Abstract
Purpose of review
Compared with the conventional forms of partial support, neurally adjusted ventilatory assist was repeatedly shown to improve patient–ventilator synchrony and reduce the risk of overassistance, while guaranteeing adequate inspiratory effort and gas exchange. A few animal studies also suggested the potential of neurally adjusted ventilatory assist in averting the risk of ventilator-induced lung injury. Recent work adds new information on the physiological effects of neurally adjusted ventilatory assist.
Recent findings
Compared with pressure support, neurally adjusted ventilatory assist has been shown to improve patient–ventilator interaction and synchrony in patients with the most challenging respiratory system mechanics, such as very low compliance consequent to severe acute respiratory distress syndrome and high resistance and air trapping due to chronic airflow obstruction; enhance redistribution of the ventilation in the dependent lung regions; avert the risk of patient–ventilator asynchrony due to sedation; avoid central apneas; limit the risk of high (injurious) tidal volumes in patients with acute respiratory distress syndrome of varied severity; and improve patient–ventilator interaction and synchrony during noninvasive ventilation, irrespective of the interface utilized.
Summary
Several studies nowadays prove the physiological benefits of neurally adjusted ventilatory assist, as opposed to the conventional modes of partial support. Whether these advantages translate into improvement of clinical outcomes remains to be determined.
Keywords: acute respiratory failure, mechanical ventilation, neurally adjusted ventilatory assist, noninvasive ventilation, patient–ventilator interaction
INTRODUCTION
First described in its general principles 15 years ago [1], neurally adjusted ventilatory assist (NAVA) is a mode of partial ventilatory assistance that has become commercially available in the last few years. With proportional assist ventilation (PAV), NAVA is the only mode of ventilation delivering assistance in proportion to a patient's demand [2]. Although with both PAV and NAVA the assistance remains under the patient's control, PAV utilizes ‘conventional’ pneumatic signals, such as flow and volume, whereas NAVA has the unique feature to control ventilator functioning through the electrical activity of the diaphragm (EAdi). In NAVA, in fact, the mechanical support is on-triggered and off-triggered by the EAdi, as assessed by transesophageal electromyography, and is proportional to EAdi throughout each inspiration [1]. The EAdi signal is obtained through a dedicated feeding tube, mounting a distal array of multiple electrodes, and processed to provide the highest possible quality of signal [1]. EAdi, expressed in microvolts, is multiplied by a user-controlled gain factor, the NAVA level (NAVAL), whose unit is cmH2O/μV. The airway pressure applied by the ventilator depends on the magnitude of both EAdi and NAVAL. For a given NAVAL, the airway pressure varies breath-by-breath in proportion to EAdi, whose profile resembles as mirror image.
Since its introduction in clinical use, a growing number of studies investigating the effects of NAVA have been performed in animal models, healthy individuals, and adult and pediatric patients during both invasive and noninvasive ventilation (NIV) (Fig. 1). Because the studies dealing with pediatric patients represent nowadays a consistent fraction of the overall published articles on NAVA, we prefer not to include them in the present review article, leaving the pediatric population to further dedicated work. Accordingly, the present review addresses the current knowledge on NAVA in adult intensive care unit (ICU) patients, either intubated or receiving NIV, focusing, in particular, on the most recent studies.
FIGURE 1.
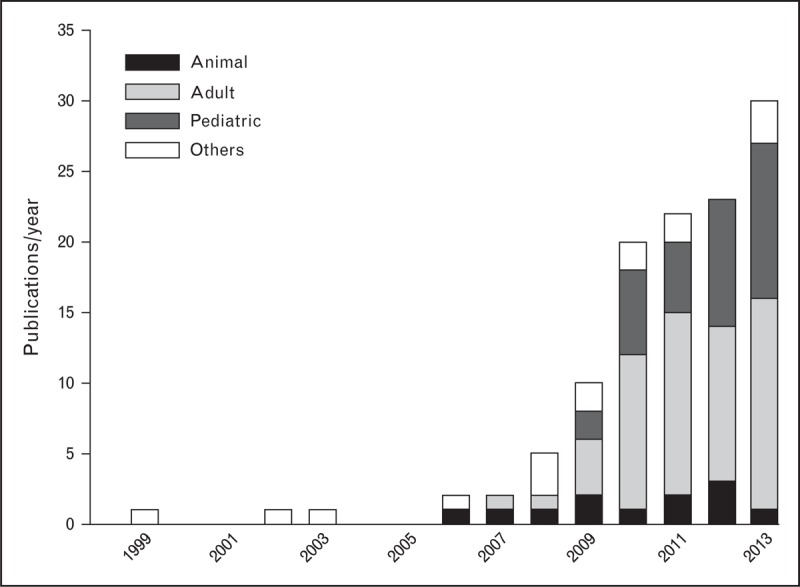
Publications on neurally adjusted ventilatory assist (NAVA) from 1999 to 2013. The studies related to NAVA yearly published from 1999 (first description of the technique) to 2013 are shown as a whole and divided according to the type of study: animal (black), adult (light gray) and pediatric patients (dark gray), and others (white), including reviews, editorials, and investigations on healthy individuals. After the introduction of NAVA in clinical use in 2008, the studies related to this mode progressively increased every year, either overall or considering the studies performed on adult and pediatric patients.
Box 1.

no caption available
NEURALLY ADJUSTED VENTILATORY ASSIST IN INTUBATED PATIENTS
The effects of NAVA have been assessed with respect to several physiological outcomes.
Respiratory drive and effort
EAdi is the best (i.e., closest to respiratory centers) signal available for clinical assessment of the respiratory drive, and provides an estimate of the pressure generated by the principal inspiratory muscle [3]. As depicted in Fig. 2, during partial ventilatory assistance, EAdi is influenced by multiple factors, including the amount of assistance and sedation [4,5▪▪]. With NAVA and PAV, the mechanical support delivered by the ventilator is directly (NAVA) or indirectly (PAV) driven and regulated by the effort exerted by the respiratory muscles [2]; this does not occur with the other assisted modes, in which the mechanical support is not affected by the patient's drive and effort.
FIGURE 2.
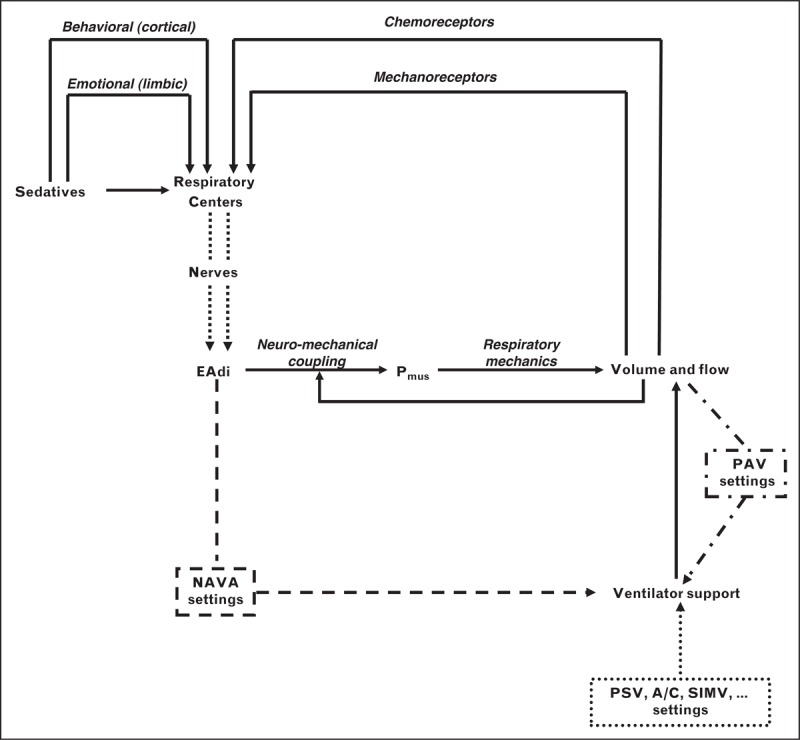
Relation between patient's demand and ventilator support with different modes of partial assistance. The figure depicts the composite interplay among respiratory drive, pressure generated by the respiratory muscles (Pmus), and ventilatory output (i.e., volume and flow) during partial ventilator assist with different modes of ventilation. The output of the respiratory centers is modulated by stimuli from mechanoreceptors and chemoreceptors, and the cortical or limbic system. Sedatives also affect, directly or indirectly, the output of the respiratory centers. The amount of assistance delivered by the ventilator with the conventional modes (single dotted line), such as pressure support (PSV), assist control (A/C), and synchronized intermittent mandatory ventilation (SIMV), is not influenced by either drive or effort or ventilator output, which exposes this mode to the risk of overassistance. In contrast, with the proportional modes, the delivered support is affected by patient's demand indirectly, by the ventilatory output in proportional assist ventilation (PAV) (dashed-dotted line), or directly, by the neural output of the respiratory centers, as obtained by the electrical activity of the diaphragm (EAdi), in neurally adjusted ventilatory assist (NAVA) (dashed line). With NAVA, moreover, a changed neuromechanical coupling, changes in respiratory mechanics, or air leaks may not disturb the relation between neural output and mechanical support. See text for further explanations. Modified with permission from [2].
When the assistance delivered is insufficiently low, regardless of the mode, the respiratory muscles are not efficiently unloaded and the drive remains high. When the assistance is high, the overall effect on drive and effort varies with the different forms of partial assistance. As indicated in Fig. 2, with NAVA and PAV increasing, ventilator assistance decreases drive, effort, and ventilatory output, which reduce in turn the ventilator support, thereby resetting the equilibrium between effort and assistance at a different point. With the other forms of partial assistance, conversely, an excessive support decreases the drive and makes respiratory muscle effort small, often just sufficient to trigger the ventilator and sometime even insufficient at that purpose [6,7]. In patients with acute respiratory failure (ARF) of different causes, NAVA has been demonstrated to efficiently unload the diaphragm [4,8,9,10▪,11]; differently from pressure support, however, the reduction of EAdi obtained with NAVA is contained and never excessive, and the risk of overassistance is therefore averted [4,8,11]. Stepwise increase of NAVAL produces a progressive decrease in EAdi that was shown to be approximately 50% at the highest NAVAL in a mixed population of patients with ARF [12].
EAdi, however, is not affected only by the amount of ventilator support. The output of the respiratory centers is also influenced, with respect to both drive and timing, by sedative administration, through either direct (respiratory centers) or indirect (cortical and limbic) effects (Fig. 2). As a consequence of the direct and tight relation between neural drive and delivered support, NAVA could, in principle, be more detrimentally affected by sedatives than pressure support, assist/control, and the other conventional forms of partial assistance. In a recent study [5▪▪], three levels of sedation (none, light, and deep), obtained by varying rates of propofol infusion, were evaluated and compared in ICU patients with ARF undergoing NAVA and pressure support administered at comparable levels of assistance. With both modes increasing, propofol infusion progressively decreased EAdi (drive) with no effect on the neural duty cycle (timing). Although gas exchange was not significantly different between the two modes, at deep sedation EAdi was lower in pressure support than in NAVA, which led to ineffective triggering in some patients with the former mode, but not with the latter [5▪▪]. Unpublished data presented in abstract form suggest different effects on drive and timing by varying doses of other sedatives.
Breathing pattern and lung volumes
NAVA being driven by the patient's drive, concerns exist on the possibility of excessively high tidal volumes (VT), not suited for protective ventilation strategies [13]. In an animal model of acute respiratory distress syndrome (ARDS), incrementing NAVAL reduced EAdi with minimal changes in respiratory rate and VT, which remained on average less than 4 ml/kg [14]. These results were subsequently confirmed in the same animal model: EAdi was also reduced when increasing pressure support, but VT significantly rose at increasing pressure support levels [15]. After injuring the lungs of 27 rabbits, Brander et al.[16] compared, at the same positive end-expiratory pressure (PEEP), NAVA and the low-VT strategy (6 ml/kg) with injurious ventilation (15 ml/kg and no PEEP). Lung injury and nonpulmonary organ dysfunction were significantly lower in both groups, as opposed to injurious ventilation. In the NAVA group, VT was 3.1 ± 0.9 ml/kg; compared with the conventional low VT strategy, respiratory rate, arterial oxygen (Pao2), and carbon dioxide (Paco2) partial pressures were higher, whereas lung wet-to-dry ratio and bronchoalveolar fluid and systemic biomarkers were similar between the two groups.
Differences in breathing pattern between NAVA and pressure support have been repeatedly shown in mechanically ventilated ICU patients [4,8,11,17▪▪,18,19▪,20]. Colombo et al.[4] first demonstrated, in intubated patients with ARF of different causes, the breathing pattern to be differently affected when varying the amount of assistance with the two modes. Increasing the amount of assistance by 50% determined different rises in VT, from 6.2 to 9.1 ml/kg in pressure support, whereas from 6.4 to 7.1 ml/kg in NAVA. Moreover, both spontaneous and mechanical respiratory rate and duty cycles decreased during pressure support, but not in NAVA. The ability of NAVA to maintain a reduced (protective) VT was subsequently confirmed in patients with ARDS in the acute phase [21,22▪▪], in the most severe patients undergoing extracorporeal membrane oxygenation [17▪▪,23], and during recovery [8]. The same was found in other patient populations such as postoperative [20] and chronic obstructive pulmonary disease [11]. Recently, in a mixed population of patients with ARF, Patroniti et al.[10▪] increased NAVAL (from 0.5 up to 5 cmH2O/μV) and found that, on average, VT did not exceed 6 ml/kg. In 30% of patients, nonetheless, at the highest NAVAL, periodical delivery of elevated (>8 ml/kg) VT was observed; in three patients, VT exceeded 10 ml/kg.
In 10 patients with mild to moderate ARDS, Blankman et al.[22▪▪] evaluated the aeration of the dependent and nondependent lung regions by means of electrical impedance tomography at varying levels of NAVA and pressure support. Although confirming with an analogous study design the reduced risk of overassistance with NAVA described by Colombo et al.[4], this study showed a beneficial effect on the ventilation of the dependent lung region with NAVA, as opposed to pressure support.
Compared with pressure support, VT variability was repeatedly found to be higher in NAVA [4,9,10▪], resembling the variability observed in healthy individuals [24]. Conversely, EAdi variability was similar [9] or even higher [4,20] with pressure support than NAVA. Variability might improve oxygenation while reducing the proinflammatory response [25]. The preserved breathing pattern variability associated with the reduced risk of overassistance in NAVA has been proposed to explain the absence of central apneas during weaning in nonsedated patients, as opposed to pressure support [26▪].
Arterial blood gases
One of the primary reasons to institute mechanical ventilation is to improve arterial blood gases. Considering that NAVA is characterized by high VT variability [4,9,20], shown in other settings to improve arterial oxygenation [25], and redistribution of the ventilation in the dependent lung regions [22▪▪], an improvement in oxygenation would be somewhat expected. Most of the studies, nonetheless, report no differences in oxygenation between NAVA and pressure support [4,5▪▪,8,9,11,17▪▪,27]. These studies, however, were short term (the time of the experimental trial varying between 10 and 30 min), which may explain why no improvement in oxygenation was observed. The effects of both VT variability and redistribution of ventilation are related to parenchymal recruitment, which takes time. Indeed, in the only study reporting an improvement in arterial oxygenation with NAVA [20], as compared with pressure support, the two modes were delivered for 24-h periods.
NAVA has been repeatedly shown to be as effective as pressure support in guaranteeing adequate values of Paco2 and pH [4,8,11,17▪▪,20,27]. Vaschetto et al.[5▪▪] found the Paco2 equally increased with NAVA and pressure support in patients receiving deep sedation by propofol.
Dyspnea
Surprisingly, no study has so far specifically evaluated in intubated patients the effects of NAVA on dyspnea, although Vagheggini et al.[28] used the Borg scale to determine comparable levels of pressure support and NAVA in patients with prolonged weaning.
Patient–ventilator synchrony
During partial ventilatory assistance, the ventilator is driven by the patient's spontaneous breathing activity. An optimal interaction between the patient and the ventilator, however, is not guaranteed. In particular, when patient effort and ventilator support lack concurrence in time, asynchronies occur. Patient–ventilator asynchrony is increasingly recognized as a clinical problem. In fact, patients with a rate of asynchronous breaths exceeding 10% of the overall breath count are characterized by worsened outcome (i.e., longer durations of mechanical ventilation [29–31] and ICU stay [30,31], reduced number of ventilator-free days [31], higher rate of tracheotomy [30], and lower probabilities of survival [29] and home discharge [31]). It remains unclear whether asynchronies are just markers of changed respiratory function in the most severe patients, or rather cause themselves the increased morbidity by prolonging the duration of mechanical ventilation [32]. In the latter case, reducing the rate of asynchrony would determine an improvement of patients’ outcomes. Visual inspection of the ventilator waveforms was shown inaccurate in detecting asynchronies, suggesting the use of an additional signal for recognizing their occurrence, such as EAdi or esophageal pressure [7].
Compared with conventional modes of ventilation, NAVA has been repeatedly demonstrated to improve the patient–ventilator synchrony in different clinical conditions [4,5▪▪,8,17▪▪,18,19▪]. Incremental ventilator assistance affects the patient's effort during conventional modes, whereas NAVA limits EAdi reduction and the risk of excessively low efforts [4,8,11].
Mechanical properties of the respiratory system may also affect patient–ventilator synchrony. In patients with low respiratory system compliance, pressure support is characterized by a high incidence of premature cycling (i.e., the mechanical breath is shorter than the patient's inspiration) [33]. This was recently confirmed, irrespective of the cycling-off settings, in patients with severe ARDS breathing in pressure support while undergoing extracorporeal membrane oxygenation; in the same patients, NAVA was able to improve patient–ventilator synchrony to suboptimal level [17▪▪]. Some premature cycling, autotriggering, and double triggering prevented an optimal patient–ventilator synchrony [17▪▪]. Ineffective triggering and delayed cycling are common during pressure support in patients with airway obstruction, determining dynamic hyperinflation and auto-PEEP [34]. In pressure support, applying PEEP helps reduce ineffective efforts and varying the cycling-off setting to a higher flow threshold limits delayed cycling. Compared with pressure support, NAVA was shown to eliminate ineffective efforts and drastically decrease on-trigger and off-trigger delays, regardless of the level of assistance [11]. Consistently, in a mixed population including approximately 30% of patients with chronic obstructive pulmonary disease, Piquilloud et al.[18] observed neither ineffective efforts nor delayed cycling during NAVA, although a few premature cycling and double triggering were detected. Recently, in a population with clinical suspicion of air trapping, Bellani et al.[19▪] confirmed that NAVA is able to improve triggering performance at different levels of applied PEEP, in comparison with pressure support.
NONINVASIVE NEURALLY ADJUSTED VENTILATORY ASSIST
The use of NIV to avoid ARF deterioration and avert the need for endotracheal intubation and invasive ventilation has markedly increased [35,36]. NIV is delivered most commonly in pressure support mode using automated software for air-leak compensation. Recent work indicates rates of asynchrony as high as 40% during NIV [37].
Beck et al.[38] first described noninvasive NAVA in 2008 in an experimental animal model of ARDS; NAVA, delivered through a single nasal prong, efficiently unloaded the respiratory muscles [38]. At increasing level of assistance, in contrast to NIV delivered by conventional modes, NAVA has been shown to avoid glottis closure during inspiration in lambs [39].
Cammarota et al.[40▪] compared NAVA with pressure support in patients with postextubation hypoxemic ARF receiving NIV through a helmet, a well tolerated interface often characterized by high rate of asynchronies [41]. Respiratory rate, EAdi, and blood gases were no different with the two modes. Compared with pressure support, however, NAVA reduced the inspiratory trigger delay, prolonged the time of inspiration during which the diaphragm was active and the ventilator was concurrently delivering assistance, and eliminated the asynchronies [40▪].
These findings were subsequently confirmed to varying extent during NIV delivered by mask [42▪–44▪]. Piquilloud et al.[42▪] compared pressure support and NAVA in delivering NIV via face mask in a series of patients with ARF or at risk of postextubation respiratory failure. They [42▪] also found EAdi and arterial blood gases no different between the two modes, and the trigger delays and asynchronies significantly improved with NAVA, compared with pressure support.
In patients receiving postextubation prophylactic NIV, Schmidt et al.[43▪] delivered both pressure support and NAVA either with or without automatic air-leak compensation. No differences in breathing pattern and EAdi were found among the four tested combinations; regardless of the algorithm for air-leak compensation, NAVA reduced the delays and improved synchrony, as opposed to pressure support [43▪]. Noteworthy, the NIV algorithm significantly reduced the incidence of asynchronous events during pressure support, but not with NAVA [43▪]. Comfort and dyspnea, as assessed by a Visual Analogue Scale, were no different among conditions [43▪]. A further study in a population of patients with ARF of varying cause [44▪] confirmed similar breathing pattern and improved patient–ventilator interaction and synchrony in NAVA, compared with pressure support.
CONCLUSION
NAVA is a novel form of proportional assistance offering several physiological advantages, compared with the conventional modes of partial support, during either invasive ventilation or NIV. In particular, NAVA improves patient–ventilator interaction, averting the risk of overassistance and limiting the occurrence of asynchronies. Whether these physiological improvements translate into clinical benefits remains to be determined by randomized trials assessing clinical outcomes.
Acknowledgements
None.
Financial support and sponsorship
The present work did not receive funds from any organization.
Conflicts of interest
Paolo Navalesi contributed to the development of a new interface for noninvasive ventilation (not mentioned in the present work), whose license for patent belongs to Intersurgical S.p.A., and receives royalties for that invention. His research laboratory has received equipment and grants from Maquet Critical Care and Intersurgical S.p.A. He also received honoraria/speaking fees from Maquet Critical Care, Covidien AG, Breas, Hill-Rom, and Linde AG. Federico Longhini has no conflict of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Sinderby C, Navalesi P, Beck J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med 1999; 5:1433–1436. [DOI] [PubMed] [Google Scholar]
- 2.Navalesi P, Costa R. New modes of mechanical ventilation: proportional assist ventilation, neurally adjusted ventilatory assist, and fractal ventilation. Curr Opin Crit Care 2003; 9:51–58. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Mauri T, Coppadoro A, et al. Estimation of patient's inspiratory effort from the electrical activity of the diaphragm. Crit Care Med 2013; 41:1483–1491. [DOI] [PubMed] [Google Scholar]
- 4.Colombo D, Cammarota G, Bergamaschi V, et al. Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 2008; 34:2010–2018. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Vaschetto R, Cammarota G, Colombo D, et al. Effects of propofol on patient–ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 2014; 42:74–82. [DOI] [PubMed] [Google Scholar]; In patients receiving partial ventilatory assistance for ARF of varied causes, propofol affects gas exchange and breathing pattern to an extent that varies with the depth of sedation and the mode of ventilation. EAdi is decreased at increasing levels of sedation with both pressure support and NAVA, although to varying extent. Deep propofol sedation deteriorates patient–ventilator interaction and may cause asynchrony during pressure support, but not in NAVA.
- 6.Berger KI, Sorkin IB, Norman RG, et al. Mechanism of relief of tachypnea during pressure support ventilation. Chest 1996; 109:1320–1327. [DOI] [PubMed] [Google Scholar]
- 7.Colombo D, Cammarota G, Alemani M, et al. Efficacy of ventilator waveforms observation in detecting patient–ventilator asynchrony. Crit Care Med 2011; 39:2452–2457. [DOI] [PubMed] [Google Scholar]
- 8.Terzi N, Pelieu I, Guittet L, et al. Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med 2010; 38:1830–1837. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Demoule A, Cracco C, et al. Neurally adjusted ventilatory assist increases respiratory variability and complexity in acute respiratory failure. Anesthesiology 2010; 112:670–681. [DOI] [PubMed] [Google Scholar]
- 10▪.Patroniti N, Bellani G, Saccavino E, et al. Respiratory pattern during neurally adjusted ventilatory assist in acute respiratory failure patients. Intensive Care Med 2012; 38:230–239. [DOI] [PubMed] [Google Scholar]; In patients with ARF, increasing NAVA levels were associated with decreased EAdi, and, differently from pressure support, with small changes in tidal volume, but higher tidal volume variability. At the highest NAVA levels, the elevated tidal volume variability was associated with increased incidence of tidal volumes exceeding 8 and 10 ml/kg.
- 11.Spahija J, de Marchie M, Albert M, et al. Patient–ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 2010; 38:518–526. [DOI] [PubMed] [Google Scholar]
- 12.Brander L, Leong-Poi H, Beck J, et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest 2009; 135:695–703. [DOI] [PubMed] [Google Scholar]
- 13.Gama de Abreu M, Belda FJ. Neurally adjusted ventilatory assist: letting the respiratory center take over control of ventilation. Intensive Care Med 2013; 39:1481–1483. [DOI] [PubMed] [Google Scholar]
- 14.Allo JC, Beck JC, Brander L, et al. Influence of neurally adjusted ventilatory assist and positive end-expiratory pressure on breathing pattern in rabbits with acute lung injury. Crit Care Med 2006; 34:2997–3004. [DOI] [PubMed] [Google Scholar]
- 15.Beck J, Campoccia F, Allo JC, et al. Improved synchrony and respiratory unloading by neurally adjusted ventilatory assist (NAVA) in lung-injured rabbits. Pediatr Res 2007; 61:289–294. [DOI] [PubMed] [Google Scholar]
- 16.Brander L, Sinderby C, Lecomte F, et al. Neurally adjusted ventilatory assist decreases ventilator-induced lung injury and nonpulmonary organ dysfunction in rabbits with acute lung injury. Intensive Care Med 2009; 35:1979–1989. [DOI] [PubMed] [Google Scholar]
- 17▪▪.Mauri T, Bellani G, Grasselli G, et al. Patient–ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med 2013; 39:282–291. [DOI] [PubMed] [Google Scholar]; In patients with severe ARDS breathing in pressure support while undergoing extracorporeal membrane oxygenation, the authors found a high incidence of premature cycling, irrespective of the cycling-off settings; in the same patients, NAVA was able to improve patient–ventilator synchrony to suboptimal level.
- 18.Piquilloud L, Vignaux L, Bialais E, et al. Neurally adjusted ventilatory assist improves patient–ventilator interaction. Intensive Care Med 2011; 37:263–271. [DOI] [PubMed] [Google Scholar]
- 19▪.Bellani G, Coppadoro A, Patroniti N, et al. Clinical assessment of auto-positive end-expiratory pressure by diaphragmatic electrical activity during pressure support and neurally adjusted ventilatory assist. Anesthesiology 2014; 121:563–571. [DOI] [PubMed] [Google Scholar]; In intubated patients with auto-PEEP, the neural control of ventilation decreased the required pressure to overcome the auto-PEEP, in comparison with pressure support.
- 20.Coisel Y, Chanques G, Jung B, et al. Neurally adjusted ventilatory assist in critically ill postoperative patients: a crossover randomized study. Anesthesiology 2010; 113:925–935. [DOI] [PubMed] [Google Scholar]
- 21.Wu XY, Huang YZ, Yang Y, et al. Effects of neurally adjusted ventilatory assist on patient–ventilator synchrony in patients with acute respiratory distress syndrome. Zhonghua Jie He He Hu Xi Za Zhi 2009; 32:508–512. [PubMed] [Google Scholar]
- 22▪▪.Blankman P, Hasan D, van Mourik MS, Gommers D. Ventilation distribution measured with EIT at varying levels of pressure support and neurally adjusted ventilatory assist in patients with ALI. Intensive Care Med 2013; 39:1057–1062. [DOI] [PubMed] [Google Scholar]; In patients with ARDS of varied severity, NAVA was characterized by enhanced distribution of ventilation in the dependent lung regions, as opposed to pressure support. Also, NAVA resulted in a reduced risk of overassistance.
- 23.Karagiannidis C, Lubnow M, Philipp A, et al. Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med 2010; 36:2038–2044. [DOI] [PubMed] [Google Scholar]
- 24.Tobin MJ, Mador MJ, Guenther SM, et al. Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol (1985) 1988; 65:309–317. [DOI] [PubMed] [Google Scholar]
- 25.Spieth PM, Carvalho AR, Pelosi P, et al. Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med 2009; 179:684–693. [DOI] [PubMed] [Google Scholar]
- 26▪.Delisle S, Terzi N, Ouellet P, et al. Effect of ventilatory variability on occurrence of central apneas. Respir Care 2013; 58:745–753. [DOI] [PubMed] [Google Scholar]; In critically ill patients during the weaning process, compared with pressure support, NAVA avoided the episodes of overassistance during sleep, which corresponded to eliminating central apneas.
- 27.Barwing J, Linden N, Ambold M, et al. Neurally adjusted ventilatory assist vs. pressure support ventilation in critically ill patients: an observational study. Acta Anaesthesiol Scand 2011; 55:1261–1271. [DOI] [PubMed] [Google Scholar]
- 28.Vagheggini G, Mazzoleni S, Vlad Panait E, et al. Physiologic response to various levels of pressure support and NAVA in prolonged weaning. Respir Med 2013; 107:1748–1754. [DOI] [PubMed] [Google Scholar]
- 29.Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Patient–ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 1997; 112:1592–1599. [DOI] [PubMed] [Google Scholar]
- 30.Thille AW, Rodriguez P, Cabello B, et al. Patient–ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006; 32:1515–1522. [DOI] [PubMed] [Google Scholar]
- 31.de Wit M, Miller KB, Green DA, et al. Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med 2009; 37:2740–2745. [DOI] [PubMed] [Google Scholar]
- 32.Navalesi P. On the imperfect synchrony between patient and ventilator. Crit Care 2011; 15:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cereda M, Foti G, Marcora B, et al. Pressure support ventilation in patients with acute lung injury. Crit Care Med 2000; 28:1269–1275. [DOI] [PubMed] [Google Scholar]
- 34.Nava S, Bruschi C, Rubini F, et al. Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med 1995; 21:871–879. [DOI] [PubMed] [Google Scholar]
- 35.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013; 188:220–230. [DOI] [PubMed] [Google Scholar]
- 36.Demoule A, Girou E, Richard JC, et al. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med 2006; 32:1747–1755. [DOI] [PubMed] [Google Scholar]
- 37.Vignaux L, Vargas F, Roeseler J, et al. Patient–ventilator asynchrony during noninvasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med 2009; 35:840–846. [DOI] [PubMed] [Google Scholar]
- 38.Beck J, Brander L, Slutsky AS, et al. Noninvasive neurally adjusted ventilatory assist in rabbits with acute lung injury. Intensive Care Med 2008; 34:316–323. [DOI] [PubMed] [Google Scholar]
- 39.Hadj-Ahmed MA, Samson N, Bussieres M, et al. Absence of inspiratory laryngeal constrictor muscle activity during nasal neurally adjusted ventilatory assist in newborn lambs. J Appl Physiol (1985) 2012; 113:63–70. [DOI] [PubMed] [Google Scholar]
- 40▪.Cammarota G, Olivieri C, Costa R, et al. Noninvasive ventilation through a helmet in postextubation hypoxemic patients: physiologic comparison between neurally adjusted ventilatory assist and pressure support ventilation. Intensive Care Med 2011; 37:1943–1950. [DOI] [PubMed] [Google Scholar]; Compared with pressure support, NAVA dramatically improves patient–ventilator interaction and synchrony during NIV delivered by helmet, with no differences in gas exchange and neural drive.
- 41.Navalesi P, Costa R, Ceriana P, et al. Noninvasive ventilation in chronic obstructive pulmonary disease patients: helmet versus facial mask. Intensive Care Med 2007; 33:74–81. [DOI] [PubMed] [Google Scholar]
- 42▪.Piquilloud L, Tassaux D, Bialais E, et al. Neurally adjusted ventilatory assist (NAVA) improves patient–ventilator interaction during noninvasive ventilation delivered by face mask. Intensive Care Med 2012; 38:1624–1631. [DOI] [PubMed] [Google Scholar]; Compared with pressure support, NAVA improved patient–ventilator interaction and reduced the asynchronous events in patients undergoing NIV through a facial mask. Gas exchange and neural drive were no different with the two modes.
- 43▪.Schmidt M, Dres M, Raux M, et al. Neurally adjusted ventilatory assist improves patient–ventilator interaction during postextubation prophylactic noninvasive ventilation. Crit Care Med 2012; 40:1738–1744. [DOI] [PubMed] [Google Scholar]; During NIV, the application of a leak-compensating software significantly improved patient ventilator synchrony during pressure support, but produced little or no benefit in NAVA. With NAVA, the asynchronies caused by air leaks were irrelevant and significantly lower than in pressure support. There were more episodes of double triggering with NAVA than with pressure support.
- 44▪.Bertrand PM, Futier E, Coisel Y, et al. Neurally adjusted ventilatory assist vs pressure support ventilation for noninvasive ventilation during acute respiratory failure: a crossover physiologic study. Chest 2013; 143:30–36. [DOI] [PubMed] [Google Scholar]; This study confirms in patients receiving NIV through a facial mask that NAVA improves the patient–ventilator interaction and synchrony and results in similar values of arterial blood gases and diaphragm electrical activity, as compared with pressure support.


