Abstract
OBJECTIVE
The objective of this study is to investigate the expression and concentration of ligand receptor activator of NFkB (RANKL) and osteoprotegerin (OPG) in human periodontal ligament (hPDL) with orthodontic forces of different magnitudes.
METHODS
Right premolars in 32 patients were loaded with 4oz or 7oz of orthodontic force for 7 days. Left first premolars were not loaded. After 7 days, premolars were extracted for treatment as indicated. OPG and RANKL mRNA expressions were measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR), and ELISA was used to assess OPG and RANKL protein concentration in compression and tension sides of PDL. Data were subjected to analysis of variance and Tukey tests.
RESULTS
There was statistically significant difference in RANKL concentration on comparing control teeth with tension and compression sides of the experimental teeth (P < 0.0001). The expression of mRNA RANKL was increased in the tension and compression sides with 4oz (P < 0.0001). OPG did not show statistically significant association with any group. Changes in RANKL/OPG protein ratio in experimental and control groups showed statistically significant difference (P < 0.0001).
CONCLUSIONS
RANKL protein levels are elevated in hPDL loaded with orthodontic forces, suggesting that RANKL protein contributes to bone modeling in response to the initial placement of orthodontic force.
Keywords: OPG, RANKL, orthodontic forces, bone modeling
Introduction
Orthodontic forces generate cellular and molecular responses, resulting in tooth movement. A difference in the expression pattern of bone modeling markers and histological changes is reasonable assuming that molecular changes in the compression zone are different than those in the tension zone of the periodontal ligament (PDL) and that these changes may depend on the magnitude and duration of orthodontic force. The biological response in PDL and bone has been interpreted as an aseptic inflam matory process mediated by prostaglandins and cytokines.1
Osteoprotegerin (OPG) and the ligand receptor activator of NFkB (RANKL) have an important role in orthodontic tooth movement.2 The key-signaling pathway of RANK–RANKL–OPG explains how the osteoblast lineage regulates the differentiation and activity of osteoclasts in physiologic and pathologic conditions and also in orthodontic movement.3
OPG is a decoy receptor produced by osteoblasts that inhibits terminal stages of osteoclast differentiation, suppresses activation of matrix osteoclasts, and competes with RANK for RANKL binding, accelerating osteoclast apoptosis.4 OPG and RANKL interact with interleukin (IL)-1α, IL-1β, tumor necrosis factor (TNF) α, TNFβ, bone morphogenetic protein 2, and prostaglandin E2 (PGE2) in bone modeling5; however, it has been proposed that TNFα and IL-1β regulate osteoclast differentiation and function through a mechanism independent of the RANKL–RANK interaction.6 In orthodontic tooth movement, OPG gene transfer inhibits RANKL-mediated osteoclastogenesis, preventing experimental tooth movement.7
The RANKL is a member of the membrane-associated TNF ligand family, expressed by mesenchymal cells of osteo-blast lineage and activated T cells (soluble RANKL) in a state of skeletal inflammation.8 RANKL expression is stimulated in osteoblast cells by most of the factors that are known to stimulate osteoclast formation and activity and is a downstream regulator of osteoclast formation and activation.9 RANKL has an important role in osteoclastogenesis when it binds to the RANK receptor in osteoclasts lineage cells, stimulating them to assume the osteoclast phenotype through interaction with multiple hormones and cytokines.10
RANKL has been associated with PDL fibroblasts, osteoblasts, and osteocytes during orthodontic tooth movement.11 In vitro, PDL cells exposed to compressive forces express RANKL.12 Nishijima et al.13 showed an increased concentration of RANKL in gingival crevicular fluid during orthodontic tooth movement, and in human PDL (hPDL) cells exposed to compression force, in a time- and force magnitude-dependent manner. Additionally, it has been shown that local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement12 and is expressed early in the compression side of teeth being moved orthodontically.14
To our knowledge, no published study has compared the expression and concentrations of OPG and RANKL in hPDL loaded with orthodontic forces of different magnitude. Therefore, the aim of the present study was to investigate the differential expression and concentration of the RANKL and OPG in the hPDL with orthodontic force.
Materials and Methods
Study population
This study was conducted according to a protocol reviewed by the ethics committee of the Dentistry Faculty at the Pontificia Universidad Javeriana. The research was conducted in accordance with the principles of the Declaration of Helsinki. Thirty-two subjects ranging in age from 15 to 25 years who required premolar extraction as part of their standard orthodontic care were enrolled in the study. Each subject exhibited good general health, healthy periodontal tissues, and was without caries in the premolars selected for extraction. Patients were excluded if they had taken any medications for up to 4 months before the study or if they had a metabolic pathology or syndrome.
Experimental design
Using a split mouth experimental design, the maxillary right first premolar of each subject was treated as the experimental group and the maxillary left first premolar was utilized as a control. Edgewise brackets were placed in both arches; however, teeth in the control group were not bracketed. After obtaining informed consent from the subjects, the maxillary right first premolar of each subject was loaded with 4oz or 7oz orthodontic force for 7 days. The force applied to the premolars in each group was measured using Dontrix (Dentspaly®). The experimental teeth were moved to the buccal with appliances designed specifically for this study (Fig. 1). After 7 days of force application to the experimental teeth, both maxillary first premolars were extracted as indicated for treatment. Immediately after tooth extraction, the PDL was recovered from the middle zone of the pressure side (labial) and the middle zone of the tension side (palatal) of each experimental tooth with a curette (Hu-Friedy). The PDL was recovered from the same areas of each control tooth. A portion of each PDL sample was stored in phosphate-buffered saline (PBS) at −70 °C for protein determination by ELISA, and a portion was stored in RNAlater® Stabilization Solution (Ambion/Life Technologies) at −70 °C for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis.
Figure 1.
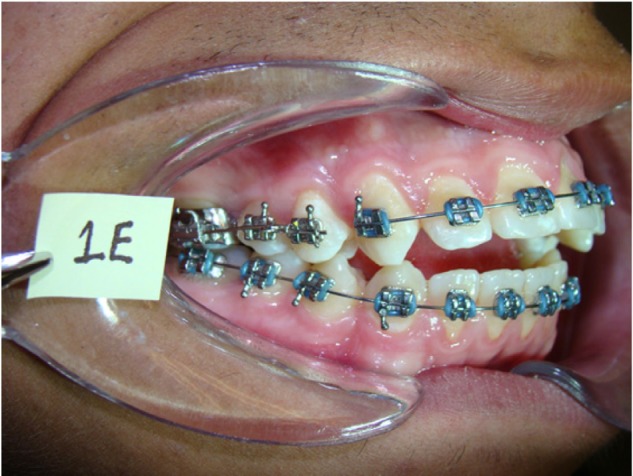
Experimental appliance. The force applied to the premolars in each group was measured using Dontrix (Dentspaly®).
RANKL and OPG protein determinations
The PDL tissues in PBS were mechanically disaggregated and homogenized by Ultrasonic Processor S-2028-130 (ISC BioExpress). Then, 250 µL of acetic acid was added and boiled in water bath for 10 minutes. The disaggregated tissue was centrifuged at 3,500 rpm for 45 minutes in aGS-6KR Centrifuge (Beckman). Supernatants were transferred to another tube. The suspensions was incubated for 2 hours and centrifuged at 5,000 rpm for 1 hour. The samples were suspended in a cocktail of proteases inhibitors (Sigma®). The presence of protein was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and protein concentration was determined by fluorescence spectroscopy (Quibt®). Total protein of 10 µg/mL was used with the ELISA kits for OPG and RANKL (R&D Systems®) according to the manufacturer’s instructions. All samples and standards were assayed twice and reported as concentration (µg/mL).
mRNA expression evaluation
OPG and RANKL mRNA expression was quantitatively measured by qPCR. The sequences of the genes OPG, RANKL, and β-actin were obtained from National Centre for Biotechnology Information, NIH, with accession numbers NM_002546.3, NM_003701.3, and NM_001101.3, respectively. The primers were designed using the data available at http://www.ncbi.nlm.nih.gov/tools/primer-blast/ (Table 1).
Table 1.
Primers sequences of qRT-PCR.
| GENE | T° ANILLING | SEQUENCE PRIMERS 5′ TO 3′ |
|---|---|---|
| OPG | 62 °C | R: ACGCGGTTGTGGGTGCGATT F: AAGACCGTGTGCGCCCCTTG |
| β-Actin | 63 °C | R: AGGGGCCGGACTCGTCAT F: GCCCTGGCACCCAGCACAAT |
| RANKL | 63 °C | R: CAGAAGATGGCACTCACTGCA F: CACCATCGCTTTCTCTGCTCT |
All tissues were digested with 20 mg/mL proteinase K (Invitrogen®) for 1 hour at 55 °C, RNA was then extracted from the samples using TRIzol Reagent (Invitrogen™/Life Technologies™) according to the manufacturer’s instructions. RNA was suspended in 30 µL diethylpyrocarbonate (DEPC) water and quantified with Nanodrop1000® Spectophotometry. DNase I (Invitrogen™/Life Technologies™) was then added to 500 ng of RNA. Complementary DNA (cDNA) was synthesized using SuperScript® III First-Strand Synthesis SuperMix and oligo(dT)20 (Invitrogen™) according to the manufacturer’s instructions.
qRT-PCR was performed using the SYBRGreen I Master (Roche®) for the target genes OPG and RANKL and for the β-actin housekeeping gene. All samples and standards were assayed in triplicate. The final concentrations were 1× master mix, 20 nM primer, and 50 ng cDNA. The negative controls used were a mixture of PCR reagents and DEPC water without RNA and without reverse transcriptase.
The standard PCR conditions were 95 °C (10 minutes, one cycle), and then 40 cycles of 95 °C (10 seconds), 63 °C for β-actin, 62 °C for OPG, 58 °C for RANKL (10 seconds), and an extension step, 72 °C (15 seconds). The melting curve conditions were 95 °C for 5 seconds, 65 °C for 1 minute, and 40 °C for 10 seconds (LightCycler® 480 system; Roche). Relative genetic expression of target gene was calculated using 2ΔCT formula with normalization of housekeeping gene β-actin.
Statistical analysis
To identify possible differences in the RANKL/OPG mRNA expression and RANKL/OPG levels between controls, tension, and compression samples, an analysis of variance test was performed as the Gaussian distribution of the data was normal. Tukey test for multiple comparisons was employed. For all the tests performed, values of P < 0.05 were considered statistically significant. GraphPad Prism 4.0 software (GraphPad Software Inc.) was used for statistical tests.
Results
RANKL and OPG mRNA expression levels
The qRT-PCR analysis was performed to compare protein concentrations of RANKL and OPG with the mRNA expression in PDL. The housekeeping gene amplification, β-actin, was 26 ± 3 cycles showing the cDNA homogeneity in the concentrations of all samples. The Gaussian distribution of the data appeared to be widespread, and the results were analyzed by Kruskal–Wallis test.
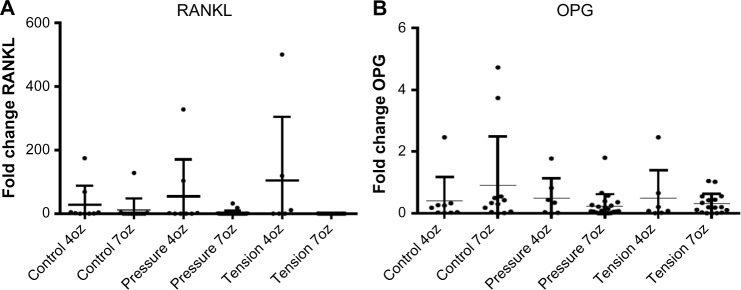
The mRNA expression of RANKL was greater in tension side followed by the pressure sides loaded with 4oz orthodontic force when compared with that in the control teeth (P < 0.0001); however, there was no statistically significant difference among the control, pressure, and tension sides (P = 0.85). In contrast, mRNA expression of OPG did not show statistically significant difference when compared among the tension, pressure, and control sides loaded with 4oz and 7oz orthodontic force (P = 0.31) and when compared between tension/compression sides of the experimental and control teeth (P = 0.20) (Fig. 2).
Figure 2.
mRNA expression levels of RANKL and OPG. (A) Comparison of mRNA expression of RANKL in tension/compression sides of the experimental teeth loaded with 4oz of force (P < 0.0001) and control teeth. (B) Comparison of mRNA expression of OPG in tension/compression sides of the experimental teeth loaded with 4oz and 7oz of force (P = 0.31) and control teeth.
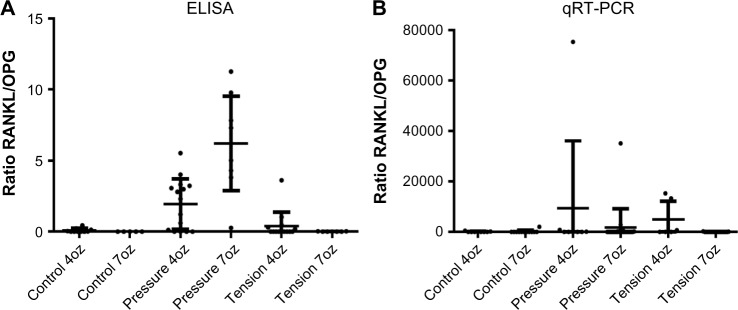
Changes in RANKL/OPG protein ratio in experimental and control groups showed statistically significant difference (P < 0.0001) but ratio of expression of RANKL/OPG did not show statistically significant differences (P > 0.05).
RANKL and OPG protein levels
As the OPG and RANKL proteins are regulators of extracellular matrix and bone metabolism, the presence of both proteins in pressure and tension sides of hPDL under orthodontic forces was evaluated.
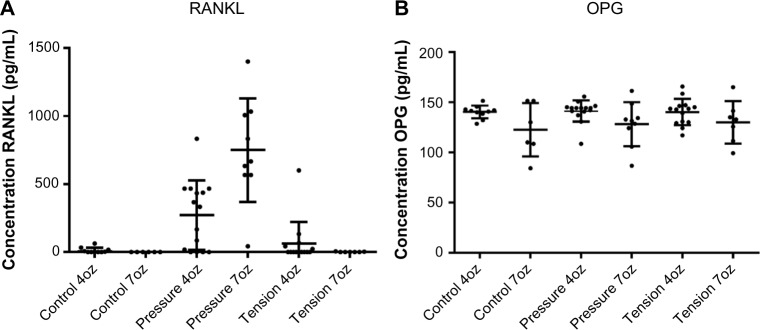
The ELISA test results showed that RANKL protein level was significantly greater in the pressure sides with 7oz (P < 0.0001) and with 4oz of force when compared with tension sides in the experimental group and with the control group. Therefore, RANKL protein levels in compression sides in the experimental teeth were higher than those in the tension side and control teeth in all groups (P < 0.0001). The protein concentrations of RANKL ranged from 0 to 1,160 µg/mL. In contrast, there were no statistically significant differences in OPG level on comparing force magnitudes (P = 0.07) and on comparing pressure and tension sides in the experimental teeth with those in the control teeth (P = 0.83). The protein concentrations of OPG ranged from 85 to 165 µg/mL (Fig. 3).
Figure 3.
RANKL and OPG protein levels. (A) Comparison of RANKL protein in tension/compression sides of the experimental teeth loaded with 4oz and 7oz of force (P < 0.0001) and control teeth. (B) Comparison of OPG protein in tension/compression sides of the experimental teeth loaded with 4oz and 7oz of force (P = 0.07) and control teeth.
Discussion
Although the concept of tension and pressure sides of a tooth generated by orthodontic forces simplifies the biological response in the PDL and bone, differential expression of bone modeling marker has been observed in vivo according to the pattern of tooth displacement.15 The tension side has been characterized by an increase in osteoblast number and pressure side by osteoclasts and bone resorption.
The present study showed an increased protein concentration of RANKL in compressed hPDL cells in a force magnitude-dependent manner (RANKL was increased in pressure side loaded with 7oz of force). This finding was reported previously in hPDL in vitro,11,16 in hPDL in vivo,17 in compressed cells of human crevicular fluid,18–21 in loaded miniscrew in humans,22 and in rat PDL.20–23 These findings support the role of RANKL in osteoclastogenesis mediated by PDL cells subjected to compressive forces. In contrast, the expression of RANKL showed statistically significant difference (P < 0.0001) in tension and pressure sides loaded with 4oz force compared with control group (unloaded teeth). These finding were reported previously in human17 and mice24 and were explained based on the expression of RANKL in osteoblasts, fibroblasts, and osteoclasts in resorption lacunae. Then, the number of osteoblasts present in the tension side loaded with light force (4oz) could be higher than those present in the tension side loaded with 7oz force showing a similar expression of RANKL in the side with more osteoblasts (tension, 4oz) and in the pressure side.
Although increased RANKL has been demonstrated in loaded hPDL and crevicular fluid in vivo, and in loaded fibroblasts and osteoblasts in vitro, the effect of force magnitude in the response of RANKL is not yet clear. Nettelhoff et al.25 compared the response of hPDL fibroblasts and osteoblasts after the application of compressive force at two different strengths in vitro (2 and 4 cN/mm2) over 12 hours and reported the highest mRNA expression of RANKL after 2 cN/mm2 of compressive force in hPDL fibroblasts and osteoblast, but mRNA expression of RANKL did not increase when the magnitude of compressive force was increase to 4 cN/mm2. Nishijima et al.13 retracted experimental canines using an elastomeric chain that delivered an initial force of 250 g for 1, 24, and 168 hours and applied compression force of different magnitudes (0.5, 1.0, 2.0, or 3.0 g/cm2) to culture hPDL. They reported the highest concentration of RANKL after 24 hours in vivo with 2 g/cm2 in vitro. In our study, we demonstrated an increase in RANKL when orthodontic force augmented but we did not know whether the expression of RANKL is also time dependent. Therefore, further studies are necessary to confirm whether the response of RANKL in compressed hPDL cells is time and force magnitude dependent.
In contrast, our study did not demonstrate an effect on OPG expression secondary to orthodontic force since the presence and expression of OPG were similar in all groups (experimental and control). Some studies demonstrated a significant decrease in OPG in compressed cells,26,27 and an increase in OPG in the tension sides in a time- and force magnitude-dependent manner.18,19,28 However, other studies did not find differences in the amount of OPG around the loaded and unloaded miniscrew implants after different times of application force21 and in OPG expression in human primary cementoblasts under compression forces in vitro compared with control group.29 The biological function of OPG in the OPG/RANK/RANKL system is the inhibition of osteoclast function and the acceleration of osteoclast apoptosis.30 Similar amount of OPG in tension, compression, and unloaded zones could suggest that the number of osteoclasts in pressure side should be maintained to get adequate bone remodeling after application of orthodontic forces.
The present study showed statistically significant difference in RANKL/OPG protein ratio. The increased ratio of RANKL/OPG in teeth loaded with orthodontic forces has been showed in several investigations.31 It seem that teeth in young people move faster than in older people because RANKL/OPG ratio measured by ELISAs is higher in young people.32 However, in our study the significance of ratio was a con sequence of the increase in the mean concentration of RANKL, no for decrease in OPG concentration. The increased expression of RANKL is regulated by inflammatory chemokines such as PGE2,33 MCP-1, MIP-1a, SDF-1 and RANTES,17 and TRAIL.34 Further, two major signaling pathways could regulate the osteoblasts: OPG/RANK/RANKL and Wnt/β-catenin35; and the activation of osteoclasts could occur through the ATP⁄P2XR7/IL-1β inflammation modulation pathway36,37 or through the OPG/RANK/RANKL network. Further studies comparing different age groups, magnitudes, and duration of orthodontic forces should be performed to clarify the exact role of RANKL and OPG in orthodontic movement.
Although the literature about the exact role of OPG/RANK/RANKL system revealed high heterogeneity in the study design, it is clear that orthodontic forces activate this system and release inflammatory bone resorptive mediators such as IL-1β and TNF-α. TNFα, IL-1β, and IL-6 can act directly on osteoclasts independent of RANKL.38 However, osteotropic hormones and cytokines regulate the concentration of OPG, RANKL, glucocorticoids, and inflammatory cytokines (IL-1β, IL-4, IL-6, IL-11, IL-17, and TNF-α) stimulating osteoclastogenesis by induction of RANKL expression.39
Using animal models, local OPG gene transfer has been used to inhibit relapse after orthodontic movement,12,40 and local RANKL gene transfer has been used to accelerate orthodontic tooth movement in rats.7 These findings demonstrate the therapeutic potential of RANKL and OPG protein and highlight the importance of the research in this topic for orthodontics and the importance to study the role of OPG and RANKL in orthodontic movement. The knowledge of the role of OPG/RANK/RANKL and ATP/P2XR7/IL-1β in orthodontic response could be use in the biomarkers for orthodontic treatment41 and will allow the design of medications or other interventions to affect the rate of tooth movement and the root resorption concurrent with orthodontics.
Conclusions
RANKL expression and concentration are increased in compression side as a consequence of application of orthodontic force. This finding suggests that RANKL contributes to bone modeling in response to the initial placement of orthodontic force. Further studies are necessary to clarify the role of RANKL and OPG in human orthodontic movement.
Figure 4.
Changes in RANKL/OPG protein (P < 0.0001) and mRNA (P > 0.05) ratios in experimental and control groups.
Footnotes
ACADEMIC EDITOR: James Willey, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 708 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by a Grant from Pontificia Universidad Javeriana. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: LO. Analyzed the data: DAG. Wrote the first draft of the manuscript: LO. Contributed to the writing of the manuscript: LWB. Agree with manuscript results and conclusions: DAG, LWB. Jointly developed the structure and arguments for the paper: LO, DAG, LWB. Made critical revisions and approved final version: LO. All authors reviewed and approved of the final manuscript.
RFERENCES
- 1.Tzannetou S, Efstratiadis S, Nicolay O, Grbic J, Lamster I. Comparison of levels of inflammatory mediators IL-1beta and betaG in gingival crevicular fluid from molars, premolars, and incisors during rapid palatal expansion. Am J Orthod Dentofac Orthoped. 2008;133(5):699–707. doi: 10.1016/j.ajodo.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Grzibovskis M, Pilmane M, Urtane I. Today’s understanding about bone aging. Stomatologija. 2010;12(4):99–104. [PubMed] [Google Scholar]
- 3.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–46. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12(2):113–9. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 5.Jurado S, Garcia-Giralt N, Diez-Perez A, Esbrit P, Yoskovitz G, Agueda L, et al. Effect of IL-1beta, PGE(2), and TGF-beta1 on the expression of OPG and RANKL in normal and osteoporotic primary human osteoblasts. J Cell Biochem. 2010;110(2):304–10. doi: 10.1002/jcb.22538. [DOI] [PubMed] [Google Scholar]
- 6.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanzaki H, Chiba M, Arai K, Takahashi I, Haruyama N, Nishimura M, et al. Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther. 2006;13(8):678–85. doi: 10.1038/sj.gt.3302707. [DOI] [PubMed] [Google Scholar]
- 8.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170(2):427–35. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Tsurukai T, Udagawa N, Matsuzaki K, Takahashi N, Suda T. Roles of macrophage-colony stimulating factor and osteoclast differentiation factor in osteoclastogenesis. J Bone Miner Metab. 2000;18(4):177–84. doi: 10.1007/s007740070018. [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara T, Yoshimine Y, Kiyoshima T, Kobayashi I, Matsuo K, Akamine A, et al. In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J Periodontal Res. 2004;39(1):42–9. doi: 10.1111/j.1600-0765.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 12.Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83(12):920–5. doi: 10.1177/154405910408301206. [DOI] [PubMed] [Google Scholar]
- 13.Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006;9(2):63–70. doi: 10.1111/j.1601-6343.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 14.Brooks PJ, Nilforoushan D, Manolson MF, Simmons CA, Gong SG. Molecular markers of early orthodontic tooth movement. The Angle orthodontist. 2009;79(6):1108–3. doi: 10.2319/121508-638R.1. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):469.e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Wang CL, Koyama Y, Mitsui N, Shionome C, Sanuki R, Suzuki N, Mayahara K, Shimizu N, Maeno M. Compressive force stimulates the gene expression of IL-17s and their receptors in MC3T3-E1 cells. Connect Tissue Res. 2010;51(5):359–69. doi: 10.3109/03008200903456942. [DOI] [PubMed] [Google Scholar]
- 17.Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci. 2007;115(5):355–62. doi: 10.1111/j.1600-0722.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006;9(2):63–70. doi: 10.1111/j.1601-6343.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki K, Takahashi T, Yamaguchi M, Kasai K. Effects of aging on RANKL and OPG levels in gingival crevicular fluid during orthodontic tooth movement. Orthod Craniofac Res. 2006;9(3):137–42. doi: 10.1111/j.1601-6343.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol. 2007;52(3):244–50. doi: 10.1016/j.archoralbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Grant M, Wilson J, Rock P, Chapple I. Induction of cytokines, MMP9, TIMPs, RANKL and OPG during orthodontic tooth movement. Eur J Orthod. 2013;35(5):644–51. doi: 10.1093/ejo/cjs057. [DOI] [PubMed] [Google Scholar]
- 22.Enhos S, Veli I, Cakmak O, Ucar FI, Alkan A, Uysal T. OPG and RANKL levels around miniscrew implants during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2013;144(2):203–9. doi: 10.1016/j.ajodo.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Shiotani A, Shibasaki Y, Sasaki T. Localization of receptor activator of NFkappaB ligand, RANKL, in periodontal tissues during experimental movement of rat molars. J Electron Microsc (Tokyo) 2001;50(4):365–9. doi: 10.1093/jmicro/50.4.365. [DOI] [PubMed] [Google Scholar]
- 24.Oshiro T, Shiotani A, Shibasaki Y, Sasaki T. Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin–deficient mice. Anat Rec. 2002;266:218–25. doi: 10.1002/ar.10061. [DOI] [PubMed] [Google Scholar]
- 25.Nettelhoff L, Grimm S, Jacobs C, et al. Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin Oral Investig. 2015 Aug 6; doi: 10.1007/s00784-015-1542-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Toygar HU, Kircelli BH, Bulut S, Sezgin N, Tasdelen B. Osteoprotegerin in gingival crevicular fluid under long-term continuous orthodontic forcé application. Angle Orthod. 2008;78(6):988–3. doi: 10.2319/100507-483.1. [DOI] [PubMed] [Google Scholar]
- 27.Barbieri G, Solano P, Alarcón JA, Vernal R, Rios-Lugo J, Sanz M, Martín C. Biochemical markers of bone metabolism in gingival crevicular fluid during early orthodontic tooth movement. Angle Orthod. 2013;83(1):63–9. doi: 10.2319/022812-168.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kook SH, Jang YS, Lee JC. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-alpha-mediated activation of CD4+ T cells. J Cell Biochem. 2011;112(10):2891–901. doi: 10.1002/jcb.23205. [DOI] [PubMed] [Google Scholar]
- 29.Diercke K, Kohl A, Lux CJ, Erber R. IL-1β and compressive forces lead to a significant induction of RANKL-expression in primary human cementoblasts. J Orofac Orthop. 2012;73(5):397–412. doi: 10.1007/s00056-012-0095-y. [DOI] [PubMed] [Google Scholar]
- 30.Tyrovola JB. The “Mechanostat Theory” of Frost and the OPG/RANKL/RANK System. J Cell Biochem. 2015 doi: 10.1002/jcb.25265. [DOI] [PubMed] [Google Scholar]
- 31.Rody WJ, Wijegunasinghe M, Wiltshire WA, Dufault B. Differences in the gingival crevicular fluid composition between adults and adolescents undergoing orthodontic treatment. Angle Orthod. 2014;84:120–26. doi: 10.2319/012813-85.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor P, Kharbanda OP, Monga N, Miglani R, Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod. 2014;15:65. doi: 10.1186/s40510-014-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayahara K, Yamaguchi A, Takenouchi H, Kariya T, Taguchi H, Shimizu N. Osteoblasts stimulate osteoclastogenesis via RANKL expression more strongly than periodontal ligament cells do in response to PGE(2) Arch Oral Biol. 2012;57(10):1377–84. doi: 10.1016/j.archoralbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Zauli G, Rimondi E, Stea S, et al. TRAIL inhibits osteoclastic differentiation by counteracting RANKL-dependent p27Kip1 accumulation in pre-osteoclast precursors. J Cell Physiol. 2008;214(1):117–25. doi: 10.1002/jcp.21165. [DOI] [PubMed] [Google Scholar]
- 35.Boyce BF, Xing L, Chen D. Osteoprotegerin, the bone protector, is a surprising target for beta-catenin signaling. Cell Metab. 2005;2(6):344–5. doi: 10.1016/j.cmet.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kradin RL, Sakamoto H, Jain F, Zhao LH, Hymowitz G, Preffer F. IL-10 inhibits inflammation but does not affect fibrosis in the pulmonary response to bleomycin. Exp Mol Pathol. 2004;76(3):205–11. doi: 10.1016/j.yexmp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Hartsfield JK., Jr Pathways in external apical root resorption associated with orthodontia. Orthod Craniofac Res. 2009;12(3):236–42. doi: 10.1111/j.1601-6343.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32(1):1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 39.Stejskal D, Bartek J, Pastorkova R, Ruzicka V, Oral I, Horalik D. Osteoprotegerin, RANK, RANKL. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145(2):61–4. doi: 10.5507/bp.2001.013. [DOI] [PubMed] [Google Scholar]
- 40.Zhao N, Lin J, Kanzaki H, Ni J, Chen Z, Liang W, Liu Y. Local osteoprotegerin gene transfer inhibits relapse of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2012;141(1):30–40. doi: 10.1016/j.ajodo.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 41.Flórez-Moreno GA, Isaza-Guzmán DM, Tobón-Arroyave SI. Time-related changes in salivary levels of the osteotropic factors sRANKL and OPG through orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2013;143(1):92–100. doi: 10.1016/j.ajodo.2012.08.026. [DOI] [PubMed] [Google Scholar]