Abstract
Purpose
To examine ocular growth in nonhuman primates (NHPs) from measurements on ex vivo eyes.
Methods
We obtained NHP eyes from animals that had been killed as part of other studies or because of health-related issues. Digital calipers were used to measure the horizontal, vertical, and anteroposterior globe diameters as well as corneal horizontal and vertical diameters of excised globes from 98 hamadryas baboons, 551 cynomolgus monkeys, and 112 rhesus monkeys, at ages ranging from 23 to 360 months. Isolated lens sagittal thickness and equatorial diameter were measured by shadowphotogrammetry. Wet and fixed dry weights were obtained for lenses.
Results
Nonhuman primate globe growth continues throughout life, slowing toward an asymptotic maximum. The final globe size scales with negative allometry to adult body size. Corneal growth ceases at around 20 months. Lens diameter increases but thickness decreases with increasing age. Nonhuman primate lens wet and dry weight accumulation is monophasic, continuing throughout life toward asymptotic maxima. The dry/wet weight ratio reaches a maximum of 0.33.
Conclusions
Nonhuman primate ocular globe and lens growth differ in several respects from those in humans. Although age-related losses of lens power and accommodative amplitude are similar, lens growth and properties are different indicating care should be taken in extrapolating NHP observations to the study of human accommodation.
Keywords: nonhuman primates, NHP, baboon, cynomolgus monkey, rhesus monkey, ocular biometry, aging, lens, globe, cornea
Nonhuman primates (NHPs), including baboons, cynomolgus and rhesus monkeys, have been used in numerous studies as models for human visual functions such as accommodation and its loss with increasing age.1–13 However, relatively little is known about the growth of the nonhuman primate eye and in particular the lens, which is a key component of the accommodative system. Limited data are available for rhesus monkeys and virtually none for baboons and cynomolgus monkeys.
Several studies on accommodation in the rhesus monkey have yielded data for in vivo lens dimensions and surface curvatures.3–7,10–13 Unfortunately, there is little agreement between them. Estimates of disaccommodated lens thickness vary from 3 to 4.5 mm at any age between 1 and 30 years, with no clearly discernible age-related trend in many of the reported data sets. Some data were interpreted as showing an increase with age: others as a decrease. Similar high variability was observed in a study on lens diameters.13 It is not obvious why there are such large variations, not only between investigators, but also within the same study. Some may reflect individual differences while others may be due to differences in measuring techniques. Consequently, it is not possible to state with certainty what the dimensions of different NHP lenses may be. In addition, it has not been established how the dimensions change with age, information which is critical to understanding presbyopia development. The variability and lack of certainty makes it very difficult to compare humans and other primates.
It has been pointed out previously that while in vivo observations can provide important information on the functioning of the lens in the ocular environment, they are less useful for determining biometric properties.14 Some of these would be influenced by the surrounding tissues, the accommodative state of the eye and any corrections applied for optical or sonic distortion during measurements. Age-related changes in lens properties can only be studied effectively with ex vivo tissues. There are potential sources of error with in vitro measurements, as well. These relate predominantly to postmortem changes in the lens resulting from inappropriate handling or prolonged storage of the tissue as observed with human15 and sheep16 lenses. Furthermore, postmortem changes due to inappropriate handling may also alter the dimensions of the globe and cornea.
As part of a study on isolated lens power, it was observed that lens diameter increases with increasing age while thickness decreases in baboons, cynomolgus and rhesus monkeys.17 This differs from humans where both diameter and thickness increase.14,18 However, most of the data were obtained from animals aged under 10 years, equivalent to humans younger than 30 to 40 years and, therefore, not particularly relevant to presbyopia development.
Over a large number of years, it has been possible to accumulate ex vivo data in our laboratory, albeit limited in some cases, on changes in NHP eye dimensions as a function of age. There appears little chance that significantly more can be collected. Ethical and financial considerations make it extremely difficult to obtain such information these days. Therefore, the currently available data are presented in the hope that they may be of value to others and/or stimulate further research in this area.
Materials and Methods
No animals were killed solely for this study. All tissues were byproducts from procedures performed elsewhere and for other purposes. Eyes from baboons (Papio hamadryas), rhesus (Macaca mulatta) and cynomolgus monkeys (Macaca fascicularis) were obtained through the division of veterinary resources at the University of Miami as part of a university-wide tissue-sharing protocol and were used in accordance with institutional animal care and use guidelines. The animals, which were used by the Diabetic Research Institute of the University of Miami Miller School of Medicine, were mainly pancreas donors plus a few which had to be euthanized because of uncontrollable diarrhea. All of the rhesus monkeys were from the Mannheimer Foundation and were of Indian origin. The cynomolgus monkeys were descended from a small founder population obtained from Mauritius. Fixed baboon eyes were also obtained from the Australian National Baboon Breeding Colony from animals which had died or were euthanized for health reasons. Sex, age, and body weight were recorded for most animals. All procedures were carried out in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approval for the project was obtained through the Animal Ethics Committees at the University of Miami (animal care and use committee protocols #01-228, 04-073, 07-089, 10-116, and 13-075) and the Princes Alexandra Hospital (Sydney South West Area Health Service Animal Welfare Committee Project 013C).
All measurements on fresh eyes were performed at the Ophthalmic Biophysics Center of the Bascom Palmer Eye Institute between 2001 and 2014 using the same procedures and the same instruments over the whole period. All optoelectromechanical instruments used were dedicated to this study and protected. These were routinely checked for calibration every 3 months by metrology-trained biomedical engineers. The instruments remained stable during that time and no recalibration was required. As described in detail previously,18,19 precision chrome alloy steel gauge balls (F.V. Fowler Co., Inc., Newton, MA, USA) were used to calibrate the calipers and shadowgraph. Edge detection was accurate to 12 μm and the precision was always better than ±0.01 mm.
All fresh NHP eyes were enucleated no later than 5 minutes post mortem and transported, on saline-soaked gauze, to the laboratory within 10 minutes. Globe IOP was checked by an ophthalmic surgeon and any perforated globes were discarded. Global horizontal, vertical, and anteroposterior diameters and corneal (white to white) horizontal and vertical dimensions were measured within 2 hours of death, before any other procedures and after removing surrounding fat and extraocular muscle stumps, using self-calibrating digital Vernier calipers (Absolute Digimatic Digital Calipers; Mitutoyo America Co., Aurora, IL, USA) as first described by Nakagawa et al.21 This instrument is more precise than Castroviejo calipers.19 It was not necessary to inflate the globes since none had collapsed or deflated. Care was taken to ensure proper orientation of the globe for the horizontal and vertical measurements. Lens dimensions were then determined within 4 hours using shadowphotogrammetry, as described below. When both eyes from an animal were obtained at the same time, the second eye was stored at 4°C in a sealed vial with a wet gauze to prevent dehydration, and lens dimensions were measured the following morning. Comparison of the fresh and stored tissues revealed no significant differences in lens dimensions or weights.
The posterior of the eye was removed by circumferential dissection and the lens was extracted with a lens spoon after freeing it from the zonular attachments with Vannas scissors. Lenses were visually assessed under an operation microscope equipped with a slit lamp illuminator (OMS300; Topcon, Tokyo, Japan) for the presence of opacities or precataractous changes. None were found. Central lens thickness and equatorial diameter were measured using shadowphotogrammetry.20 Lenses were then carefully blotted dry and weighed to the nearest 0.01 mg (PM400 electronic balance; Mettler-Toledo, Columbia, MD, USA) before being placed in 10% buffered formalin. After at least 1 week in the formalin, lenses were rinsed with water and then dried at 80°C until constant weight was achieved, usually after approximately 2 weeks.
The 79 baboon eyes collected in Australia were placed directly into 10% buffered formalin and, after a minimum of 2 months, the lens was removed through an incision in the posterior sclera and dried as before.
Not all parameters could be measured for each eye or lens. Where two eyes were available from any donor, data were averaged for all statistical evaluations. Any value more than 2.5 SD from the mean for that age, or its corresponding estimated value under the nonlinear regression analysis in cases of logistic model fitting (see later in the Methods section) was considered to be due to tissue damage and was eliminated from these calculations. This entailed no more than six values in any of the baboon and rhesus monkey data sets and 12 for the cynomolgus monkeys. All data are shown in the figures but those eliminated from the calculations are identified. Fourteen baboon lens wet weights were discarded because the ratio of dry weight/wet weight was more than 3 SD below the mean for that age, indicative of water uptake.15,16
All statistics were calculated using statistical software (XLStat for Excel, version 2014, 4.05; Addinsoft, New York, NY, USA) add-in and macros developed in a programming environment (Visual Basic for Applications in Excel 2013; Microsoft Corp., Redmond, WA, USA). Sex differences in globe and corneal dimensions were compared using the 2-tailed t-test and the Wilcoxon Mann-Whitney U test when the distribution was not normal. For the nonparametric analyses, the P values were computed using a distribution-free Monte Carlo method with 10,000 simulations.
The effects of age and sex on the various ocular dimensions and lens weights were examined under the two-parameter logistic model
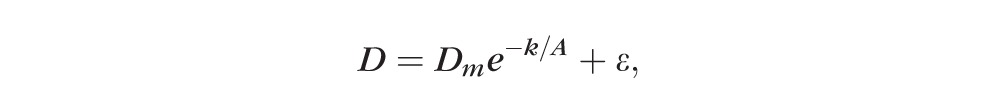 |
where D is the dimension or weight, Dm is the maximum (asymptotic) dimension or weight, A is the age since conception, k is the growth constant and ε the error (residual) term. Data were fitted to the model (without logarithmic transformation) using a nonlinear regression technique to minimize the sum of square of residual. Nonlinear regression was implemented with Solver in a spreadsheet program (Excel; Microsoft Corp.), setting Dm and k as variables using the generalized reduced gradient method for solution (precision and convergence set to 10−6 and 10−5, respectively). Data are presented in some figures according to the transformed logistic equation
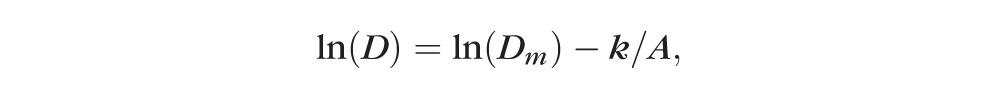 |
since this better illustrates the continued growth. For all analyses, the probability of a Type I error was well below 0.01. Data were also tested against a model based on the two-parameter logistic equation with an added linear component of slope k2 to identify possible nonasymptotic continuous growth:
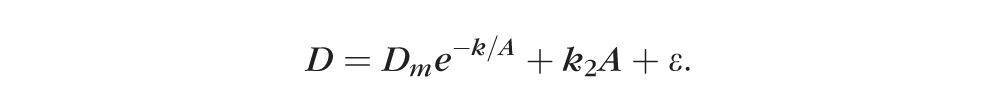 |
Adult body weights and life spans were obtained from the listing of Grzimek.22
Results
Globe
External dimensions of the globe and cornea were obtained for 98 baboon, 112 rhesus and 551 cynomolgus monkey eyes. It was not possible to collect tissues covering the whole of the animals' possible life spans but enough were obtained to allow comparisons and reasonable conclusions. Postnatal ages ranged from 20 to 360 months for the baboons, 17 to 196 months for the cynomolgus monkeys and 9 to 210 months for the rhesus monkeys. These represent ∼70%, 45%, and 55%, respectively, of their possible lifespans. Since ocular growth commences before birth, the age since conception has been used throughout.
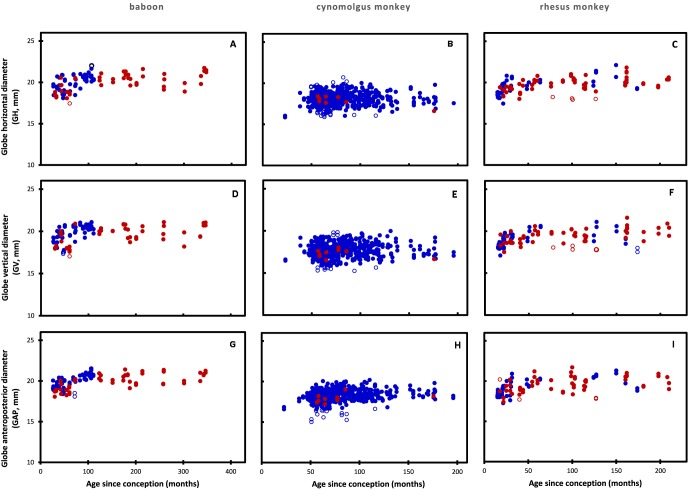
The horizontal (GH), vertical (GV) and anteroposterior (GAP) diameters for the globe as a function of age are presented in Figure 1.
Figure 1.
Globe horizontal, GV, and GAP diameters as a function of age in (A, D, G) baboons, (B, E, H) cynomolgus (C, F, I) and rhesus monkeys. Solid blue circles: males. Solid red circles: females. Open circles: outlier data not used in the statistical analyses.
There were no differences between males and females in baboon GH (P = 0.72), GV (P = 0.46), and GAP (P = 0.47), as well as rhesus monkey GH (P = 0.08) and GAP (P = 0.19). There appeared to be a statistically significant difference for the rhesus monkey GV (P = 0.033). Insufficient female data, with the same age distribution as for the males (17 female, 468 male), were available for assessment of cynomolgus dimensions. Male and female globe data were combined for further analyses.
Although the increase is small, the data suggest that globe dimensions continue to increase throughout the period examined. This was explored by fitting the data to a two-parameter logistic growth curve using nonlinear regression. Reasonable logistic fits (R2 = 0.43–0.55) were obtained with the baboon and rhesus monkey data for each dimension, indicating that globe dimensions continue to increase throughout adult life toward an asymptotic maximum. It was not possible to be certain about cynomolgus monkey dimensions since the logistic fits were poor (R2 = < 0.1).
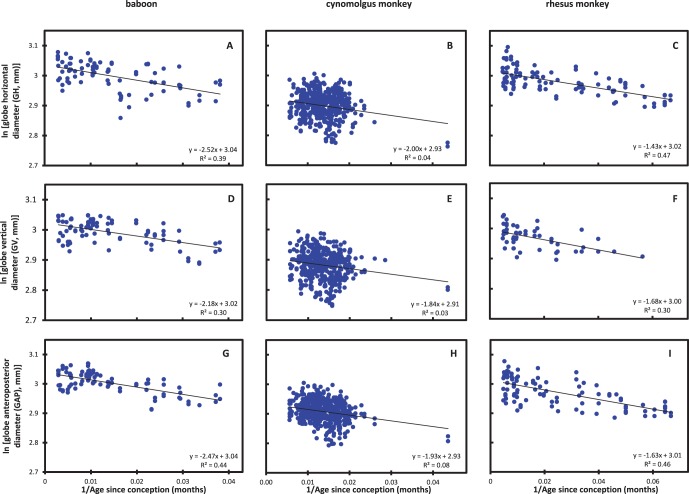
The data are plotted according to the transformed form of the logistic equation in Figure 2.
Figure 2.
Logistic analysis of the changes in GH, GV, and GAP diameters with age in (A, D, G) baboons, (B, E, H) cynomolgus and (C, F, I) rhesus monkeys. Male and female data are combined. Open circles: data excluded from the analyses.
The negative slopes of the straight lines of best fit indicate that growth is self-limiting continuing throughout life toward an asymptotic maximum. The abscissa intercepts correspond to the asymptotes in Figure 1. The maxima are summarized in the Table. Corresponding data for humans, taken from the study by Augusteyn et al.,23 have been included for comparative purposes.
Table.
Globe and Cornea Dimensions
The maximum horizontal globe diameters and anteroposterior lengths are very similar in each of the three nonhuman primates. However, the vertical diameters are significantly lower (GV/GH = 0.98; P < 0.001 by both 2-tailed, paired t-test and Wilcoxon signed-rank test) for all species, indicative of a slight flattening of the globe. This differs from the human globe where the three dimensions are not statistically significantly different from each other.23
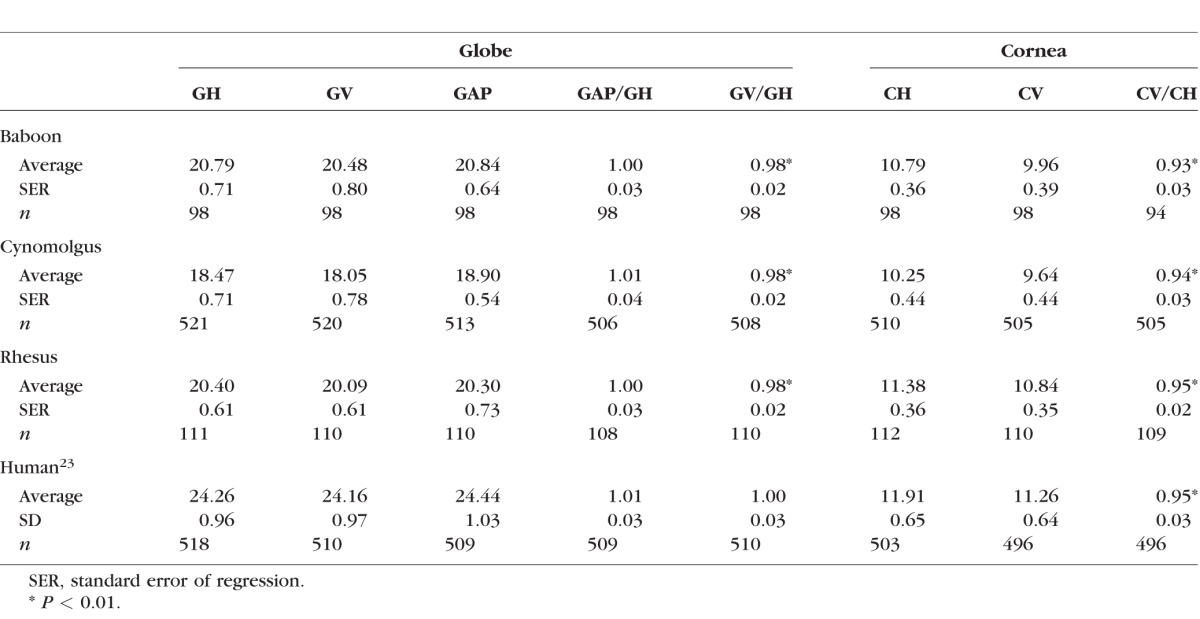
Allometric phylogenetic analysis (Fig. 3) indicates that primate adult eye volume, calculated from the dimensions in the Table, scales with negative allometry relative to male adult body weight with an exponent of 0.33 (R2 = 0.96). An exponent of 0.104 (R2 = 0.96) was determined for the allometric relationship between each of the three globe diameters and body weight.
Figure 3.
Allometric analysis of the relationship between maximum globe volume and male adult body weight. Globe volume was calculated from the maximum dimensions in the Table.
Cornea
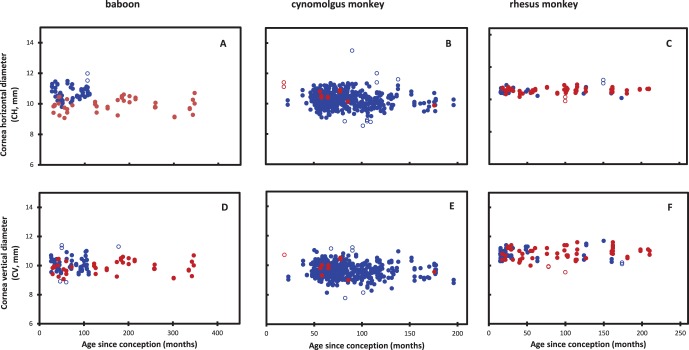
The nonhuman primate corneal horizontal (CH) and vertical (CV) diameters are presented in Figure 4.
Figure 4.
Corneal horizontal and CV diameters (white to white) as a function of age in (A, D) baboons, (B, E) cynomolgus and (C, F) rhesus monkeys. Solid blue circles: males. Solid red circles: females. Open circles: Outlier data not used in the statistical analyses.
No differences were observed between males and females in baboon CH (P = 0.23) and CV (P = 0.25) and in rhesus monkey CH (P = 0.87) and CV (P = 0.93). Insufficient female data were available for assessment of the cynomolgus corneal dimensions.
Corneal dimensions did not increase continuously with age. Corneal horizontal and vertical diameters reach maximum values sometime near postnatal 20 months. This was confirmed with logistic analysis which yielded plots with zero slopes (not shown) after this age. The average adult dimensions are listed in the Table. Similar CH values were obtained by Kaufman et al.24 from in vivo MRI measurements on eight adolescent and young adult monkeys.
Corneal horizontal and vertical diameters are significantly different (P < 0.0001) in all three NHPs. To verify that this was not an artefact of the handling procedures, several cynomolgus monkeys were examined prior to enucleation of the eye. The corneal vertical and horizontal ratio of 0.93 to 0.95 is similar to that observed with human corneas.23
Corneal dimensions scale with negative allometry relative to head diameter with an exponent of around 0.2 (R2 = 0.62) for the baboon, cynomolgus monkey, and humans. However, the rhesus monkey corneal dimensions were over 10% larger than what might be expected from the allometric relationship.
Lens
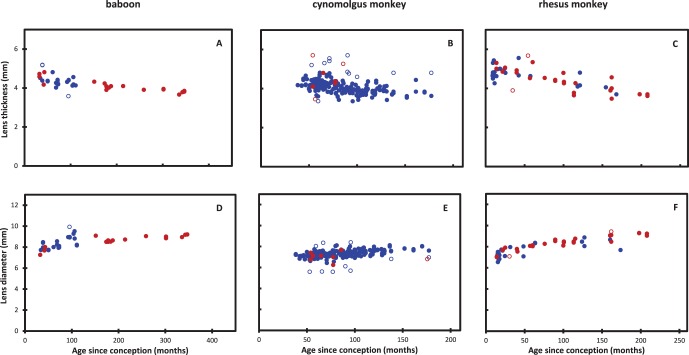
Dimensions were determined for 41 baboon, 294 cynomolgus and 58 rhesus monkey lenses. They are presented in Figure 5.
Figure 5.
Lens thickness and diameter as a function of age in (A, D) baboons, (B, E) cynomolgus and (C, F) rhesus monkeys. Solid blue circles: males. Solid red circles: females. Open circles: outlier data not used in the statistical analyses.
Male and female lens dimensions are the same for rhesus monkeys (D and T; P = 0.97 and 0.65, respectively). This differs from the report by Fernandes et al.7 that males have thicker lenses than females in vivo (by a fixed 0.1017 mm, at all ages). It was not possible to compare baboon lens dimensions because of differences in the age distributions, with all male data obtained from animals younger than 105 months while most females were older than this (Figs. 5A, 5D). However, logistic analysis of the male and female data (not shown) indicated that they could be described by the same equation. Insufficient female data were available for the cynomolgus monkeys to permit comparison.
Lens diameter increases continuously with age in all three species (Figs. 5A–C). The maximum values observed for the diameters range from around 8.2 mm for cynomolgus monkeys to 9.2 for rhesus monkeys and baboons. Logistic analysis (not shown) suggested asymptotic values would be 0.1 to 0.2 mm higher. By contrast, lens thickness decreases (Figs. 5D–F). Consequently, the aspect ratio (T/D) decreases throughout life (Fig. 6). The ratios in the three NHPs are similar and range from a high near 0.8 early in postnatal life (2-month-old rhesus monkey) to around 0.4 late in adulthood (340-month-old baboon).
Figure 6.
Lens aspect ratio (T/D) for baboons (solid blue circles), cynomolgus (solid green circles) and rhesus monkeys (solid red circles). Male and female lens data have been combined.
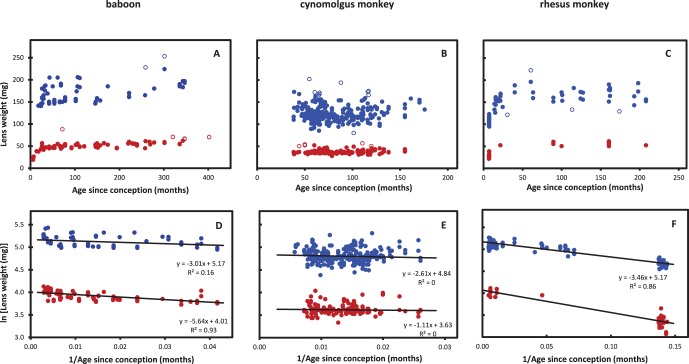
Wet and/or dry weights were obtained for many of the lenses (114 wet and 115 dry from baboons, aged 0 to 396 months; 271 wet and 161 dry from 60- to 176-month-old cynomolgus monkeys; 128 wet and 129 dry from rhesus monkeys, aged 1–208 months). No differences were found between male and female lenses so the data were combined. These are presented in Figures 7A through 7C together with logistic analyses (Figs. 7D–F). Wet weight data were more variable than the dry weights, presumably reflecting water uptake during storage and handling.15,16 When lenses had obviously taken up water, as indicated by low dry/wet weight ratios (<0.20) the wet weights were not used in the analyses. Dry weights were unaffected by prior water uptake. Because of the cubic relationship between dimensions and volume/weight, moderate water uptake (<20% of weight) had relatively little effect (average of 6%) on lens dimensions.
Figure 7.
Lens wet (solid blue circles) and dry (solid red circles) weights as a function of age in (A) baboons, (B) cynomolgus and (C) rhesus monkeys, and logistic analyses of these data in (D–F). Open circles: outlier data not used in the statistical analyses.
The lens weight data for the baboon and rhesus monkey (Figs. 7A, 7C) clearly indicate an age-dependent increase but those for the cynomolgus monkey are somewhat equivocal because of the relatively narrow age range over which data could be collected. Logistic analyses (Figs. 7D–F) of the baboon and rhesus data yield reasonably linear plots with negative slopes consistent with asymptotic growth over the whole of the available age range.25 The fits of the cynomolgus data are too poor (R2 = 0.01–0.03) to allow any conclusions. The logistic growth constants (slopes) for the baboon and rhesus monkey lens weights are very similar, ranging from 3.01 to 3.35 months for wet weight and 5.07 to 5.64 months for dry weight. The higher growth constant for dry weight accumulation compared with that for wet weight, indicates compaction is taking place so the average concentration of dry matter increases with age. The ratio of dry weight to wet weight in the youngest lenses available is 0.25. In middle age (150–250 months) the ratio is 0.32 to 0.33.
Discussion
One of the motivations for the present study was to acquire NHP data in an ex vivo regime to avoid many of the sources of error inherent with in vivo measurements. While ex vivo measurements carry their own potential sources of error, their predominant causes, inappropriate tissue handling or storage, typically result in substantial alterations in the tissue properties, especially those of the lens. We have attempted to eliminate, or at least minimize, errors due to such alterations by substantially reducing handling and storage of the eyes and by removing outliers (beyond 2.5 × SD) from the analyses.
The data presented in this communication indicate that qualitatively, the changes with age in the ocular parameters of the three commonly studied nonhuman primates are very similar but that there are substantial differences from humans.
The nonhuman primate globe continues growing throughout life toward the asymptotic maximum, differing from the human globe which, from ex vivo measurements, has been reported to stop growing around 1 year of age.23 This early stop appears to be at odds with a generally accepted view that human globe growth can continue until as late as age 13.26–31 However, this view is based on measurements of the internal axial length which do not represent growth of the globe. Rather, as discussed previously,23 they show that the retina, choroid, and outer coat become thinner with age, as indicated by the increase in vitreous depth.31
At 1 year, the NHP globe dimensions are more than 2 mm below their maximum sizes and even after sexual maturation (4–5 years) the dimensions are still 0.5 mm below the asymptotic maxima. This previously unrecognized growth could complicate studies on myopic changes in globe dimensions and/or axial length. This finding emphasizes that studies directed to understanding myopia progression must take into account the refractive contribution of multiple ocular components and their dimensional changes. Nonhuman primate globes are also slightly flattened whereas human globes are not.23,32
Globe dimensions scale with body weight with an exponent of 0.104. A similar value of 0.117 was obtained by Howland et al.33 using the axial lengths from 11 primate species. Globe dimensions also scale with exponents of around 0.33 relative to head diameter. Thus, the globes are relatively larger for animals with a smaller head or body. Because of sexual dimorphism in body weight and head size, female eyes may appear to be larger than those of males of the same age even though they are the same size.
Based on the present observations, it would appear that lens growth in the adult nonhuman primate is quite different from that in adult humans. As found for humans,14,18 isolated lens diameter increases with age in all three NHP species. Around the middle of the expected life span, lens diameter in the baboon, cynomolgus and rhesus monkey increases at approximately 0.040, 0.025, and 0.045 mm/y, respectively. These rates are considerably faster than the 0.013 mm/y for human lenses between ages 40 and 60 years.18
More notable is the observation that isolated lens thickness decreases continuously in all three NHPs, from around 5 mm early in life to 3.6 to 3.8 mm in the oldest animals examined (Fig. 5). A decrease in lens thickness with increasing age or body weight has also been observed in vivo for cynomolgus monkeys,9,24 rhesus monkeys,1–7,10–13 and marmosets.34 Qiao-Grider et al.12 found that disaccommodated rhesus monkey lens thickness increased from birth until around 15 months. In marmosets, there is an increase up to around 80 days after birth.34 These early increases probably correspond to the final stages of the prenatal growth phase, similar to that described for humans.14 Thereafter, thickness decreases, as is the case with the human lens until the late teens.14 It has been reported that, sometime between ages 4 and 12 years, in vivo NHP lens thickness starts increasing again.1–7,10–13 However, the in vivo data are highly scattered and there is little agreement between the actual values reported by different investigators for the dimensions. No indication of an increase in lens thickness, at any age, is evident in the ex vivo data reported here.
It seems possible that some of the in vivo thickness measurements may have been influenced by the conditions under which the measurements were made and these may vary with age. Most measurements were made under cycloplegia or with the eye in a relaxed condition, generally thought to represent the disaccommodated state. Therefore, they cannot be directly compared with the in vitro data which represent maximum accommodation. Furthermore, it is possible that the in vivo lenses were not completely disaccommodated. In humans, the relaxed condition is actually around 1.6 diopter (D) of accommodation and this may vary between individuals.35
Because of the opposing trends in the growth of diameter and thickness, the isolated lens aspect ratio (T/D) decreases for all three NHPs from values near 0.8 early in life to around 0.4 by the end of the age range available (Fig. 6). Thus, the NHP lens becomes flatter with increasing age. The shape of the young NHP lens is very similar to that of the young human lens and the decrease in the ratio is very similar to that observed with human lenses, both in vivo and in vitro, during the lens remodeling, which takes place between birth and the late teens, when the human ratio drops from well over 0.6 to 0.4.14,18 However, there is a transition in humans at around 18 to 20 years where the lens thickness starts to increase again and continues to do so for the rest of life. No such transition is apparent at any age in the ex vivo NHP data and the lens continues to flatten.
Lens weight accumulation in the NHPs yields linear logistic relationships indicating that growth takes place continuously throughout life toward an asymptotic maximum, as has been found for 126 diverse species.25 The human lens differs in that it exhibits biphasic growth in weight accumulation, manifest as logistic plots comprising a linear portion, reflecting self-limiting growth at high 1/age (ages under 12 months) and upwards curvature, indicative of linear growth at low 1/age (ages > 24 months).14 Different crystallin populations are produced in these two growth modes.36,37 No suggestion of upward curvature is apparent at any age in the NHP logistic plots (Fig. 7) and there appears to be little or no difference between the protein distributions in the cortex and nucleus of the rhesus monkey lens,38,39 consistent with a single growth mode.
Since the growth constant for dry weight accumulation is greater than that for wet weight, the average concentration of dry matter and, consequently, the refractive index increase with age. The 0.25 ratio of dry weight to wet weight for the youngest lenses available is the same as that observed with young human lenses14 and, together with the similarity in shape, is consistent with the similar contributions of the refractive index gradient (GRIN) to lens power (34 D for monkeys15 and 31 D for humans40). The ratios of NHP reach 0.32 to 0.33 in middle age but do not increase significantly thereafter because of the asymptotic slowing in growth. In contrast, because of the continuing linear growth in adulthood, the ratio for human lenses increases to around 0.36 at ages 40 to 60 years and to >0.38 after 80 years.14 It should be noted that these ratios represent the average for the whole lens. The ratio in the nucleus would be higher if the monkey lens had a substantial refractive index gradient, as has been demonstrated for humans and other species.41,42
Human and NHP lenses exhibit similar power losses over their life spans due to a combination of changes in lens shape (−7 D in humans and −15 D in monkeys) and changes in the GRIN (−22 D in humans and −19 D in monkeys).15,40 However, the patterns are different. In the three NHP species examined in this study lens power drops continuously up to at least age 28, close to the normal life span, whereas human lens power decreases until around age 55 when it apparently starts to increase again.40
Despite the lower concentration of dry matter, the contribution of the GRIN to lens power in middle age is higher in monkeys (20–25 D)15 than in humans (10–15 D).40 This implies there are substantial differences in the GRIN. In addition, 19- to 20-year-old rhesus monkey lenses are still capable of a ∼0.5-mm thickness change and have an accommodative amplitude of 6 to 8 D,13 whereas the change in 55- to 60-year-old human lenses is <0.2 mm and accommodative amplitude is close to zero.43 This would suggest that presbyopia development may be slower in rhesus monkeys than in humans despite the similar decrease in power. However, by age 28 to 30 years, baboon and rhesus lenses no longer respond to ex vivo stretching forces and the in vivo accommodative amplitude is zero.1,44
Overall, it would appear that there are previously unrecognized potential difficulties in using NHPs as models for examining age related changes in visual performance.
Loss of accommodative ability appears to be due to changes in the lens and the power changes are very similar in humans and NHPs. However, the underlying causes of these changes may differ because of differences in the way in which the lens grows. Human lens growth is biphasic, generating two distinct compartments, the nucleus, which is generated during prenatal growth, and the cortex produced after birth. Nonhuman primate lens growth is monophasic. Human lenses become thicker and rounder with increasing age while NHP lenses become thinner and flatter. In addition, the average concentration of dry matter in the human lens is higher than that in NHPs and continues to increase with age while that in the NHPs virtually remains constant. Yet, the NHP lens has the greater power. This would suggest that the refractive properties are quite different. In view of these differences, caution is required when extrapolating observations from NHPs to understanding human vision. Further work is required to understand these differences.
Acknowledgments
This work would not have been possible without the interest and support of our colleague Professor Brien Holden, who was lost to vision science in July 2015. Apart from being a global leader in vision care and vision research, Brien was a humanitarian who did much to promote eye health care throughout the world and especially among the poor.
The authors thank Norma Kenyon and Dora Berman-Weinberg of the Diabetes Research Institute, Julia Zaias and James Geary of the Division of Veterinary Research at the University of Miami, and Scott Heffernan of the Australian National Baboon Breeding Colony for their assistance in obtaining the primate eyes. We thank the ophthalmic surgeons (Christian Billotte, Viviana Fernandez, Joseph Stoiber, Ana Carolina Acosta, Hideo Yamamoto, Mohamed Gamil Metawalli Aly, and Esdras Arrieta) and the staff and students (Eleut Hernandez, David Denham, Alexandre Rosen, Noel Ziebarth, David Borja, Mariela Aguilar, Andres Bernal, Derek Nankivil, Heather Durkee, and Shawn Kelly) of the Ophthalmic Biophysics Center who gave surgical and technical support during this study.
Supported in part by NIH Research Grants R01EY014225, R01 EY021834, F31EY021444 (NRSA Individual Predoctoral Fellowship [BMH]); Center Grant P30EY14801; the Australian Federal Government CRC Scheme through the Vision Cooperative Research Centre; the Florida Lions Eye Bank; unrestricted funds from Research to Prevent Blindness and from Karl R. Olsen and Martha E Hildebrandt; and the Henri and Flore Lesieur Foundation (JMP).
Disclosure: R.C. Augusteyn, None; B. Maceo Heilman, None; A. Ho, None; J.-M. Parel, None
References
- 1. Bito Z,, De Rousseau CJ,, Kaufman PL,, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982; 23: 23–31. [PubMed] [Google Scholar]
- 2. Kaufman PL,, Bito LZ,, DeRousseau CJ. The development of presbyopia in primates. Trans Ophthalmol Soc U K. 1982; 102: 323–326. [PubMed] [Google Scholar]
- 3. Bito LZ,, Kaufman PL,, DeRousseau CJ,, Koretz J. Presbyopia: An animal model and experimental approaches for the study of the mechanism of accommodation and ocular ageing. Eye. 1987; 1: 222–230. [DOI] [PubMed] [Google Scholar]
- 4. Bradley DV,, Fernandes A,, Lynn M,, Tigges M,, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999; 40: 214–229. [PubMed] [Google Scholar]
- 5. Denlinger JL,, Eisner G,, Balazs EA. Age-related changes in the vitreus and lens of rhesus monkeys (Macaca mulatta). Exp Eye Res. 1980; 31: 67–79. [DOI] [PubMed] [Google Scholar]
- 6. De Rousseau CJ,, Bito LZ. Intraocular pressure of rhesus monkeys (Macaca mulatta) II Juvenile ocular hypertension and its apparent relationship to ocular growth. Exp Eye Res. 1981; 32: 407–417. [DOI] [PubMed] [Google Scholar]
- 7. Fernandes A,, Bradley DV,, Tigges M,, Tigges J,, Herndon JG. Ocular measurements throughout the adult life span of rhesus monkeys. Invest Ophthalmol Vis Sci. 2003; 44: 2373–2380. [DOI] [PubMed] [Google Scholar]
- 8. Huang J,, Hung L-F,, Ramamirtham R,, et al. Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2009; 50: 4033–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiely PM,, Crewther SG,, Nathan J,, Brennan NA,, Efron N,, Madigan MA. Comparison of ocular development of the cynomolgus monkey and man. Clin Vis Sci. 1987; 1: 269–280. [Google Scholar]
- 10. Koretz JF,, Neider MW,, Kaufman PL,, Bertasso AM,, De Rousseau CJ,, Bito LZ. Slit-lamp studies of the rhesus monkey eye, I: survey of the anterior segment. Exp Eye Res. 1987. a ; 44: 307–318. [DOI] [PubMed] [Google Scholar]
- 11. Koretz JF,, Bertasso AM,, Neider MW,, True-Gabelt BA,, Kaufman PL. Slit-lamp studies of the rhesus monkey eye II: changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987. b ; 45: 317–326. [DOI] [PubMed] [Google Scholar]
- 12. Qiao-Grider Y,, Hung LF,, Kee CS,, Ramamirtham R,, Smith EL,, III. Normal ocular development in young rhesus monkeys (Macaca mulatta). Vis Res. 2007; 47: 1424–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wendt M,, Croft MA,, McDonald J,, Kaufman PL,, Glasser A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp Eye Res. 2008; 86: 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010; 90: 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Augusteyn RC,, Rosen AM,, Borja D,, Ziebarth NM,, Parel J-M, Biometry of primate lenses during immersion in preservation media. Mol Vis. 2006; 12: 740–747. [PubMed] [Google Scholar]
- 16. Augusteyn RC,, Cake MA. Post mortem uptake of water by sheep lenses left in the eye. Mol Vis. 2005; 11: 749–751. [PubMed] [Google Scholar]
- 17. Borja D,, Manns F,, Ho A,, et al. Optical power and biometric properties of isolated non-human primate crystalline lenses. Invest Ophthalmol Vis Sci. 2010; 51: 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohamed A,, Sangwan VS,, Augusteyn RC. Growth of the human lens in the Indian adult population: preliminary observations. Indian J Ophthalmol. 2012; 60: 511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohamed A,, Nankivil D,, Veerendranath P,, Taneja M. The precision of ophthalmic biometry using callipers. Can J Ophthalmol. 2013; 48: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosen AM,, Denham DB,, Fernandez V,, et al. Ex vivo dimensions and curvatures of human lenses. Vis Res. 2006; 46: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 21. Nakagawa N,, Murray TG,, Parel J-M,, Oshima K. Effect of scleral shortening on axial length. Arch Ophthalmol. 2000; 118: 965–968. [PubMed] [Google Scholar]
- 22. Grzimek B. Grzimek's encyclopedia of mammals. New York: McGraw-Hill; 1990. [Google Scholar]
- 23. Augusteyn RC,, Mohamed A,, Nankivil D,, Maceo B,, Pierre F,, Parel, J-M, Human ocular biometry. Exp Eye Res. 2012; 102: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaufman PL,, Calkins, BT,, Erickson KA. Ocular biometry of the cynomolgus monkey. Cur Eye Res. 1981; 1: 307–309. [DOI] [PubMed] [Google Scholar]
- 25. Augusteyn RC. Growth of the lens I: Weight accumulation in multiple species. Mol Vis. 2014; 410–426. [PMC free article] [PubMed]
- 26. Duke-Elder S. Normal and abnormal development. : Smelser GK, System of Ophthalmology, volume III. St. Louis, MO: CV Mosby; 1963. [Google Scholar]
- 27. Park DJJ,, Karesh J. Chapter 1: topographic anatomy of the eye: an overview. : TD Duane,, Jaeger EA,, Tasman W, Duane's foundations of clinical ophthalmology, volume I. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 28. Dawson DG,, Watsky MA,, Geroski DH,, Edelhauser HF. Chapter 4: cornea and sclera. : Tasman W,, Jaeger EA, Duane's foundations of clinical ophthalmology, volume 2. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 29. Song HT,, Kim YJ,, Lee SJ,, Moon YS. Relations between age weight, refractive error and eye shape by computerized tomography in children. Korean J Ophthalmol. 2007; 21: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorsby A,, Benjamin B,, Refraction Sheridan M. and its components during the growth of the eye. Memo Med Res Counc. 1961; 301: 1–67. [PubMed] [Google Scholar]
- 31. Larsen JS. The sagittal growth of the eye IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthal. 1971; 49: 873–886. [DOI] [PubMed] [Google Scholar]
- 32. Atchison DA,, Markwekk EL,, Kasthurirangan S,, Pope JM,, Smith G,, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008; 8: 1–20. [DOI] [PubMed] [Google Scholar]
- 33. Howland HC,, Merola S,, Basarab JR. The allometry and scaling of vertebrate eyes. Vis Res. 2004; 44: 2043–2065. [DOI] [PubMed] [Google Scholar]
- 34. Graham B,, Judge SJ. Normal development of refractive state and ocular component dimensions in the marmoset (Callithrix jacchus). Vis Res. 1999; 39: 177–187. [DOI] [PubMed] [Google Scholar]
- 35. Wolfe JM,, O'Connell KM. Adaptation of the resting states of accommodation dark and light field measures. Invest Ophthalmol Vis Sci. 1987; 28: 992–996. [PubMed] [Google Scholar]
- 36. Thomson JA,, Augusteyn RC. Ontogeny of human lens crystallins. Exp Eye Res. 1985; 40: 393–410. [DOI] [PubMed] [Google Scholar]
- 37. Garland DL,, Duglas-Tabor Y,, Jimenez-Asensio J,, Datiles MB,, Magno B. The nucleus of the human lens: Demonstration of a highly characteristic protein pattern by two-dimensional electrophoresis and introduction of a new method of lens dissection. Exp Eye Res. 1985; 62: 285–291. [DOI] [PubMed] [Google Scholar]
- 38. Sato S,, Russell P,, Kinoshita JH. Analysis of the proteins in the rhesus monkey lens. Exp Eye Res. 1985; 41: 324–334. [DOI] [PubMed] [Google Scholar]
- 39. Rao PV,, Garrow TA,, John F,, et al. Betaine-homocysteine methyltransferase is a developmentally regulated enzyme crystallin in Rhesus monkey lens. J Biol Chem. 1998; 273: 30669–30674. [DOI] [PubMed] [Google Scholar]
- 40. Borja D,, Manns F,, Ho A,, et al. Optical power of the isolated human crystalline lens. Invest Ophthalmol Vis Sci. 2008; 49: 2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pierscionek BK,, Augusteyn RC. Species variations in optical parameters of the eye lens. Clin Exp Optom. 1993; 76: 22–26. [Google Scholar]
- 42. Pierscionek BK. Species variations in the refractive index of the eye lens and patterns of change with ageing. : Ioseliani OR, Focus on eye research. New York: Nova Science Publishers, Inc.; 2005: 91–116. [Google Scholar]
- 43. Augusteyn RC,, Mohamed A,, Nankivil D,, et al. Age-dependence of the optomechanical responses of human lenses from India and the USA, and the force required to produce these in a lens stretcher: the similarity to in vivo disaccommodation. Vis Res. 2011; 51: 1667–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maceo B,, Manns F,, Borja D,, et al. Contribution of the crystalline lens gradient refractive index to the accommodation amplitude in non-human primates: in vitro studies. J Vis. 2011; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]