, and Diameter of the Vessels
The volume density of the vessels in the NA group was significantly higher compared to the control and the base groups (63% higher than the controls and 120% higher than the base group; P < 0.05). In addition, in comparison to the control group, the length density of the vessels was lower in NA (P = 0.9) and base groups (P = 0.7). As Table 2 shows, the length density had increased in the NA group, in contrast with the base group (P = 0.8), which suggests the adverse effects of the gel base in this regard (Table 2). In comparison to the NA treated group, the mean of the vessel diameters was 71% lower in the control group (P = 0.014) and 126% lower in the base group (P = 0.001) (Table 2).
5. Discussion
Finding agents with noticeable impacts and reduced adverse effects on the process of cutaneous wound healing have been a concern of the recent studies. NA has been introduced as an anti-inflammatory, antioxidant, and anti-apoptotic agent, in a large number of researches. It is also proved to have immunomodulatory effects, as well as the ability to modulate cell energy (11). Although systemic administration of NA had shown several side effects, data on possible adverse effects of topical usage are still insufficient (12, 13). In this study, topical NA cream was used on full thickness skin wounds to determine the effectiveness of this agent on the wound healing process. Up till now, a great number of studies have been conducted on the different therapeutic aspects of topical and systemic administration of NA, among which several are related to the factors involved in wound healing. Damian et al. (2007) used topical NA as an ATP precursor, anti-inflammatory agent, and poly ADP ribose polymerase (PARP) inhibitor, on immunity suppression induced by cutaneous ultraviolet (UV) radiation. They found that NA could exert immunoprotective influence, by inhibiting the changes induced by UV radiation on the complement pathways, apoptosis, inflammation, and cellular energy pathways (14). In addition, Biedron et al. mentioned that NA metabolites, such as methylnicotinamide, have noticeable anti-inflammatory impacts in-vivo. They have also stated that NA itself had remarkable anti-inflammatory and antioxidant activities, both in-vivo and in-vitro, mostly due to better cell-membrane permeability (15). In another investigation, NA was reported to increase the synthesis of ceramides and other stratum corneum intracellular lipids, which led to keratinocytes’ apoptotic response; however, increases in the level of ceramides and other intracellular lipids resulted in an improvement in the epidermal permeability barrier deficiencies (16). Smith et al. showed that systemic NA had increased the microvasculature density in the skin and also enriched the skin density, both around and within a burn wound (17). Our study also demonstrated that topical NA had the capability to increase the healing rate of full thickness wounds. According to the results of the present study, NA enhances the healing by induction of collagen bundle synthesis, fibroblast proliferation, and revascularization. Moreover, the findings of the study showed that the closure rate of the wounds treated with NA had remarkably improved, and, based on the pathological evaluations, NA treatment led to reduced inflammation and induced the epithelization at the wound site. Briefly, topical NA was shown to have the ability to be used as a beneficial agent in the treatment of skin wounds and/or as an adjuvant therapy, since it is believed to be safe for topical administrations (18). However, further investigations are still needed in order to more clearly determine its mechanisms of action, which are beneficial in wound treatment, as well as possible adverse effects.
Acknowledgments
This study was supported by the Shiraz University of Medical Sciences, Shiraz, Iran. The authors would like to thank Dr. Vahedi and Mr. Koohi-Hosseinabadi for their generous contribution in this experimental study. The support of the Research Improvement Center of Shiraz University of Medical Sciences, Shiraz, Iran, and Ms. A. Keivanshekouh are also appreciated for improving the use of English in the manuscript.
Footnotes
Authors’ Contribution:Study concept and design: Mohammad Reza Namazi, Soheil Ashkani Esfahani; acquisition of data: Soheil Ashkani Esfahani, Elham Nadimi, Mahsima Khoshneviszadeh; analysis and interpretation of data: Soheil Ashkani-Esfahani; drafting of the manuscript: Soheil Ashkani Esfahani, Ali Noorafshan; Critical revision of the manuscript for important intellectual content: Ali Noorafshan, Soheil Ashkani Esfahani, Bita Geramizadeh; statistical analysis: Soheil Ashkani Esfahani; administrative, technical, and material support: Ali Noorafshan, Bita Geramizadeh; study supervision: Ali Noorafshan, Mohammadreza Namazi.
Funding/Support:This project was supported and funded by Shiraz University of Medical Sciences, Shiraz, Iran and SadraTech Intelligent Research Developers Company, Shiraz Medical School, Shiraz, Iran.
References
- 1.Clark R. The molecular and cellular biology of wound repair. 2th ed. New York: Plenum Press; 1996. [Google Scholar]
- 2.Lappas M, Permezel M. The anti-inflammatory and antioxidative effects of nicotinamide, a vitamin B(3) derivative, are elicited by FoxO3 in human gestational tissues: implications for preterm birth. J Nutr Biochem. 2011;22(12):1195–201. doi: 10.1016/j.jnutbio.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Virag L, Szabo C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54(3):375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 4.Myczkowski T. [Healing of the donor area after transplantation of splitted grafts]. Med Klin. 1975;70(19):861–6. [PubMed] [Google Scholar]
- 5.Collins TM, Caimi R, Lynch PR, Sheffield J, Mitra A, Stueber K, et al. The effects of nicotinamide and hyperbaric oxygen on skin flap survival. Scand J Plast Reconstr Surg Hand Surg. 1991;25(1):5–7. doi: 10.3109/02844319109034915. [DOI] [PubMed] [Google Scholar]
- 6.Iraji F, Banan L. The efficacy of nicotinamide gel 4% as an adjuvant therapy in the treatment of cutaneous erosions of pemphigus vulgaris. Dermatol Ther. 2010;23(3):308–11. doi: 10.1111/j.1529-8019.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashkani-Esfahani S, Imanieh MH, Meshksar A, Khoshneviszadeh M, Noorafshan A, Geramizadeh B, et al. Enhancement of fibroblast proliferation, vascularization and collagen synthesis in the healing process of third-degree burn wounds by topical arnebia euchroma, a herbal medicine. Galen Med J. 2013;1(2):53–9. [Google Scholar]
- 8.Ashkani-Esfahani S, Imanieh MH, Khoshneviszadeh M, Meshksar A, Noorafshan A, Geramizadeh B, et al. The healing effect of arnebia euchroma in second degree burn wounds in rat as an animal model. Iran Red Cres Med J. 2012;14(2):70–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–94. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 10.Ashkani-Esfahani S, Emami Y, Esmaeilzadeh E, Bagheri F, Namazi MR, Keshtkar M, et al. Silymarin enhanced fibroblast proliferation and tissue regeneration in full thickness skin wounds in rat models; a stereological study. J Saudi Soc Dermatol Dermatol Surg. 2013;17(1):7–12. doi: 10.1016/j.jssdds.2012.11.001. [DOI] [Google Scholar]
- 11.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–85. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, Miyamoto K, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147(1):20–31. doi: 10.1046/j.1365-2133.2002.04834.x. [DOI] [PubMed] [Google Scholar]
- 13.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43(11):1337–45. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 14.Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol. 2008;128(2):447–54. doi: 10.1038/sj.jid.5701058. [DOI] [PubMed] [Google Scholar]
- 15.Biedron R, Ciszek M, Tokarczyk M, Bobek M, Kurnyta M, Slominska EM, et al. 1-Methylnicotinamide and nicotinamide: two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch Immunol Ther Exp (Warsz). 2008;56(2):127–34. doi: 10.1007/s00005-008-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanno O, Ota Y, Kitamura N, Katsube T, Inoue S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br J Dermatol. 2000;143(3):524–31. doi: 10.1111/j.1365-2133.2000.03705.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith YR, Klitzman B, Ellis MN, Kull FJ. The effect of nicotinamide on microvascular density and thermal injury in rats. J Surg Res. 1989;47(5):465–9. doi: 10.1016/0022-4804(89)90103-0. [DOI] [PubMed] [Google Scholar]
- 18.Morganti P, Berardesca E, Guarneri B, Guarneri F, Fabrizi G, Palombo P, et al. Topical clindamycin 1% vs. linoleic acid-rich phosphatidylcholine and nicotinamide 4% in the treatment of acne: a multicentre-randomized trial. Int J Cosmet Sci. 2011;33(5):467–76. doi: 10.1111/j.1468-2494.2011.00658.x. [DOI] [PubMed] [Google Scholar]