Summary
Development of novel more efficient preventive vaccines against tuberculosis (TB) is crucial to achieve TB eradication by 2050, one of the Millennium Development Goals (MDG) for the current century. MTBVAC is the first and only live attenuated vaccine based on a human isolate of Mycobacterium tuberculosis developed as BCG-replacement strategy in newborns that has entered first-in-human adult clinical trials. In this work, we characterize the safety, immunogenicity and protective efficacy of MTBVAC in a model of newborn C57/BL6 mice. Our data clearly indicate that MTBVAC is safe for newborn mice, and does not affect animal growth or organ development. In addition, MTBVAC-vaccinated mice at birth showed enhanced immunogenicity and better protection against M. tuberculosis challenge in comparison with BCG.
Keywords: MTBVAC, Newborn mice, Tuberculosis vaccine, Neonates
1. Introduction
Tuberculosis (TB) disease causes more than one million and a half deaths per year and is one of the leading infectious diseases affecting developing countries. Thus, development of new vaccines able to prevent TB represents a global emergency [1].
The only vaccine against TB in use today, Bacille Calmette-Guerin (BCG), is a live attenuated strain from a Mycobacterium bovis strain isolated from cattle, and is worldwide administered at birth. Despite its effectiveness in reducing the incidence of the disseminated forms of TB in children, it is inconsistent in preventing pulmonary TB, the most common form of the disease in adolescents and adults, and responsible of TB transmission [2]. BCG was developed a century ago by repeated subculture. The loss of the RD1 region-the principal genetic basis for its attenuation-, encodes a secretion system to export the major T-cell antigen complex/virulence factor ESAT-6/CFP-10 [3]. In addition, when compared to clinical isolates of the Mycobacterium tuberculosis complex, BCG has more than one hundred genes deleted from its genome [4], including important immunodominant antigen proteins which contain a high proportion of epitopes recognized by HLA complex, considered important in generating effective long-lasting immune responses [5]. Some of these antigens are employed in different subunit TB vaccines for use in BCG-vaccinated individuals, currently in clinical trials [1].
MTBVAC is a live rationally-attenuated derivative of the M. tuberculosis isolate MT103, which belongs to the Lineage 4 (Euro-American), one of the most widespread lineages of M. tuberculosis [6]. MTBVAC contains two independent stable deletion mutations in the virulence genes phoP and fadD26 without antibiotic resistance marker, in accordance to the established in the Second Geneva Consensus document for progressing new live mycobacterial vaccines to advanced clinical development [7]. PhoP is a transcription factor that controls 2% of the genome of MTB including production of immunomodulatory cell-wall lipids and ESAT-6 secretion [8]. Deletion of fadD26 leads to complete synthesis abrogation of the virulence surface lipids phtioceroldimycocerosates (DIM) [9]. MTBVAC has been the first and only live attenuated M. tuberculosis vaccine approved to enter into clinical trials. A first-in-human MTBVAC clinical trial was recently conducted successfully in healthy adults in Lausanne (Switzerland) (NCT02013245) [10].
Newborn mice have been used in different works as a model to measure safety, immunogenicity and protective efficacy of BCG alone or in combination with diverse boosting approaches [11]. Given that the preclinical development of MTBVAC to date was generated in adult animal models with the objective to support the first-in-human adult Phase 1a safety and immunogenicity trial in Lausanne, the present safety and immunogenicity study in newborn mice is the first to date conducted to support progress to Phase 1b evaluation of MTBVAC in newborns.
2. Material and methods
All mice were kept under controlled conditions and observed for any sign of disease. Experimental work was conducted in agreement with European and national directives for protection of experimental animals and with approval from the competent local ethics committees.
For vaccination with lyophilized BCG Danish 1331 (Staten Serum Institute SSI) or MTBVAC (manufactured by Biofabri, Spain), approximately 5 × 106 colony-forming units (CFU) were reconstituted with 1 mL of reconstitution buffer. Groups of C57/BL6 newborn mice were vaccinated subcutaneously with 50 μl of reconstituted vaccines (2.5 × 105 CFU) in the first three days after birth. Unvaccinated controls were inoculated with 50 μl of phosphate buffered saline (PBS).
2.1. Safety
During the next eight weeks following vaccination of the newborn mice, different welfare-related parameters as weight gain, social and individual behaviour and food and water consumption were monitored. Eight weeks post-vaccination, animals were sacrificed and the target organs (spleen, kidneys, testes, ovaries, heart, lungs, liver and brain) were harvested, weighed and fixed in formaldehyde for haematoxylin-eosin staining and pathological evaluation. The experts in charge of the anatomopathological examination were blinded to the vaccine group origin of the indicated organs.
2.2. Immunogenicity
For immunogenicity assessment, 106 splenocytes per experimental point were incubated with purified-protein derivative (PPD) (Statens Serum Institut, SSI) 10 μg/ml or recombinant Ag85B (Lionex) 2 μg/ml in culture medium during 48 h. After incubation, supernatant was separated from the cellular fraction by centrifugation and IFNγ concentration determined by ELISA using a specific commercial kit (MabTech), and performed according to manufacturer instructions.
2.3. Protective efficacy
Eight weeks post-vaccination, mice were intranasally challenged with 150 CFU MTB H37Rv in 40 μl of PBS. Bacterial load from lungs was determined four weeks post–challenge by plating lung homogenates on solid 7H11 agar medium supplemented with ADC (Difco).
2.4. Statistical analysis
GraphPrism software was used for statistical analysis. For experiments with two experimental groups, unpaired t-student test was used. When three or more groups were compared, One-Way ANOVA analysis with Bonferroni post-test was performed. Differences were considered significant at p < 0.05.
3. Results and discussion
Despite the failure of the subunit vaccine MVA85A to improve BCG efficacy in an infant Phase 2b trial in South Africa, the clinical trial paved the way for testing of new TB vaccines in infants [12]. Some authors have criticized that considering the MVA85A preclinical data available the failure of this vaccine candidate was not unexpected [13], [14]. Thus a lesson to be learnt through the experience with MVA85A is the necessity to conduct rigorous preclinical characterization of vaccine candidates in models mimicking as much as possible the conditions planned for testing in the clinic. Since MTBVAC was conceived as a BCG-replacement strategy, newborns represent its main target population. Nevertheless, all the preclinical studies performed up to date with MTBVAC, or its prototype version SO2 [7], have been carried out in different adult animal models, showing an excellent safety profile in all preclinical experiments performed [7], [15]. Following the successful outcome of the first-in-human Phase 1a trial in healthy adults in Lausanne, we conducted the present study of MTBVAC in a neonatal preclinical model with the aim to support progress to clinical evaluation in healthy newborn infants.
3.1. Safety evaluation
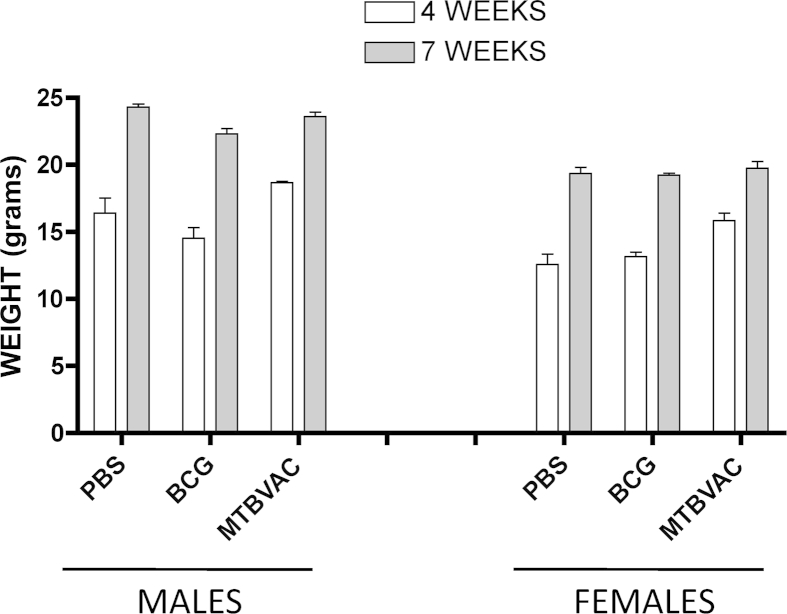
In the whole study, a total of 80 newborn mice were vaccinated with MTBVAC with no mortality or disease-associated symptomatology. As shown in Figure 1, both male and female mice vaccinated with MTBVAC gained weight in a similar way when compared to control BCG and unvaccinated groups. At eight weeks post-birth, animals were euthanatized and pivotal organs were harvested for further analysis. No significant differences with respect to organ weight were found in any of the organs studied (Supplementary Table 1). In addition, an anatomopathological blind analysis from the selected organs was performed. Our results show no histopathological differences related to vaccination in any of the studied organs. Thus, we conclude that MTBVAC did not produce structural or developmental changes in any studied organ in newborn immunized mice.
Figure 1.
Weight monitoring of C57/BL6 mice immunized at birth for safety evaluation. Groups of at least twelve newborn C57/BL6 mice were vaccinated at birth with MTBVAC, BCG or PBS. Four weeks post-birth, mice were divided by gender and weight was monitored weekly. Data showed in the figure correspond to mice weighed at four and seven weeks post-birth. A representative experiment of two is shown. Data in the graphs are represented as mean ± SEM. Statistical analysis was done using a one-way ANOVA test to compare different vaccination groups for each age and gender. No statistical differences were found.
3.2. Immunogenicity evaluation
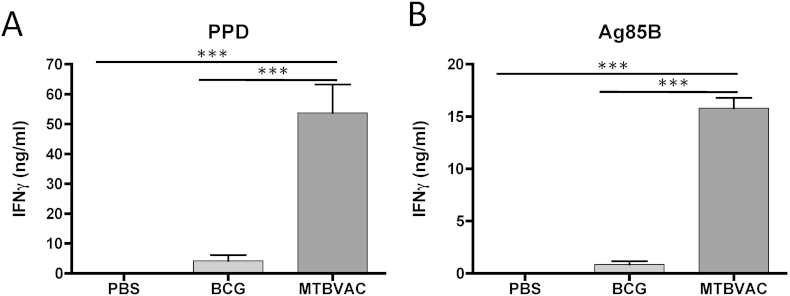
Newborn immunization takes place when the immune system is still immature which may represent an important difference to vaccination in adults. This fact could have an impact in the immune response triggered by vaccination. Indeed, Phase 2b and other MVA85A clinical studies have shown that Th1 responses induced by this vaccine are weaker in infants compared to adults [12]. To assess vaccine-induced immunogenicity with MTBVAC, we harvested splenocytes from eight-week-old mice vaccinated at birth and stimulated ex vivo cells with PPD (Figure 2A) or single antigen Ag85B (Figure 2B) for 48 h. We analysed next IFNγ production by ELISA. IFNγ production was higher in the group of MTBVAC-vaccinated mice after stimulation with PPD or Ag85B. PPD results contrasted with those obtained in adult C57/BL6 mice, where non-significant differences between MTBVAC- and BCG-vaccinated animals were found after PPD stimulation ([16], Unpublished results). The results with Ag85B may be relevant, based on data with other Ag85B-based vaccines showing improved protective efficacy when compared to BCG, suggesting that Ag85B-specific response could be protective [17]. Ag85B is one of the most antigenic proteins of M. tuberculosis, harbouring a number of experimentally confirmed human T cell epitopes. Importantly, a recent report showed that all BCG strains contain a polymorphism in ag85b gene, which triggers an amino acid substitution predicted to affect protein's structure and stability [5].
Figure 2.
Immunogenicity conferred by vaccination at birth in C57/BL6 mice. Groups of six newborn C57/BL6 mice were vaccinated with MTBVAC, BCG or PBS. Immunogenicity was studied at eight weeks post-birth. Animals were humanely sacrificed and a cellular suspension from spleen was obtained. Cells were stimulated with PPD (A) or Ag85B (B) for 48 h and subsequently IFNg analysed in cell supernatant by ELISA. A representative experiment of two is shown. Data in the graphs are represented as mean ± SEM. One-way ANOVA test with Bonferroni post analysis was performed to calculate statistical significance. *p < 0.05; **p < 0.01; ***p < 0.001.
3.3. Protective efficacy evaluation
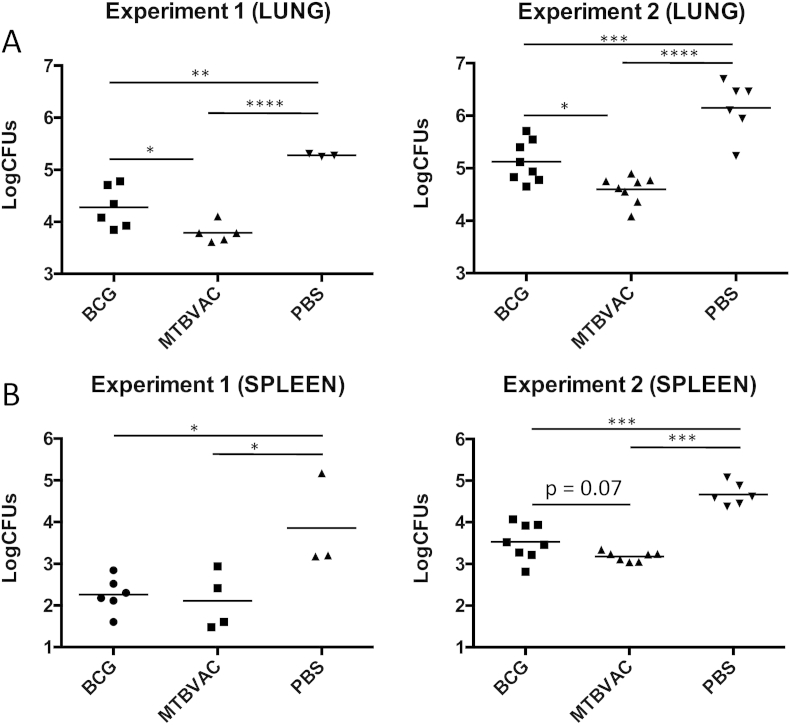
To evaluate protective efficacy, we intranasally inoculated eight-week old mice vaccinated at birth with a low dose of H37Rv and four weeks after challenge we determined bacterial load reduction in lungs and spleen. A group of ten unvaccinated mice was sacrificed one day after challenge to determine the initial number of bacteria in lungs, resulting in a mean value ± SEM of 29 CFU ± 11. The two independent experiments performed showed a similar efficacy profile in lungs and spleen, and both BCG and MTBVAC conferred significant protection in comparison with unvaccinated controls. Remarkably, bacterial reduction in lungs induced by MTBVAC was significantly higher (around 0.5 logs) than the decrease triggered by BCG. In the case of bacterial load in spleen, MTBVAC also tended to provide a better protection than BCG, although no significance was observed in this case (Figure 3).
Figure 3.
Protective efficacy conferred by vaccination at birth in C57/BL6 mice. Groups of three to nine newborn C57/BL6 mice were vaccinated with MTBVAC, BCG or PBS. At eight weeks post-birth, animals were challenged intranasally with H37Rv and four weeks later CFUs were determined in lungs (A) and spleen (B). Data of two independent experiments are shown in the figure. Data in the graphs are represented as mean ± SEM. One-way ANOVA test with Bonferroni post analysis was performed to calculate statistical significance. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. P value shown in Experiment 2 (SPLEEN) graph, comparing BCG and MTBVAC groups, was determined using a Mann–Whitney t-student test.
Since BCG is effective in preventing disseminated TB in children one of the potential issues of BCG-replacement TB vaccines is that individuals in TB-endemic countries not vaccinated with BCG at birth might be at risk of developing disseminated TB in childhood. Given that MTBVAC is aimed for use at birth, our data provide evidence for the adequate safety and improved immunogenicity and protection efficacy profile as compared to licensed BCG Danish SSI, suggesting that newborn vaccination with MTBVAC in clinic is expected to be at least as safe, immunogenic and effective as BCG.
4. Conclusions
The most important conclusion of this study is that MTBVAC is safe in newborn mice, and does not affect organ development. To our knowledge, this is the first time that a live attenuated M. tuberculosis vaccine is tested in a preclinical neonatal model, so this work can pave the way for the preclinical and clinical development of other M. tuberculosis-based vaccines targeting this population.
Acknowledgements
The authors gratefully acknowledge Biofabri and TuBerculosis Vaccine Iniciative (TBVI) Preclinical & Clinical Development Teams for their expertise and advise in the elaboration of the protocols for safety evaluation of MTBVAC in the mouse neonatal model.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.tube.2015.10.010.
Funding
This work was supported by “Fundación Española de la Ciencia y la Tecnología” [grant INNOCASH (INC-098), “Spanish Ministry of Economy and Competitiveness” [grant numbers BIO2011-23555, BIO2014-5258P], “European Commission FP7 and H2020 programs” [grant numbers NEWTBVAC 241745, TBVAC2020 643381], and “Gobierno de Aragón/Fondo Social Europeo”. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
Carlos Martin is co-inventor of the patent “tuberculosis vaccine” filled by the University of Zaragoza. There are no other conflicts of interest.
Ethical approval
Experimental work was conducted in agreement with European and national directives for protection of experimental animals and with approval from the competent local ethics committees.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Marinova D., Gonzalo-Asensio J., Aguilo N., Martin C. Recent developments in tuberculosis vaccines. Expert Rev Vaccines. 2013;12(12):1431–1448. doi: 10.1586/14760584.2013.856765. [DOI] [PubMed] [Google Scholar]
- 2.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E., Rodrigues L.C., Smith P.G., Lipman M., Whiting P.F., Sterne J.A. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 3.Pym A.S., Brodin P., Brosch R., Huerre M., Cole S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46(3):709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 4.Brosch R., Gordon S.V., Garnier T., Eiglmeier K., Frigui W., Valenti P., Dos Santos S., Duthoy S., Lacroix C., Garcia-Pelayo C., Inwald J.K., Golby P., Garcia J.N., Hewinson R.G., Behr M.A., Quail M.A., Churcher C., Barrell B.G., Parkhill J., Cole S.T. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007;104(13):5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copin R., Coscolla M., Efstathiadis E., Gagneux S., Ernst J.D. Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette–Guerin (BCG) Vaccine. 2014;32(45):5998–6004. doi: 10.1016/j.vaccine.2014.07.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brites D., Gagneux S. Co-evolution of Mycobacterium tuberculosis and homo sapiens. Immunol Rev. 2015;264(1):6–24. doi: 10.1111/imr.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbues A., Aguilo J.I., Gonzalo-Asensio J., Marinova D., Uranga S., Puentes E., Fernandez C., Parra A., Cardona P.J., Vilaplana C., Ausina V., Williams A., Clark S., Malaga W., Guilhot C., Gicquel B., Martin C. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–4873. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalo-Asensio J., Mostowy S., Harders-Westerveen J., Huygen K., Hernandez-Pando R., Thole J., Behr M., Gicquel B., Martin C. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One. 2008;3(10):e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camacho L.R., Ensergueix D., Perez E., Gicquel B., Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34(2):257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 10.Spertini F., Audran R., Chakour R., Karoui O., Steiner-Monard V., Thierry A.-C., Mayor C.E., Rettby N., Jaton K., Vallotton L., Lazor C., Doce J., Puentes E., Marinova D., Aguilo N., Martin C. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med. 2015;3(12):953–962. doi: 10.1016/S2213-2600(15)00435-X. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero G.G., Debrie A.S., Locht C. Boosting with mycobacterial heparin-binding haemagglutinin enhances protection of Mycobacterium bovis BCG-vaccinated newborn mice against M. tuberculosis. Vaccine. 2010;28(27):4340–4347. doi: 10.1016/j.vaccine.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 12.Tameris M.D., Hatherill M., Landry B.S., Scriba T.J., Snowden M.A., Lockhart S., Shea J.E., McClain J.B., Hussey G.D., Hanekom W.A., Mahomed H., McShane H. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beverley P. TB vaccine failure was predictable. Nature. 2013;503(7477):469. doi: 10.1038/503469e. [DOI] [PubMed] [Google Scholar]
- 14.Kashangura R., Sena E.S., Young T., Garner P. Effects of MVA85A vaccine on tuberculosis challenge in animals: systematic review. Int J Epidemiol. 2015 Sep 8 doi: 10.1093/ije/dyv142. pii: dyv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin C., Williams A., Hernandez-Pando R., Cardona P.J., Gormley E., Bordat Y., Soto C.Y., Clark S.O., Hatch G.J., Aguilar D., Ausina V., Gicquel B. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006;24(17):3408–3419. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Solans L., Uranga S., Aguilo N., Arnal C., Gomez A.B., Monzon M., Badiola J.J., Gicquel B., Martin C. Hyper-attenuated MTBVAC erp mutant protects against tuberculosis in mice. Vaccine. 2014;32(40):5192–5197. doi: 10.1016/j.vaccine.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz M.A., Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003;71(4):1672–1679. doi: 10.1128/IAI.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.