Abstract
Background
Lacunar infarctions are caused by small vessel disease (SVD) and branch atheromatous disease (BAD). Lacunar infarction may be classified as proximal vessel lacunar infarction (BAD) or distal vessel lacunar infarction (SVD) according to its location within the middle cerebral artery (MCA) territory in patients with normal MCA. Studies found that the lenticulostriate arteries may exist different ways and that the size of lacunar infarction may be dependent on the branching order. We investigated whether lacunar infarction size can differentiate between SVD and BAD in patients with normal MCA.
Material/Methods
We retrospectively studied 312 patients with lacunar infarction who had normal MCA on MR angiography. We found the normal flow void of the MCA on MR T2-weighted images, and the same layer on DWI was considered the level 0. The median of lowest layer of infarction lesions and the mean of lesion size were considered the cutoff point. We divided lacunar infarction into 2 groups according to cutoff point of lesion location and size. Data compared between the 2 groups included clinical information, radiography, and National Institutes of Health Stroke Scale score.
Results
Of all the 312 patients, the median of lowest layer of infarction lesions was the 3rd level. Compared to patients with BAD, according to infarct location, patients with SVD were older, more often had a history of hypertension and smoking, and had more severe leukoaraiosis and smaller infarct lesions. The mean length of lesions was 11.1 mm on DWI images. Patients with SVD, according to infarct size, had lower NIHSS scores at admission. The mean lesion height was 12.26 mm on FLAIR images. Patients with SVD were more often male, had higher prevalence of smoking, and had more severe leukoaraiosis and lower NIHSS scores at admission. The lacunar infarction diameter on DWI and FLAIR images was negatively correlated with the level of lowest layer of infarction lesions.
Conclusions
Our data suggest that infarct lesion size may be used as a method to distinguish SVD and BAD in lacunar infarction patients with normal MCA.
MeSH Keywords: Cerebral Small Vessel Diseases; Leukoaraiosis; Stroke, Lacunar
Background
Lacunar infarction is usually caused by small vessel disease (SVD) or branch atheromatous disease (BAD). In 1989, Caplan [1] proposed ‘intracranial branch atheromatous disease’ as a new pathogenesis of lacunar infarction, caused by a localized atheromatous lesion at the mouth of the perforating branches of stem arteries, and pathophysiologically distinguished from traditional SVD caused by lipohyalinosis primarily affecting the distal part of perforators.
However, branch vessels cannot be visualized by conventional imaging technologies. Caplan considered that branch disease can be inferred clinically if neuroimaging shows the infarcts abut on the basal surface. Clinically, different scholars [2–5] used different methods to define BAD and reached different conclusions. Yamamoto [2] defined BAD of the middle cerebral artery (MCA) as infarcts more than 10 mm in diameter on axial slice and visible on 3 or more axial slices at a slice thickness of 7 mm. They found that there were no significant differences in prevalence of hypertension, diabetes mellitus (DM), hyperlipidemia, history of coronary artery disease (CAD), or stroke. However, Nah [3] considered that involvement of the lowest portion of the basal ganglia was BAD and found that patients with BAD had a significantly lower prevalence of hypertension, leukoaraiosis, and microbleeds, and a higher prevalence of diabetes, compared with SVD.
Neuroanatomical studies [6,7] found that the lenticulostriate arteries may arise as an individual vessel only or by common stems, or in both ways. A recent pathological study provided a microangiographic template of the basal ganglia. This template displays first-order (proximal) to third-order (distal) branching of perforator arteries of the basal ganglia [8]. An MRI study [9] found that the size of lacunar infarction may be dependent on the branching order of the arteries involved, with first-order branches associated with largest and third-order branches associated with smallest infarct dimensions. Compared with SVD, previous research [3,10–15] showed that the infarction sizes in BAD were larger.
We hypothesized that infarct location and size in patients with lacunar infarction may suggest different stroke mechanisms (SVD or BAD). We proposed, according to the location and size of lesions, a way to differentiate the 2 different types of lacunar infarction (SVD and BAD) in patients with normal MCA (Figure 1).
Figure 1.
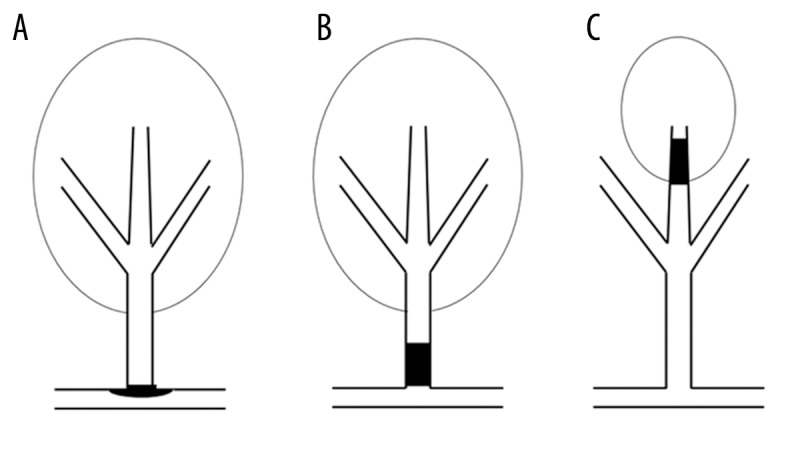
Presumed mechanism of lacunar infarction. (A) Proximal vessel lacunar infarction caused by plaque within parent artery blocking the branch orifice. (B) Proximal vessel lacunar infarction caused by microatheroma in the orifice of the branch. (C) Distal vessel lacunar infarction caused by fibrinoid necrosis or lipohyalinosis of the distal perforating artery.
Material and Methods
We retrospectively studied data from the Neurology Department of Beijing Chaoyang Hospital. Consecutive patients who were admitted between January 2011 and December 2013 were enrolled if they had a lacunar infarction in the perforator territory of the MCA on diffusion-weighted imaging (DWI).
Patients included in the study met the following criteria: (1) admitted within 1 week after stroke onset; (2) showed perforator territory infarction in the MCA territory [16] on an DWI, and (3) had MR angiography (MRA) that was normal. Patients with a definite cardioembolic source (e.g., atrial fibrillation, recent myocardial infarction, dilated cardiomyopathy, valvular heart disease, or infectious endocarditis) or ipsilateral extracranial carotid stenosis were excluded. The Institutional Review Board of Beijing Chaoyang Hospital affiliated to Capital Medical University approved the study and all subjects provided their written informed consent to participate in this study.
All patients underwent a blood test (including aspartate aminotransferase, alanine aminotransferase, urea nitrogen, creatinine, creatine kinase, glucose, cholesterol, and triglycerides) and cardiac evaluation (including electrocardiogram and heart ultrasound).
Demographic features and risk factors were recorded, including hypertension (defined as receiving medication for hypertension or blood pressure >140/90 mmHg on repeated measurements), diabetes mellitus (defined as receiving medication for diabetes mellitus or diagnosed at discharge), hyperlipidemia (defined as receiving cholesterol reducing agents or low-density lipoprotein cholesterol ≥2.6 mmol/L at the time of admission), current cigarette smoking, history of stroke, and history of coronary heart disease. National Institutes of Health Stroke Scale (NIHSS) score was measured at the time of admission and discharge.
A brain MRI scan was performed within 1 week of onset, including DWI, fluid-attenuated inversion recovery (coronal), and MRA. Brain MRI was performed with a 1.5T scanner (Signa Horizon LX, GE, American; HDx 14.0 TwinSpeed, GE Healthcare, Waukesha, WI; GE Discovery MR750; General Electric Healthcare, Waukesha, WI) with a 32-channel head coil. Imaging sequences obtained included: axial T2-weighted (repetition time (TR), 4500 ms; echo time (TE), 84 ms); T1-weighted imaging (TR, 1200 ms; TE, 11 ms); fluid-attenuated inversion recovery sequences (TR, 7000 ms; TE, 94 ms); and diffusion-weighted imaging (TR, 3000 ms; TE, 75 ms). All of the above sequences had 5-mm slice thickness, 1.5-mm interslice gap, and a 256×128 matrix. The parameters of the 3DTOF MRA were: TR/TE=22/3 ms, 15° lip angle, field of view (FOV) 220×220 mm, 1-mm thickness, no gap between slices, and 512×512 matrix.
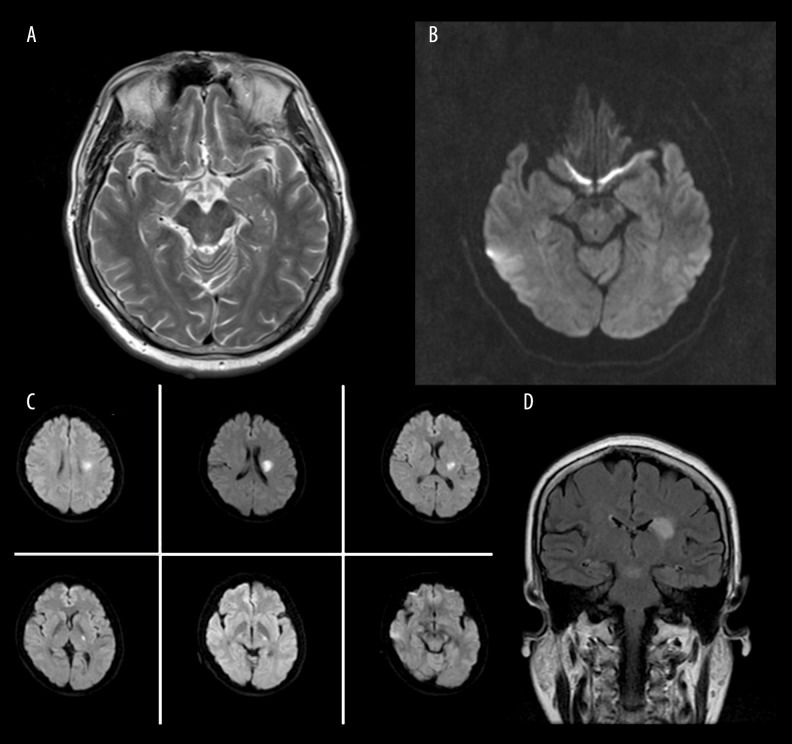
We found the normal flow void of the MCA on MR T2-weighted images. The same layer on DWI was considered the level 0. The next layer of DWI was considered the 1st level, and the rest were done in the same manner (Figure 2A–2D). We recorded the lowest layer of infarction lesions. Lesion locations were analyzed based on the axial DWIs, which ranged from the 1st level at the proximal origin of the perforators of the MCA. The median of lowest layer of infarction lesions was considered the cutoff point. We defined BAD of the middle cerebral artery (MCA) as the lowest layer of infarction lesions lower than the median, and SVD as the lowest layer of infarction lesions higher than the median.
Figure 2.
(A) Normal flow void of the MCA on MR T2-weighted images. (B) The same layer on DWI. (C) A patient with lacunar infarction on DWI. (D) The same patient with lacunar infarction on FLAIR.
The diameter in the maximally involved infarct level (axial on DWI and coronal on FLAIR) was evaluated as the parameter for the size of the infarct. The mean of lesion size was considered the cutoff point. We defined BAD of the MCA as infarcts larger than the mean size in diameter and SVD as infarcts smaller than the mean size in diameter.
WMHs were assessed by a scale of periventricular hyperintensity (PVH) and deep white-matter hyperintensity (DWMH) according to Fazekas [17]. Two investigators (neurologists) independently reviewed the MRI and MRA images. In cases of discrepancy, the third investigator (a neuroradiologist) made the final decision.
Statistical analysis
Differences in risk factors, except for age, were tested by a χ2 test for categorical variables and the Student’s t-test for continuous variables. Parameters of SVD (e.g., hypertension and WMHs) and BAD (e.g., DM, artery stenosis) among groups categorized by lesion locations or lesion size were analyzed by χ2 test. Correlations between these parameters of lesion locations or lesion size were analyzed by Pearson or Spearman correlation. Statistical analyses were performed using SPSS 20.0. P values of <0.05 were considered statistically significant.
Results
General patient characteristics
During the study period, 669 patients had lacunar infarction. Of these, we excluded 130 patients with lacunar infarction located in the basilar artery or vertebral artery territory, 116 patients with thalamus infarction, 35 patients who did not undergo MRA, 26 patients with significant stenosis in the responsible extracranial artery, 18 patients with cardiac embolic sources, and 32 patients with stenosis of MCA. Thus, our study consisted of 312 patients (215 men and 97 women) with a mean age of 62.94±11.86 years (range, 34 to 91 years).
Risk factors included hypertension in 182 patients (58%), diabetes in 95 (30%), hyperlipidemia in 50 (16%), and cigarette smoking in 160 (51%). There were 88 patients (28%) who had histories of stroke and 38 (12%) had histories of coronary heart disease. The median NIHSS score was 4 (interquartile range, 2 to 5).
The numbers of patients with lowest layer of infarction lesions from the 1st level to the 6th level were 32, 70, 81, 76, 45, and 8, respectively. Infarct dimensions for our study was as follows: anterior-posterior length (on DWI) 11.9±4.9 mm, horizontal width (on DWI) 6.8±3.2 mm, and height (on FLAIR) 12.7±6.3 mm.
Characteristics of lacunar infarction according to lesion locations
Of the 312 patients, the median lowest layer of infarction lesions was the 3rd level. Patients with the level of lowest layer of infarction lesions ≤3 were considered as BAD and other patients were SVD; 183 had BAD and 129 had SVD. Table 1 summarizes the descriptive statistics of the 2 groups. Compared to patients with BAD, patients with SVD were older, more often had a history of hypertension (P=0.027) and smoking (P=0.006), and had more severe leukoaraiosis (P<0.001) and smaller infarct lesions in FLAIR (P<0.001).
Table 1.
Characteristics of lacunar infarction according to lesion location.
| Clinical features | Lesion locations (lowest layer) | P | |
|---|---|---|---|
| ≤ The 3rd level (183) BAD | > The 3rd level (129) SVD | ||
| Age, years, mean ±SD | 61.7±12.2 | 64.7±11.2 | 0.031 |
| Male, no. (%) | 131 (71.6%) | 84 (65.1%) | 0.264 |
| Hypertension, no. (%) | 97 (53%) | 85 (65.9%) | 0.027 |
| DM, no. (%) | 51 (27.9%) | 44 (34.1%) | 0.262 |
| Hyperlipidemia, no. (%) | 25 (13.7%) | 25 (19.4%) | 0.21 |
| History of CAD, no. (%) | 19 (10.4%) | 16 (12.4%) | 0.589 |
| History of stroke, no. (%) | 49 (26.8%) | 39 (30.2%) | 0.525 |
| Smoking, no. (%) | 106 (57.9%) | 54 (41.9%) | 0.006 |
| NIHSS, median (IQR) | 4 (2–5) | 3 (1–5) | 0.201 |
| Length (DWI), mean ±SD | 12.5±5.0 | 11.1±4.6 | 0.616 |
| Height (FLAIR), mean ±SD | 14.1±6.7 | 10.7±5.3 | 0.000 |
| PVH (Fazekas) ≥2, no. (%) | 90 (49.2%) | 89 (69%) | 0.001 |
| DWMH (Fazekas) ≥2, no. (%) | 51 (27.9%) | 70 (54.3%) | 0.000 |
Characteristics of lacunar infarction according to lesion length on DWI
In the 312 patients, the mean of lesion length was 11.1 mm. According to our hypothesis that patients with smaller lesion size were SVD and patients with bigger lesions were BAD, 156 had BAD and 156 had SVD. Table 2 summarizes the descriptive statistics of the 2 groups. Patients with SVD had lower NIHSS scores at admission (P=0.027). However, there were no significant differences in prevalence of hypertension, DM, hyperlipidemia, and history of CAD or stroke.
Table 2.
Characteristics of lacunar infarction according to lesion length on DWI.
| Clinical features | Lesion size: length | P | |
|---|---|---|---|
| ≤11.1 mm (156) SVD | >11.1 mm (156) BAD | ||
| Age, years, mean ±SD | 63.5±11.1 | 62.3±12.6 | 0.376 |
| Male, no. (%) | 113 (72.4%) | 102 (65.4%) | 0.221 |
| Hypertension, no. (%) | 91 (58.3) | 91 (58.3) | 1 |
| DM, no. (%) | 53 (34%) | 42 (26.9%) | 0.219 |
| Hyperlipidemia, no. (%) | 25 (16%) | 25 (16%) | 1 |
| History of CAD, no. (%) | 15 (9.6%) | 20 (12.8%) | 0.473 |
| History of stroke, no. (%) | 41 (26.3%) | 47 (30%) | 0.529 |
| Smoking, no. (%) | 83 (53.2%) | 77 (49.4%) | 0.571 |
| NIHSS, median (IQR) | 2 (1–4) | 4 (2–6) | 0.001 |
| PVH (Fazekas) ≥2, no. (%) | 93 (59.6%) | 86 (55.1%) | 0.492 |
| DWMH (Fazekas) ≥2, no. (%) | 68 (43.6%) | 53 (34%) | 0.104 |
Characteristics of lacunar infarction according to lesion height on FLAIR
Of the 312 patients, the mean of lesion size in height was 12.26 mm. According to our hypothesis, 156 had BAD and 156 had SVD. There were no significant differences in terms of hypertension, DM, hyperlipidemia, or history of CAD and stroke between the 2 groups. However, patients with SVD were more likely to be male, have higher prevalence of smoking, more severe leukoaraiosis (DWMH ≥2, P=0.02), and lower NIHSS score at admission (P=0.015) (Table 3).
Table 3.
Characteristics of lacunar infarction according to lesion height on FLAIR.
| Clinical features | Lesion size: height (FLAIR) | P | |
|---|---|---|---|
| ≤12.26 mm (156) SVD | >12.26 mm (156) BAD | ||
| Age, years, mean ±SD | 63.6±11 | 62.3±12.65 | 0.355 |
| Male, no. (%) | 119 (76.3%) | 96 (61.5%) | 0.007 |
| Hypertension, no. (%) | 90 (57.7%) | 92 (59%) | 0.909 |
| DM, no. (%) | 55 (35.3%) | 40 (25.6%) | 0.085 |
| Hyperlipidemia, no. (%) | 31 (19.9%) | 19 (12.2%) | 0.089 |
| History of CAD, no. (%) | 18 (11.5%) | 17 (10.9%) | 1 |
| History of stroke, no. (%) | 45 (28.8%) | 43 (27.6%) | 0.9 |
| Smoking, no. (%) | 93 (59.6%) | 67 (42.9%) | 0.005 |
| NIHSS, median (IQR) | 2 (1–4) | 4 (2–6) | 0.015 |
| PVH (Fazekas) ≥2, no.(%) | 97 (62.2%) | 82 (52.6%) | 0.109 |
| DWMH (Fazekas) ≥2, no.(%) | 71 (45.5%) | 50 (32.1%) | 0.02 |
Relations of lesion locations and lesion size
The length of the maximally involved infarct on DWI was negatively correlated with the level of lowest layer of infarction lesions (r=−0.168, p=0.003; Figure 3). The height of the maximally involved infarct on FLAIR also was negatively correlated with the level of the lowest layer of infarction lesions (r=−0.299, p<0.001; Figure 4).
Figure 3.
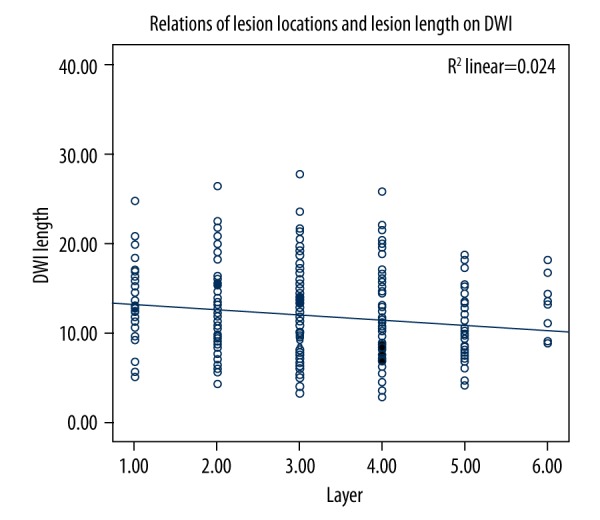
Relationship between lesion locations and lesion length on DWI.
Figure 4.
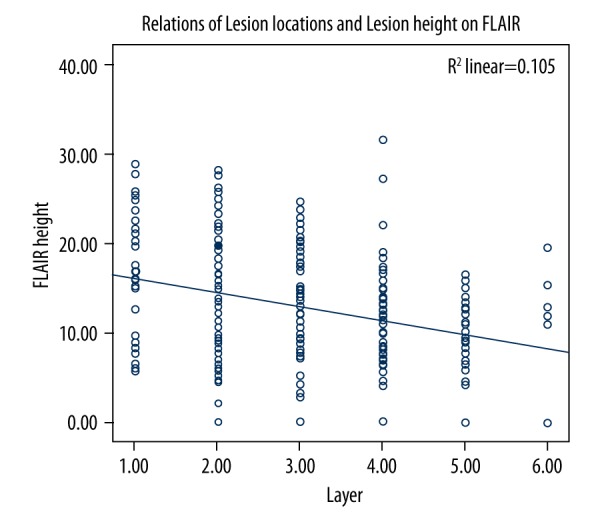
Relationship between lesion locations and lesion height on FLAIR.
Discussion
We classified patients with lacunar infarction into BAD and SVD groups according to lesion locations, and found that patients with SVD had higher prevalence of history of hypertension, significantly more severe leukoaraiosis, and larger size in FLAIR image compared to patients with BAD. These results are consistent with previous studies [3,10,12]. SVD and BAD are difficult to directly visualize in vivo. Therefore, some markers of radiological phenotypes were selected as surrogates for SVD, such as small size, deep brain infarcts, cerebral white matter lesions, deep brain hemorrhages, and cerebral microbleeds [18]. However, diabetes, cerebral atherosclerosis, and coronary heart disease may represent BAD. Our results support that distal vessel lacunar infarction is more closely related to SVD compared with proximal vessel lacunar infarction.
In 1989, Caplan [1] proposed ‘intracranial branch atheromatous disease’, based on 3 autopsied cases with pontine infarcts. However, only the third patient had pathologic evidence of a perforator artery. One artery of the patient was found to be a microdissection that created a crevice in a plaque located at the orifice of a branch. The concept of basilar artery branch disease was then extended to the idea of intracranial BAD, including not only basilar artery branch disease, but also other specific arteries of larger caliber, such as lenticulostriate arteries.
Because lacunar infarcts and white matter lesions are easily detected by neuroimaging but small branch vessels are not, some alternative indicators of parenchyma lesions were used to describe the underlying small branch vessel alterations. In addition, various scholars used different definitions for perforator artery disease and BAD. Yamamoto et al. [2] defined BAD of the lenticulostriate arteries as infarcts more than 10 mm in diameter on axial slice and visible on 3 or more axial slices at a slice thickness of 7 mm. Nakase et al. [4] defined as BAD intracerebral lesions with a diameter of ≥15 mm visible on more than 3 slices, or lesions extending to the surface of the pontine base. Jeong. [5] defined BAD when lesions were visible in 4 axial MRI cuts at a slice thickness of 5 mm in the lenticulostriate territory. In addition, Nah et al. [3] considered that involvement of the lowest portion of the basal ganglia was an extension to the basal surface (BAD). This hypothesis seems to be optimal, and it has caused a wide range of discussion in China. However, anatomical study of the perforator arteries suggested that the clinical hypothesis may not be exactly correct.
Neuroanatomical studies [6,7] found that the lenticulostriate arteries may arise as an individual vessel only or by common stems, or in both ways. Feekes et al. [8] investigated microvascular territories of perforator arteries (including the lenticulostriate arteries), and found that branching patterns of first-, second-, and third-order vessels leading to circumscribed terminal vascular beds could structurally account for “lacunar” infarcts. Phan et al. [9] found that lacunar infarcts with first-order branch involvement were associated with largest infarct size and that smallest infarct size was associated with third-order branch involvement. Compared with hypertensive arteriolar sclerosis, previous studies [3,10–14] have shown that BAD infarctions were larger. Infarct size is correlated with worse short-term functional outcome [3,10–15].
We hypothesized that involvement of the lowest portion of the basal ganglia and larger infarct size may denote an extension to the basal surface. For the first time, we classified patients with lacunar infarction into either BAD or SVD groups according to lesion size and found that patients with SVD had significantly more severe leukoaraiosis (DWMH) compared to patients with BAD. We also found that the lesions were significantly larger (both in length and height) in BAD than in SVD patients. However, there were no significant differences in prevalence of hypertension, DM, hyperlipidemia, and history of CAD or stroke. These results are consistent with a previous study [2], but not consistent with other studies [3,10,12].
These differences seem to be associated with the different definitions of SVD and BAD. Previous studies of lacunar infarction included lesions in the territory of the MCA and basilar artery, which may have affected the results. Therefore, we chose just the MCA for this research in order to exclude this effect.
However, our results showed that the diameter in the maximally involved infarct on DWI and FLAIR was negatively correlated with the level of the lowest layer of infarction lesions. These results indicate that larger infarcts tend to be closer to the vessel trunk. The size of the infarcts may also be used as a method to distinguish between SVD and BAD.
It is important to differentiate between the 2 pathogeneses of lacunar infarction – small vessel occlusion vs. branch atheromatous disease – because prognoses and treatment strategies differ between SVD and BAD. Patients with BAD have worse prognoses compared with SVD [2,5,14,19,20].
A deep subcortical infarct in the perforating arterial territory <15 mm in diameter has been called a ‘lacunar infarct’ due to small vessel occlusion. The criterion of 15 mm originated from previous autopsy results. In the era of MRI, some studies [2,3,21] attempted to use a criterion of 20 mm diameter as the cutoff point of lacunar infarct. However, some research considered that reliance on an axial dimension of 15–20 mm may not be the best approach in classifying lacunar infarcts. A recent pathology study showed first-order (proximal) to third-order (distal) branching of perforator arteries of the basal ganglia [6]. An MRI study [7] found that lacunar infarction size may be dependent on the branching order of arteries involved, with first-order branches associated with largest infarct dimensions and SSSI sizes of up to 30 mm. Therefore, in our study we did not set the cutoff value.
From a pathological point of view, SVD (also referred to as hypertensive SVD) is mainly characterized by loss of smooth muscle cells from the tunica media, deposits of fibrohyaline material, narrowing of the lumen, and thickening of the vessel wall. This form of the disease is a common and systemic type that also affects the kidneys and retinas and is strongly associated with ageing, diabetes, and, in particular, hypertension [22]. However, pathological documentation of BAD is difficult to obtain. Based on a pathology study of 3 patients, Caplan reported that the orifices of the perforator artery could be blocked by atheroma in the trunk, atheroma could originate in the trunk and extend into the branch (so-called junctional atheromatous plaques), or microatheromas could arise at the origin of the branch itself.
In clinical settings, it is possible to easily distinguish microatheromas if the MRA is normal [3,11]. However, this method may be flawed. Tatsumi presented a case with MR images compatible with BAD, but the histopathological findings were those of a large artery atherothrombotic infarct23. Recent research [13,24] showed that high-resolution MRI (HR MRI) can identify MCA plaques in patients with SSSI, even if the patient’s MRA findings are normal. It was hard to obtain pathological documentation of SVD and BAD, but it is possible to image intracerebral branch atheromatous disease using HR MRI. Using HR MRI will lead to a better understanding of the mechanisms involved in stroke.
Our study had some differences from previous studies. First, we classified patients with lacunar infarction into either proximal vessel lacunar infarction (BAD) or distal vessel lacunar infarction (SVD) groups according to lesion size in addition to lesion location. Second, previous studies on lacunar infarction included lesions in the territory of the MCA and basilar artery, but our research focused on the territory of the MCA. Third, infarct size of patients in our study was not confined to 15 mm or 20 mm. Our study has certain limitations. First, we could not rule out whether lacunar infarction were due to cardiac embolism or artery-to-artery embolism. Second, ours was a retrospective hospital-based study, and selection bias was inevitable. Third, the MCA was not examined by high-resolution MRI, so we cannot exclude the presence of mild atherosclerotic plaque that did not cause significant luminal stenosis.
Conclusions
According to the MRI findings at acute phase, if the lacunar infarction size is larger or the location of SSSI with normal MCA is closer to the orifice of the perforating artery, the pathogenesis of the ischemic lesion will probably be diagnosed as BAD.
Footnotes
Source of support: Supported by the National Natural Science Foundation of China (Grant No. 81271309)
References
- 1.Caplan LR. Intracranial branch atheromatous disease: A neglected, understudied, and underused concept. Neurology. 1989;39:1246–50. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Ohara T, Hamanaka M, et al. Characteristics of intracranial branch atheromatous disease and its association with progressive motor deficits. J Neurol Sci. 2011;304:78–82. doi: 10.1016/j.jns.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease: Analysis of indicators for small vessel disease and atherosclerosis. Stroke. 2010;41:2822–27. doi: 10.1161/STROKEAHA.110.599464. [DOI] [PubMed] [Google Scholar]
- 4.Nakase T, Yoshioka S, Sasaki M, Suzuki A. Clinical evaluation of lacunar infarction and branch atheromatous disease. J Stroke Cerebrovasc Dis. 2013;22:406–12. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Jeong HG, Kim BJ, Yang MH, et al. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke. 2015;46:687–91. doi: 10.1161/STROKEAHA.114.007466. [DOI] [PubMed] [Google Scholar]
- 6.Djulejic V, Marinkovic S, Malikovic A, et al. Morphometric analysis, region of supply and microanatomy of the lenticulostriate arteries and their clinical significance. J Clin Neurosci. 2012;19:1416–21. doi: 10.1016/j.jocn.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Marinkovic SV, Milisavljevic MM, Kovacevic MS, Stevic ZD. Perforating branches of the middle cerebral artery. Microanatomy and clinical significance of their intracerebral segments. Stroke. 1985;16:1022–29. doi: 10.1161/01.str.16.6.1022. [DOI] [PubMed] [Google Scholar]
- 8.Feekes JA, Hsu SW, Chaloupka JC, Cassell MD. Tertiary microvascular territories define lacunar infarcts in the basal ganglia. Ann Neurol. 2005;58:18–30. doi: 10.1002/ana.20505. [DOI] [PubMed] [Google Scholar]
- 9.Phan TG, van der Voort S, Beare R, et al. Dimensions of subcortical infarcts associated with first- to third-order branches of the basal ganglia arteries. Cerebrovasc Dis. 2013;35:262–67. doi: 10.1159/000348310. [DOI] [PubMed] [Google Scholar]
- 10.Zhang CQ, Wang YL, Zhao XQ, et al. Distal single subcortical infarction had a better clinical outcome compared with proximal single subcortical infarction. Stroke. 2014;45:2613–19. doi: 10.1161/STROKEAHA.114.005634. [DOI] [PubMed] [Google Scholar]
- 11.Ryoo S, Park JH, Kim SJ, et al. Branch occlusive disease clinical and magnetic resonance angiography findings. Neurology. 2012;78:888–96. doi: 10.1212/WNL.0b013e31824c4699. [DOI] [PubMed] [Google Scholar]
- 12.Cho HJ, Roh HG, Moon WJ, Kim HY. Perforator territory infarction in the lenticulostriate arterial territory: Mechanisms and lesion patterns based on the axial location. Eur Neurol. 2010;63:107–15. doi: 10.1159/000276401. [DOI] [PubMed] [Google Scholar]
- 13.Yoon Y, Lee DH, Kang DW, et al. Single subcortical infarction and atherosclerotic plaques in the middle cerebral artery high-resolution magnetic resonance imaging findings. Stroke. 2013;44:2462–67. doi: 10.1161/STROKEAHA.113.001467. [DOI] [PubMed] [Google Scholar]
- 14.Yang XM, Pu YH, Liu LP, et al. The infarct location predicts the outcome of single small subcortical infarction in the territory of the middle cerebral artery. J Stroke Cerebrovasc Dis. 2014;23:1676–81. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Asdaghi N, Pearce LA, Nakajima M, et al. Clinical correlates of infarct shape and volume in lacunar strokes the secondary prevention of small subcortical strokes trial. Stroke. 2014;45:2952–58. doi: 10.1161/STROKEAHA.114.005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: Cerebral hemispheres. Neurology. 1998;50:1699–708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 17.Fazekas F, Chawluk JB, Alavi A, et al. Mr signal abnormalities at 1.5 t in alzheimer’s dementia and normal aging. Am J Roentgenol. 1987;149:351–56. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 18.Moran C, Phan TG, Srikanth VK. Cerebral small vessel disease: A review of clinical, radiological, and histopathological phenotypes. Int J Stroke. 2012;7:36–46. doi: 10.1111/j.1747-4949.2011.00725.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Ohara T, Hamanaka M, et al. Predictive factors for progressive motor deficits in penetrating artery infarctions in two different arterial territories. J Neurol Sci. 2010;288:170–74. doi: 10.1016/j.jns.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 20.Men XJ, Li JJ, Zhang BJ, et al. Homocysteine and c-reactive protein associated with progression and prognosis of intracranial branch atheromatous disease. PLoS One. 2013;8(9):e73030. doi: 10.1371/journal.pone.0073030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longstreth WT, Jr, Bernick C, Manolio TA, et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: The cardiovascular health study. Arch Neurol. 1998;55:1217–25. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 22.Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi S, Yamamoto T. An autopsied case of an apparent pontine branch atheromatous disease. Eur Neurol. 2010;63:184–85. doi: 10.1159/000290248. [DOI] [PubMed] [Google Scholar]
- 24.Chung JW, Kim BJ, Sohn CH, et al. Branch atheromatous plaque: A major cause of lacunar infarction (high-resolution mri study) Cerebrovasc Dis Extra. 2012;2:36–44. doi: 10.1159/000341399. [DOI] [PMC free article] [PubMed] [Google Scholar]