Abstract
Purpose
To assess the pathological macular changes with optical coherence tomography (OCT) before the removal of silicone oil (SiO) in eyes that had undergone pars plana vitrectomy for complicated forms of retinal detachment (RD).
Patients and methods
Subjects included 48 patients (51 eyes) with complicated RD including proliferative vitreoretinopathy, proliferative diabetic retinopathy, recurrent RD, penetrating trauma, uveitis, giant retinal tears, and macular holes. All the eyes had undergone SiO injection. Furthermore, all eyes had been planned for the removal of SiO 6–12 months after the primary surgery. Finally, all eyes had a fundus examination and OCT examination before the silicone oil removal.
Results
OCT findings indicated epiretinal membrane in 41% of the eyes, macular edema in 17%, macular detachment in 13.5%, macular thinning in 13.5%, macular holes in 10%, and subretinal membranes in 2%. Preoperative OCT was normal in only 12% of the eyes, while a clinical fundus examination was normal in 43% (P<0.001). Eyes with normal OCT had significantly better mean logMAR (0.35) than eyes with pathological changes detected through OCT (1.28; P<0.001). Surgical modifications were made during the removal of SiO in 74.5% of the eyes.
Conclusion
OCT detected significantly more pathological changes than a clinical fundus examination. This had an impact on both surgical step modification during the removal of SiO and predictability of visual outcome after the removal of SiO.
Keywords: optical coherence tomography, silicone oil, pars plana vitrectomy, proliferative vitreoretinopathy, proliferative diabetic retinopathy
Introduction
Optical coherence tomography (OCT) is a noninvasive technique that provides high-resolution images of the macular layers.1 Pars plana vitrectomy (PPV) with silicone oil (SiO) tamponade has been used for complicated retinal detachment (RD) with proliferative vitreoretinopathy (PVR) and proliferative diabetic retinopathy (PDR).2–4 SiO is subsequently removed before the occurrence of complications, such as dense complicated cataracts, secondary glaucoma, keratopathy, and emulsification.5–9 However, other complications such as epiretinal membrane (ERM), macular holes, macular edema, sensory detachment, and thinning of the retinal layers can clinically be easily missed. These complications, if present, can affect visual outcome, and some of them require modification of the surgical maneuver during the removal of SiO.5–9 Few studies have used OCT as a routine tool to assess the macula before the removal of SiO. OCT can be used in SiO-filled eyes to examine the posterior segments when the media are clear.10 We set out to evaluate the role of preoperative OCT in the detection of macular changes when used routinely before the removal of SiO.
Patients and methodology
This study was a cross-sectional study performed between July 2011 and September 2014. This study adhered to the Tenets of the Declaration of Helsinki. The Research Ethics Committee, Faculty of Medicine, Ainshams University (FMAUS REC) approved the protocol of this study from the ethical point of view. Written informed patient consent was also obtained.
Inclusion criteria
PPV and SiO 2000 injection.
Different indications including PVR B and C, PDR, penetrating trauma, uveitis, recurrent RD, giant retinal tears (GRT), and macular holes.
Plan for the removal of SiO with or without phacoemulsification to be performed 6–12 months after primary surgery.
Exclusion criteria
Media opacity as a cataract or corneal opacity that prevents OCT imaging.
SiO present for >12 months.
SiO total emulsification or hemorrhage opacifying the view.
SiO-induced glaucoma.
An opthalmological examination including indirect ophthalmoscopy, slit lamp biomicroscopy, and preoperative and postoperative visual acuity was carried out on all eyes. All surgical details were recorded on the patient follow-up chart. Biometry was performed using a Ziess IOLMaster SiO formula.
Examination using OCT (Ziess Cirrus, software version 4.5; Carl Zeiss Meditec AG, Jena, Germany) was performed for the macular area by scanning across a series of six radiating cross-sectional B scans of 6 mm with the center of each scan placed at the center of the fovea using a fast macula map including central macular thickness. Macular GUBE-5 raster lines were used in the eyes with neurosensory RD and ERM with traction. Macular edema was defined as increased retinal thickness calculated with an OCT algorithm at the central fovea between the inner retinal surface and the retinal pigment epithelium. Patients with significant cataracts underwent microcoaxial phacoemulsification using an Infiniti® machine (Alcon Laboratories, Inc., Fort Worth, TX, USA). Hydrophobic acrylic intraocular lenses with appropriate power were implanted.
All patients had standard three-port sclerotomies in the pars plana, 3.5 mm from the limbus. SiO was left to flow passively under the effect of infusion fluid pressure. Endoillumination using the halogen light of a CONSTELLATION Vitrectomy Machine® (Alcon Laboratories, Inc.) was used to examine the macular region after the removal of SiO. The macular pathology found in the OCT examination was checked and dealt with intraoperatively. Eyes with significant ERM traction needed peeling after the removal of SiO. Eyes with macular edema needed pharmacotherapy, and eyes with macular RD needed drainage of subretinal fluid. Eyes that needed further tamponade were injected with perfluoropropane (C3F8). All eyes were refracted to achieve the best-corrected visual acuity (BCVA) after 4 weeks. Postoperative measurements of visual acuity (logMAR) were recorded. Statistical analysis was performed to evaluate OCT findings in correlation with preoperative fundus appearance, surgical findings, and visual outcome.
Statistical analysis
The following formula was used for sample calculation:
| (1) |
An adequate sample should be at least 40 eyes.
Data were collected, coded, revised, and entered into the Statistical Package for Social Science (SPSS) version 20. Qualitative data were presented as number and percentages and compared using the chi-square test. Quantitative data were presented as means, standard deviations, and ranges and compared using an independent t-test. Confidence intervals were set to 95%, and the margin of error accepted was 5%. The P-value was considered significant at a level of <0.05.
Results
Results included 51 eyes from 48 patients. Of those patients, 20 (41.6%) were females and 28 (58.4%) were males. Original diagnoses before performing PPV and SiO are given in Table 1.
Table 1.
Original diagnosis before pars plana vitrectomy (PPV)
| Diagnosis | Number | Percent |
|---|---|---|
| PVR | 15 | 29.4 |
| PDR | 12 | 23.5 |
| Penetrating trauma | 8 | 15.7 |
| Recurrent RD | 5 | 9.8 |
| Uveitis | 4 | 8 |
| GRT | 4 | 8 |
| Macular hole | 3 | 5.9 |
Abbreviations: PVR, proliferative vitreoretinopathy; PDR, proliferative diabetic retinopathy; RD, retinal detachment; GRT, giant retinal tear.
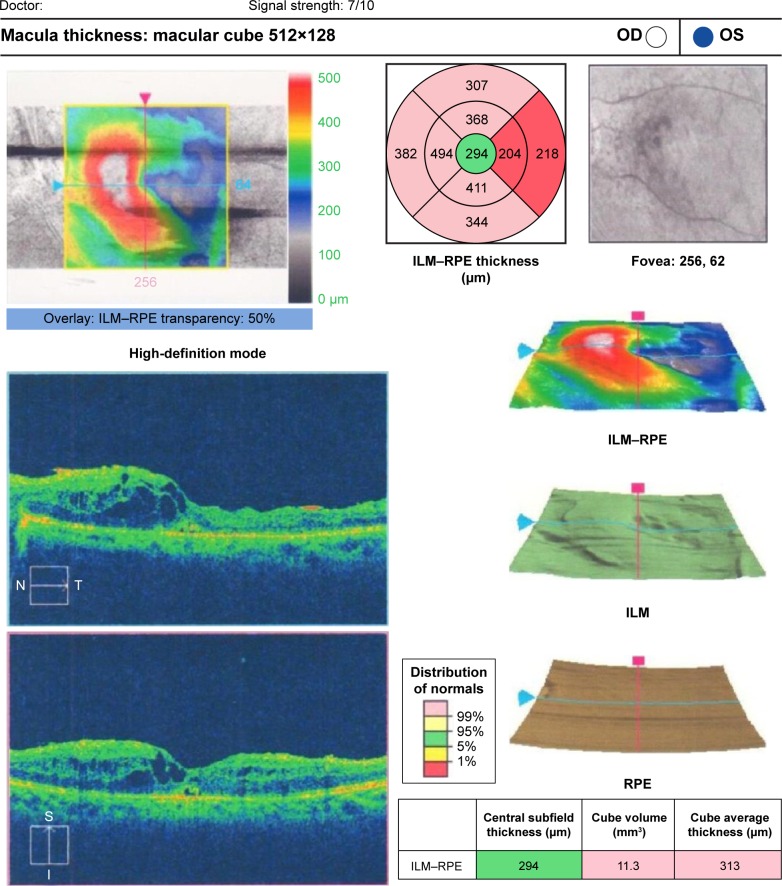
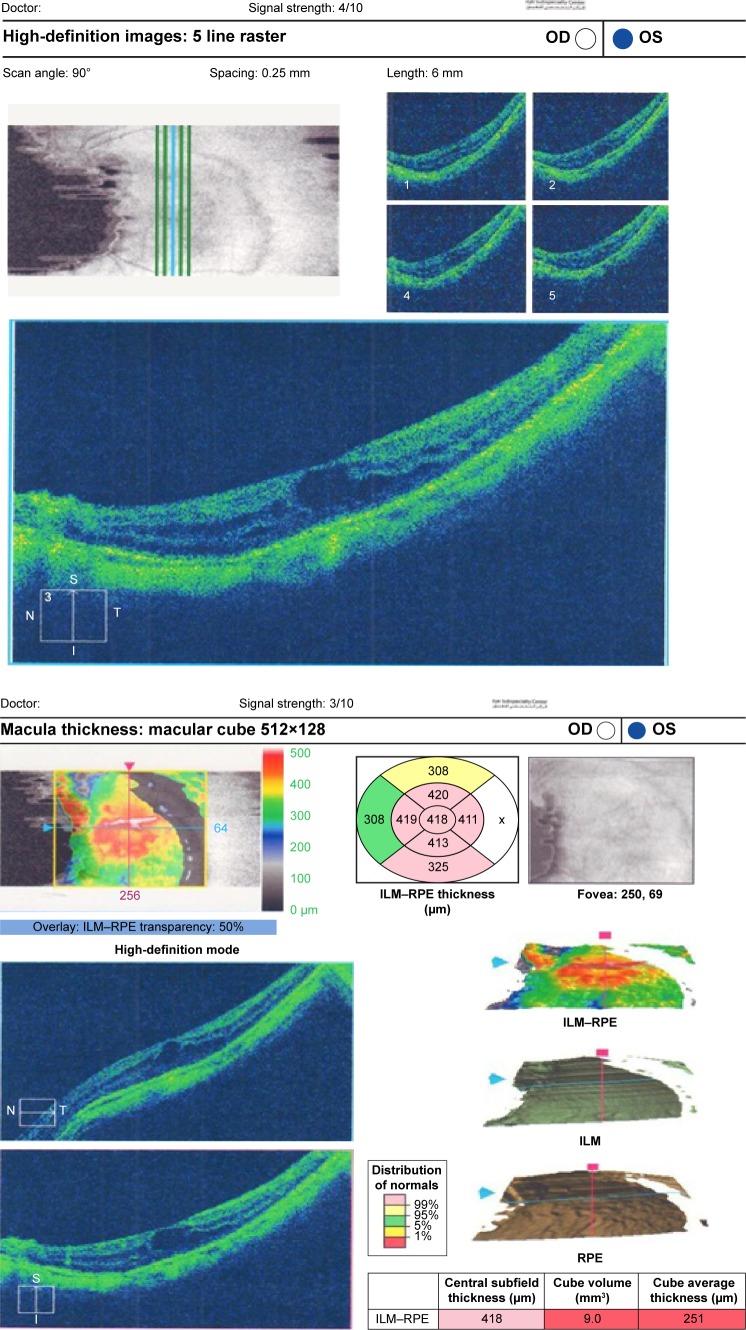
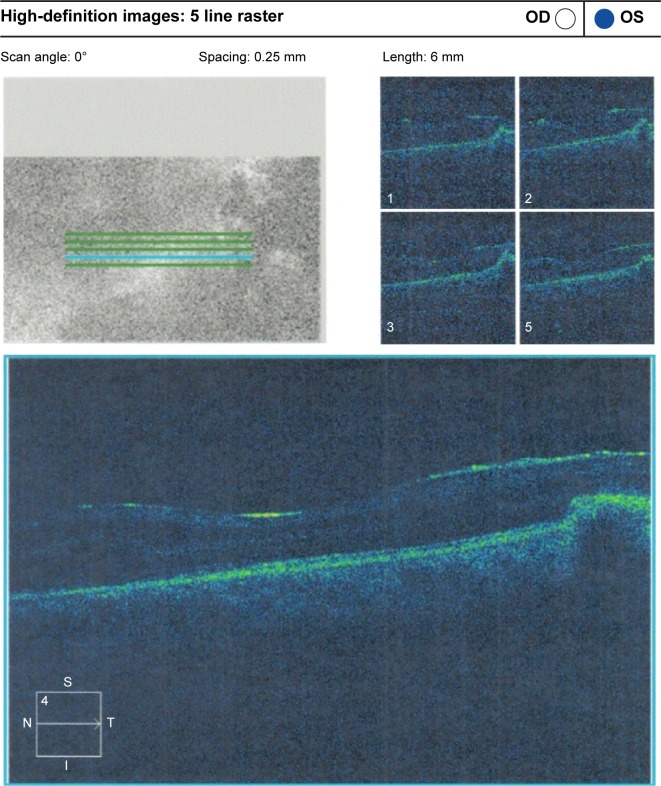
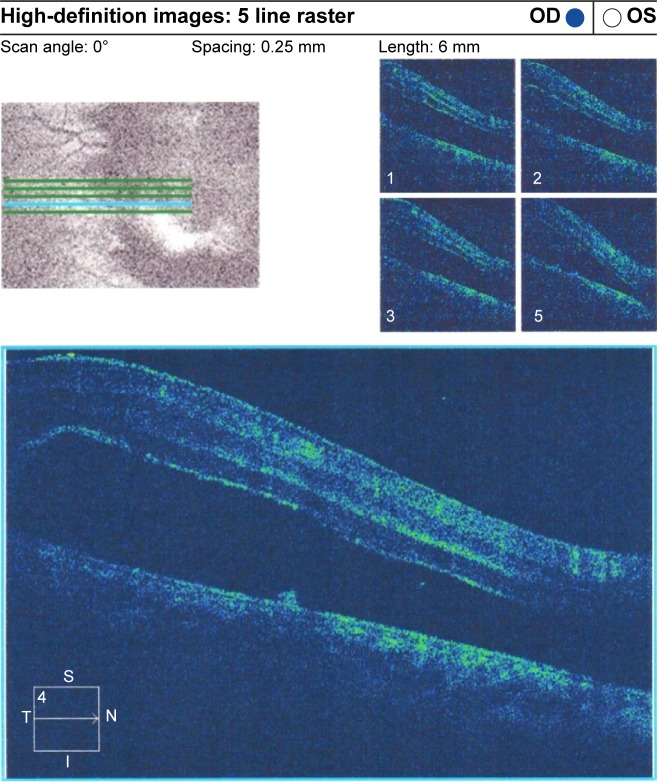
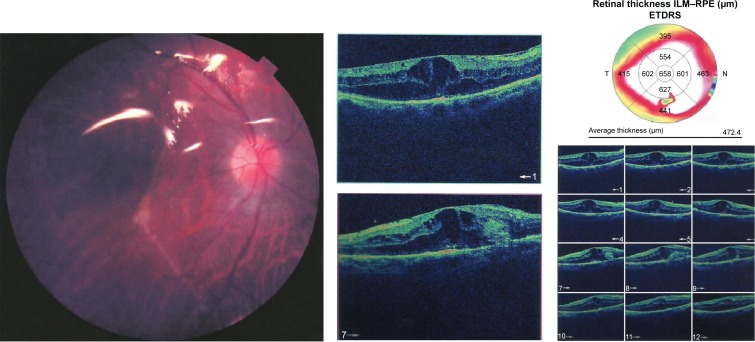
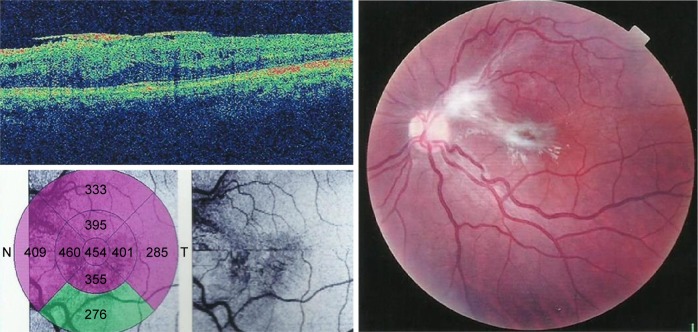
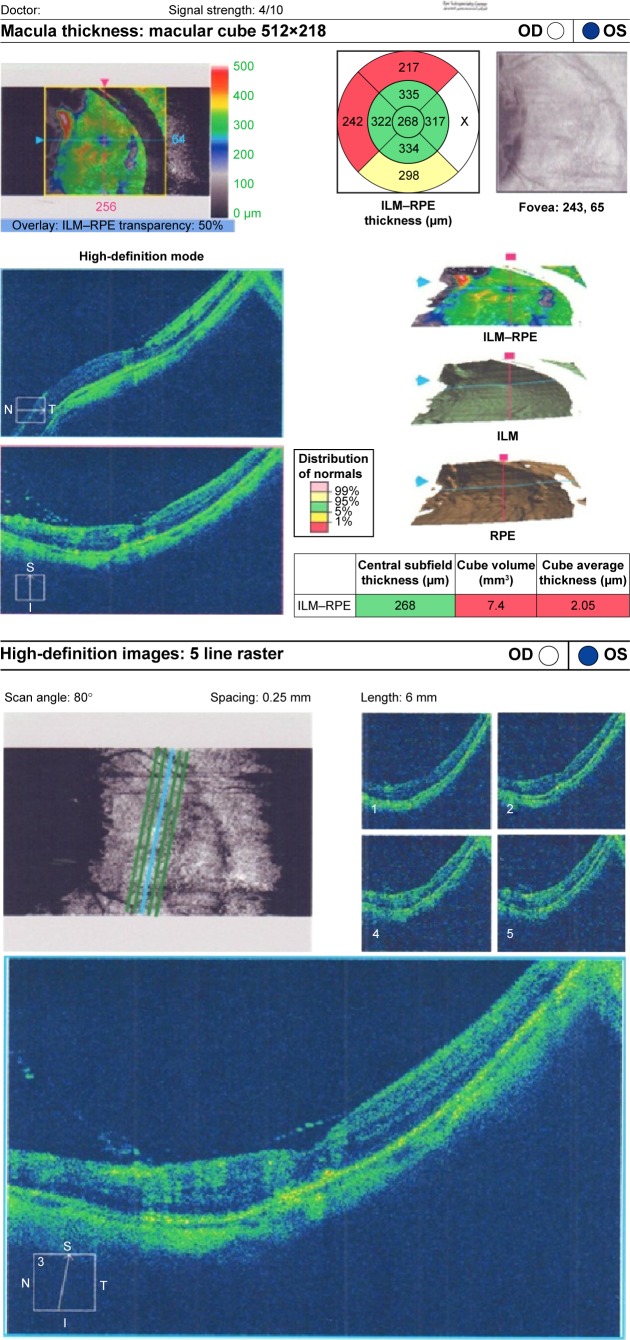
Preoperative OCT examination of the macula showed macular edema in 14 eyes (27.5%); this was diabetic macular edema (Figure 1) in seven eyes (13.7%) and cystoid macular edema (CME) in seven eyes (13.7%; Figure 2). CME was present in five out of fifteen (33.3%) of PVR eyes, one of GRT eyes, and one out of four uveitic eyes (25%). Diabetic macular edema was found seven out of twelve (58.3%) of PDR eyes. ERMs were detected by OCT in 21 eyes (41.2%); this included fine ERM in 13 eyes (25.5%; Figure 3) and ERM with traction in eight eyes (15.7%; Figure 4). Neurosensory RD was found in seven eyes (13.7%; Figure 5), macular thinning was found in seven eyes (13.7%; Figure 6), and macular holes were found in five eyes (9.8%; Figure 7). Emulsification of SiO at the macula was detected by OCT in two eyes (4%; Figure 8), and one eye (2%) had a subretinal band (Figure 9). Overall, abnormal OCT findings were present in 45 eyes (88.2%) and normal OCT findings were found in six eyes (11.8%; Figure 7; Table 2).
Figure 1.
Diabetic macular edema under SiO.
Abbreviations: SiO, silicone oil; OD, right; OS, left; ILM, internal limiting membrane; RPE, retinal pigmented epithelium; t, temporal; S, superior; I, inferior; N, nasal.
Figure 2.
(Top) CME under SiO in GRT. (Bottom) CME under SiO in GRT.
Abbreviations: CME, cystoid macular edema; SiO, silicone oil; GRT, giant retinal tear; OD, right; OS, left; ILM, internal limiting membrane; RPE, retinal pigmented epithelium; t, temporal; S, superior; I, inferior; N, nasal.
Figure 3.
ERM without traction.
Abbreviations: ERM, epiretinal membrane; OD, right; OS, left; t, temporal; S, superior; I, inferior; N, nasal.
Figure 4.
ERM with traction RD.
Abbreviations: ERM, epiretinal membrane; RD, retinal detachment; OD, right; OS, left; t, temporal; S, superior; I, inferior; N, nasal.
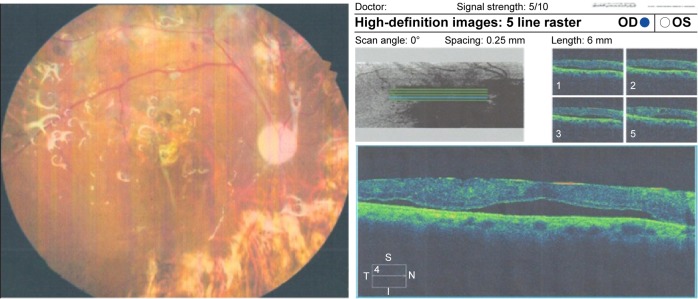
Figure 5.
Left fundus photo of a diabetic patient with PPV and SiO and OCT of the same patient with shallow macular detachment.
Notes: (Left) Colored fundus picture from a diabetic patient who underwent PPV with SiO injection. (Right) OCT raster lines of the same patient showing shallow RD and fine ERM.
Abbreviations: PPV, pars plana vitrectomy; SiO, silicone oil; OCT, optical coherence tomography; RD, retinal detachment; ERM, epiretinal membrane; OD, right, OS, left; t, temporal; S, superior; I, inferior; N, nasal.
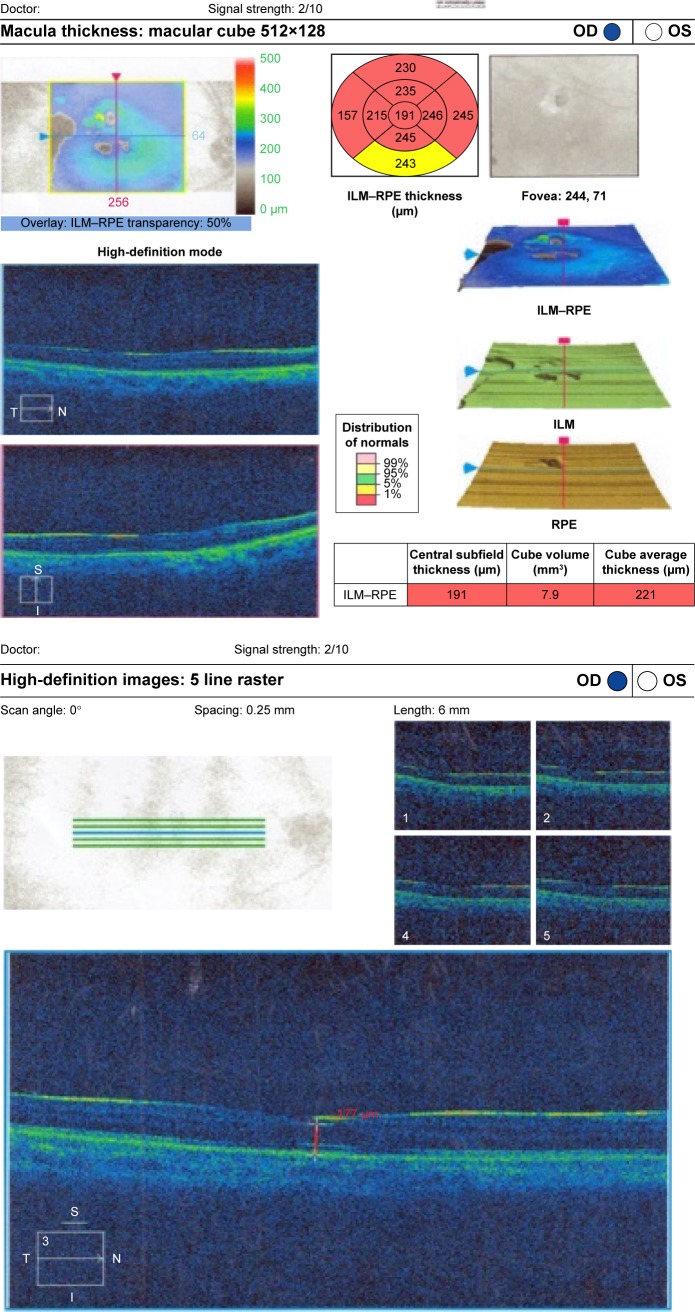
Figure 6.
(Top) Patient fast thickness map. (Bottom) Macular thinning in SiO-filled eye with normal fundus raster lines.
Abbreviations: SiO, silicone oil; OD, right; OS, left; t, temporal; S, superior; I, inferior; N, nasal; ILM, internal limiting membrane; RPE, retinal pigmented epithelium.
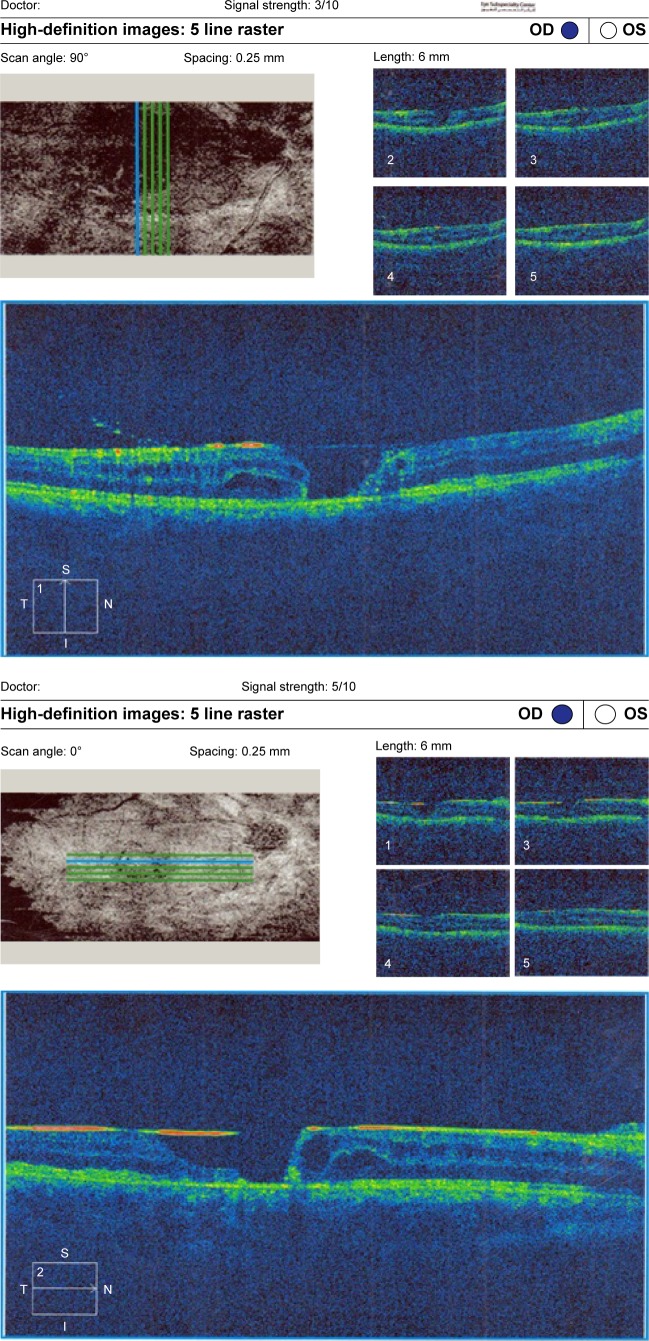
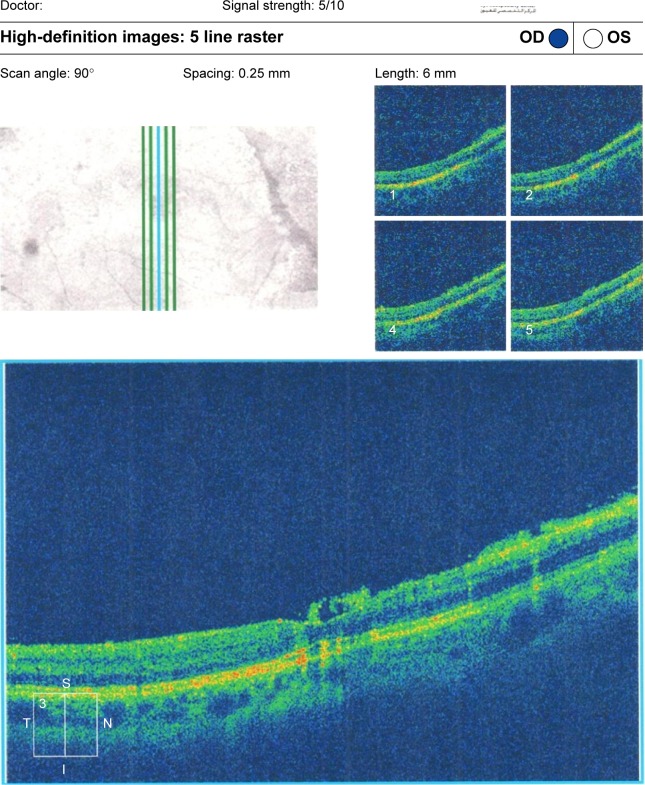
Figure 7.
(Top) Macular hole with macular edema and posterior silicone face. (Bottom) Another raster line of the same patient.
Abbreviations: OD, right; OS, left; t, temporal; S, superior; I, inferior; N, nasal.
Figure 8.
Raster OC with premacular emulsified SiO in an eye that underwent PPV for recurrent RD.
Abbreviations: OC, optical coherence; SiO, silicone oil; PPV, pars plana vitrectomy; RD, retinal detachment; OD, right; OS, left; t, temporal; S, superior; I, inferior; N, nasal.
Figure 9.
(Left) Colored fundus photograph of PVRC that underwent PPV. (Right) The same patient with ERM and a subretinal band.
Abbreviations: PVR, proliferative retinopathy; PPV, pars plana vitrectomy; ERM, epiretinal membrane; ETDRS, early treatment of diabetic patient study.
Table 2.
OCT findings before the removal of SiO
| OCT findings | Number | Percent |
|---|---|---|
| Macular edema | 14 | 27.5 |
| DME | 7 | 13.7 |
| CME | 7 | 13.7 |
| ERM | 21 | 41.2 |
| Fine | 13 | 25.5 |
| Traction | 8 | 15.7 |
| Neurosensory retinal detachment | 7 | 13.5 |
| Macular thinning | 7 | 13.5 |
| Macular hole | 5 | 13.5 |
| Subretinal band | 1 | 9.8 |
| Normal | 6 | 2 |
Abbreviations: OCT, optical coherence tomography; SiO, silicone oil; DME, diabetic macular edema; CME, cystoid macular edema; ERM, epiretinal membrane.
Clinically, normal macula were seen in 22 eyes (43%) before the removal of SiO. Preoperative OCT examination of these eyes with clinically normal macula was normal in only four eyes (18%).
Table 3 shows the abnormalities detected by preoperative OCT in those eyes with clinically normal macula.
Table 3.
Preoperative OCT abnormalities in eyes with clinically normal macula
| Findings | Number | Percent |
|---|---|---|
| Macular edema | 5 | 22.7 |
| Neurosensory retinal detachment | 3 | 13.6 |
| Macular thinning | 3 | 13.6 |
| Fine ERM | 2 | 9 |
| Emulsified silicon | 1 | 4.5 |
| Normal OCT | 4 | 18 |
Abbreviations: OCT, optical coherence tomography; ERM, epiretinal membrane.
OCT detected macular pathological changes occurred in 88.24% of all eyes. Clinical fundus examination detected fewer changes (56.86%). The difference in the detection ability was highly significant (P<0.001; Table 4; Figure 10).
Table 4.
Comparison between preoperative fundus examination and detection of OCT abnormalities
| OCT
|
Clinical fundus
|
Chi-square test
|
||||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | χ2 | P-value | |
| Normal | 6 | 11.76 | 22 | 43.14 | 11.076 | <0.001 |
| Abnormal | 45 | 88.24 | 29 | 56.66 | ||
Abbreviation: OCT, optical coherence tomography.
Figure 10.
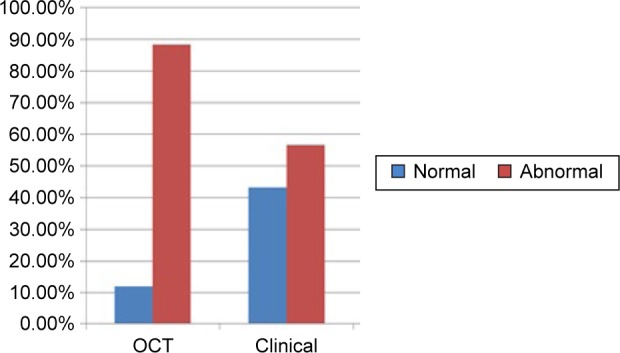
OCT detects more abnormalities in the macula than clinical examination.
Abbreviation: OCT, optical coherence tomography.
Mean logMAR after the removal of SiO was 0.35 (20/45 Snellen’s acuity) in eyes with preoperative normal OCT. It was 1.22 (20/380 Snellen’s acuity) in eyes with abnormal OCT before the removal of SiO. The difference was highly significant (P<0.001; Table 5; Figure 11).
Table 5.
Comparison of postoperative mean logMAR in eyes with preoperative normal and abnormal OCT
| LogMAR
|
Independent t-test
|
|||
|---|---|---|---|---|
| Mean | SD | t | P-value | |
| Normal | 0.35 | 0.22 | 5.866 | <0.001 |
| Abnormal | 1.28 | 0.37 | ||
Abbreviations: OCT, optical coherence tomography; SD, standard deviation.
Figure 11.
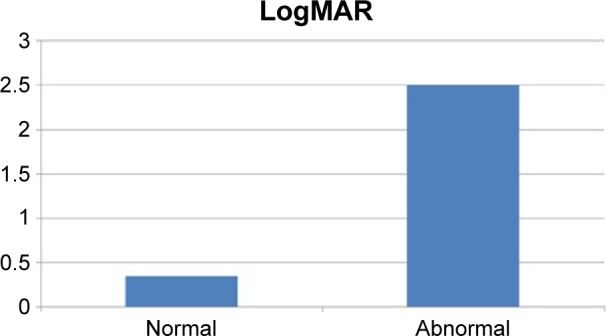
Mean logMAR after the removal of SiO in eyes with normal and abnormal preoperative OCT.
Abbreviations: SiO, silicone oil; OCT, optical coherence tomography.
OCT findings were less than expected from clinical examination in three of 51 eyes (5%; Figure 12). Surgical findings during the removal of SiO corresponded to preoperative OCT examination in 37 of 51 eyes (72.5%). Details of the surgical findings corresponding to preoperative OCT in each subgroup are described in Table 6.
Figure 12.
(Left) OCT of a patient with ERM with no tangential traction. (Right) Colored fundus photograph of the same patient with PVR filled with SiO with ERM with tangential traction.
Abbreviations: OCT, optical coherence tomography; ERM, epiretinal membrane; PVR, proliferative retinopathy; SiO, silicone oil.
Table 6.
Correspondence of intraoperative findings during the removal of SiO to preoperative OCT findings
| Surgical findings corresponding to OCT
|
||
|---|---|---|
| Number | Percent | |
| PVR | 15/15 | 100 |
| PDR | 5/12 | 41.6 |
| Trauma | 7/15 | 87.5 |
| GRT | 2/4 | 50 |
| Macular hole | 2/3 | 66.6 |
| Recurrent detachment | 3/5 | 60 |
| Uveitis | 3/4 | 75 |
| Total | 37/51 | 72.5 |
Abbreviations: SiO, silicone oil; OCT, optical coherence tomography; PVR, proliferative vitreoretinopathy; PDR, proliferative diabetic retinopathy; GRT, giant retinal tear.
Surgical plans during the removal of SiO were changed based on preoperative OCT findings in 38 of 51 eyes (74.5%). Details of the changes in surgical plans for PVR and PDR eyes are described in Table 7.
Table 7.
Surgical plan changes during the removal of SiO in PVR and PDR eyes based on preoperative OCT findings
| Number | Percent | ERM dissection | Pharmacotherapy | |
|---|---|---|---|---|
| PVR | 11/15 | 73.3 | 33.3% | 40% |
| PDR | 9/12 | 75 | 25% | 50% |
Abbreviations: SiO, silicone oil; PVR, proliferative vitreoretinopathy; PDR, proliferative diabetic retinopathy; OCT, optical coherence tomography; ERM, epiretinal membrane.
In trauma, GRT, macular holes, and recurrent detachment, the surgical plan was changed in 100% of eyes with abnormal OCT results.
Discussion
OCT operates through a mechanism similar to ultrasonography by replacing ultrasound waves with infrared waves.1 It provides higher resolution images than ultrasonography. This enables OCT to assess SiO and detailed macular pathology in eyes with clear media.11 We included eyes that needed SiO tamponade for 6–12 months because of their complicated RD. Our concern was the ability of OCT to detect macular pathological changes in the presence of SiO and its correlation to clinical and surgical findings. Maia et al12 studied the changes in silicone-filled eyes. They included fewer eyes (n=28), and details of some examples of the cases were described. However, the study concluded that systematic use of OCT was useful in recognizing retinal changes.
Avitabile et al13 found that the presence of SiO does not affect measurements of foveal thickness during OCT. They excluded eyes with macular holes and ERM and performed OCT only 3 months after SiO injection. They did not report the percentage of each macular abnormality. We compared the abnormal findings in the macula detected during clinical examination of the fundus to those detected by preoperative OCT. Numbers of pathological macular changes detected by OCT were highly significantly greater than those detected by a clinical fundus examination. No previous studies had compared preoperative OCT findings to clinical findings. Some of the findings detected by preoperative OCT examination could not be detected during surgery for the removal of SiO. This included macular edema in 27.5% of all eyes and macular atrophy in 13.5% of all eyes. Macular edema was the most common abnormality in all eyes (27.5%) and in eyes with clinically normal macula under SiO removal (22.7%). This means there is difficulty in clinical detection of macular edema in the presence of SiO. In PVR eyes 33% had CME and in PDR eyes 58.3% had DME. Theses findings raise the question about the value of pharmacotherapy under SiO. In our study we used pharmacotherapy in all eyes at the time of SiO removal.
We found that OCT examination before the removal of SiO helped to predict visual outcome. We found a highly significant difference in visual outcome in eyes with normal OCT compared with eyes with abnormal OCT before the removal of SiO (P<0.001). The difference in the visual outcome is explained by the fact that findings detected by OCT affect postoperative visual prognoses. Eyes with normal preoperative OCT had a better postoperative visual outcome. Accordingly, OCT examination can partially explain visual loss not related to cataractogenesis-associated SiO. Avitable et al13 similarly found that postoperative BCVA was significantly correlated to increased and decreased foveal thickness as assessed by OCT, compared to eyes with normal thickness. However, they measured BCVA in the presence of SiO, so it could have been affected by cataract or glaucoma. They only correlated BCVA to thickness, while we correlated BCVA to all OCT abnormalities. We noted that intraoperative findings during the removal of SiO were identical to preoperative OCT examination in 72.5% of all eyes. Surgical plans were changed from simple removal of SiO to the addition of other steps in 74.5% of all eyes according to preoperative OCT examination.
In this study, OCT identified treatable macular pathology in most of the eyes. These included macular edema treated by pharmacotherapy (Figure 13) in 13.5% and macular sensory neural detachment in 2%. Accordingly, implementation of OCT may help in making earlier decisions for the removal of SiO to manage underlying macular pathologies for the prevention of visual loss. Macular thinning was present in 13.5% of the eyes. This may be induced by affection of macular microcirculation due to SiO that was reported by a laser scanning study.14 Three eyes (6%) had highly elevated macula by OCT. We changed biometry according to the amount of subretinal fluid. In PVR eyes, 100% had surgical findings corresponding to OCT and the surgical decision was changed in 73%. Findings were related to PVR in 60% of the eyes (ERM in 40% and macular RD in 20%).
Figure 13.
(Top) Fast thickness map improvement of the patient with GRT after pharmacotherapy treatment of CME. (Bottom) Raster line of the same patient.
Abbreviations: GRT, giant retinal tear; CME, cystoid macular edema; OD, right; OS, left; t, temporal; S, superior; I, inferior; N, nasal; ILM, internal limiting membrane.
Martinez-Castillo et al15 reported ERM in 10% of the PVR eyes. This was different from our prevalence because this study was dependent on a fundus examination for detection of ERM and used OCT only to confirm clinical diagnosis of ERM. It also reported only eyes that needed surgical intervention to remove ERM; 15% of the eyes in our study required surgical removal. In recurrent RD eyes, 60% had surgical findings corresponding to OCT. This means that the attachment of the retina and SiO injection did not stop the process of PVR. In PDR, 41% of the eyes had surgical findings corresponding to OCT, while in 75% of the eyes, there was modification of the surgical plan including pharmacotherapy. This disparity occurred because macular edema is not detected during surgery. ERM was removed in 15% of the eyes.
Messmer et al16 reported the recurrence of ERMs as being significantly associated with poor vision following PPV for PDR and SiO injection. This study specifically focused on eyes with tractional RD. In eyes with penetrating trauma, 62% had ERM and 37% had RD. This was similar to the incidence of RD after PPV (41%) reported in a study for penetrating trauma for intraocular foreign bodies.22 The latter study did not report any cases of postoperative ERM. This is explained by our detection using OCT, while the comparative study17 did not use OCT. In GRT, 66% had surgical findings corresponding to OCT and related to the original disease. In uveitic eyes, OCT detected ERM in 75% and CME in 50%.
Another study18 reported postvitrectomy CME in 11% and RD in 7% of the eyes.18 They included a greater number of eyes (n=28). They did not report ERM in any eye. Again, they did not use OCT as a postoperative examination tool. Another study19 reported 13.7% RD and CME in uveitic eyes. They also included more eyes (n=51) but did not use OCT to examine the macula postoperatively. This may explain the underestimation of ERM and CME in both studies.
Conclusion
We suggest that performing noncontact OCT before the removal of SiO is important. It helps to predict visual outcome, which we found was related to cataract density and macular pathologies detected by OCT. It helps to change the surgical plan of the removal of SiO to manage treatable macular pathologies. It also helps to detect macular edema and thinning that could not be detected intraoperatively. This procedure should be performed routinely before the removal of SiO whenever the media allow. We recommend the removal of SiO earlier whenever possible before the development of dense cataracts that prevent OCT performance as a baseline care.
Limitations and flaws to our study
There was a wide spectrum of diagnoses before the use of an SiO tamponade, including PVR, PDR, recurrent RD, trauma, uveitis, and GRT. We concentrated on abnormalities under SiO rather than on the primary diagnosis.
A small number of eyes were studied in the uveitis and GRT groups.
Footnotes
Author contributions
MAR participated in the conception and design of the study, intervention, data collection and statistical analysis, manuscript drafting and revising, and editing and approval of the final manuscript. AAM participated in conception and design, data collection, editing and approval of the final manuscript. AIA participated in the data collection, statistical analyses, drafting the manuscript, and editing and approval of the final manuscript. All authors read and approved the final manuscript.
Disclosure
The authors report that they have no conflicts of interest in this work.
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cibis PA, Becker B, Okun E, Cowan S. The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol. 1962;681:590–599. doi: 10.1001/archopht.1962.00960030594005. [DOI] [PubMed] [Google Scholar]
- 3.Scott JD. The treatment of massive vitreous traction by the separation of pre-retinal membranes using liquid silicone oil. Mod Probl Ophthalmol. 1975;15:285–290. [PubMed] [Google Scholar]
- 4.Watzke RC. Silicone retinopiesis for retinal detachment: along-term clinical evaluation. Arch Ophthalmol. 1967;77:185–196. doi: 10.1001/archopht.1967.00980020187008. [DOI] [PubMed] [Google Scholar]
- 5.Cockerham WD, Schepens CL, Freeman HM. Silicone injection in retinal detachment. Arch Ophthalmol. 1970;83:704–712. doi: 10.1001/archopht.1970.00990030704006. [DOI] [PubMed] [Google Scholar]
- 6.Barr CC, Lai MY, Lean JS, et al. Postoperative intraocular pressure abnormalities in the Silicone Study: Silicone Study report 4. Ophthalmology. 1993;100:1629–1635. doi: 10.1016/s0161-6420(93)31425-9. [DOI] [PubMed] [Google Scholar]
- 7.Hutton WL, Azen SP, Blumenkranz MS, et al. The effects of silicone oil removal: Silicone Study report 6. Arch Ophthalmol. 1994;112:778–785. doi: 10.1001/archopht.1994.01090180076038. [DOI] [PubMed] [Google Scholar]
- 8.Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology. 1988;95:870–876. doi: 10.1016/s0161-6420(88)33080-0. [DOI] [PubMed] [Google Scholar]
- 9.Ober RR, Blanks JC, Ogden TE, Pickford M, Minckler DS, Ryan SJ. Experimental retinal tolerance to liquid silicone. Retina. 1983;3:77–85. doi: 10.1097/00006982-198300320-00002. [DOI] [PubMed] [Google Scholar]
- 10.Mastropasqua L, Carpineto P, Ciancaglini M, Falconio G, Harris A. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography in silicone oil-filled eyes. Ophthalmologica. 2001;215(2):91–96. doi: 10.1159/000050836. [DOI] [PubMed] [Google Scholar]
- 11.Satchi K, Patel CK. Posterior chamber compartments demonstrated by optical coherence tomography, in silicone-filled eyes, following macular hole surgery. Clin Experiment Ophthalmol. 2005;33:619–622. doi: 10.1111/j.1442-9071.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 12.Maia OO, Jr, Takahashi WY, Nakashima Y, Primiano Júnior HP, Takahashi BS, Nakashima AF. Optical coherence tomography macular study on eyes filled with silicone oil. Arq Bras Oftalmol. 2007;70(2):281–285. doi: 10.1590/s0004-27492007000200017. [DOI] [PubMed] [Google Scholar]
- 13.Avitabile T, Bonfiglio V, Sanfilippo M, Torrisi B, Reibaldi A. Correlation of optical coherence tomography pattern and visual recovery after vitrectomy with silicone oil for retinal detachment. Retina. 2006;26(8):917–921. doi: 10.1097/01.iae.0000250007.76155.74. [DOI] [PubMed] [Google Scholar]
- 14.Kubicka-Trzaska A, Kobylarz J, Romanowska-Dixon B. Macular microcirculation blood flow after pars plana vitrectomy with silicone oil tamponade. Klin Oczna. 2011;113(4–6):146–148. [PubMed] [Google Scholar]
- 15.Martinez-Castillo V, Boixedara A, Distefano L, Zapata M, Garcia-Arumi J. Epiretinal membrane after pars plana vitrectomy for primary pseudophakic or aphakic rhegmatogenous retinal detachment: incidence and outcome. Retina. 2012;32(7):1350–1355. doi: 10.1097/IAE.0b013e318242b965. [DOI] [PubMed] [Google Scholar]
- 16.Messmer E, Bronfeld N, Oehlschӧlger U, Heinrich T, Foester MH, Wessing A. Epiretinal membrane formation after pars plana vitrectomy in proliferative diabetic retinopathy. Klin Monbl Augenheilkld. 1992;200(4):207–272. doi: 10.1055/s-2008-1045750. [DOI] [PubMed] [Google Scholar]
- 17.Mahapatra SK, Rao NG. Visual outcome of pars plana vitrectomy with intraocular foreign body removed through sclerocorneal tunnel and sulcus-fixated intraocular lens implantation as a single procedure in cases of metallic intraocular foreign body with traumatized cataract. India J Ophthalmol. 2010;58(2):115–118. doi: 10.4103/0301-4738.60077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pion B, Valyi ZS, Jansseens X, et al. Vitrectomy in uveitis patients. Bull Soc Belge Opthalmol. 2013;322:55–61. [PubMed] [Google Scholar]
- 19.Bovey EH, Herbot CP. Vitrectomy in the management of Uveitis. Ocul Immunol Inflamm. 2000;8:285–291. doi: 10.1076/ocii.8.4.285.6456. [DOI] [PubMed] [Google Scholar]