Abstract
Purpose
We compared corneal backscatter estimated from a Scheimpflug camera with backscatter estimated from a clinical confocal microscope across a wide range of corneal haze.
Methods
A total of 59 corneas from 35 patients with a range of severity of Fuchs' endothelial corneal dystrophy and 15 corneas from 9 normal participants were examined using a Scheimpflug camera (Pentacam) and a confocal microscope (ConfoScan 4). The mean image brightness from the anterior 120 μm, midcornea, and posterior 60 μm of the cornea across the central 2 mm recorded by the Scheimpflug camera and analogous regions from the confocal microscope were measured and standardized. Differences between instruments and correlations between backscatter and disease severity were determined by using generalized estimating equation models.
Results
Backscatter measured by the two instruments in the anterior and midcornea were correlated (r = 0.67 and 0.43, respectively, P < 0.001), although in the posterior cornea they were not correlated (r = 0.13, P = 0.66). Measured with the Scheimpflug camera, mean backscatter from the anterior and midcornea were greater, whereas backscatter from the posterior cornea was lower (P < 0.001) than that measured by the confocal microscope. Backscatter from the anterior cornea was correlated with disease severity for both instruments (Scheimpflug, r = 0.55, P < 0.001; confocal, r = 0.49, P = 0.003).
Conclusions
The Scheimpflug camera and confocal microscope should not be used interchangeably to measure corneal haze. The ability to detect changes in backscatter with disease severity is superior with the Scheimpflug camera. However, the confocal microscope provides higher resolution of corneal structure.
Keywords: confocal microscopy, Scheimpflug photography, corneal haze, backscattered light
Corneal haze, the pathologic light scattered back to an observer during an examination, has been used as a means of assessing the condition and optical quality of the cornea.1–4 In a normal cornea, backscattered light typically is low, but in corneal dystrophies or after injury, corneal haze can be associated with pathology that often indicates the corneal structures responsible for poor vision. Changes in haze can be used to track progression of disease or response to treatment.1 Although corneal haze is associated with pathology, it cannot in itself degrade vision; only light that is scattered forward to the retina degrades vision by increasing glare around bright objects. However, observation of haze is valuable because the same processes that produce haze often are responsible for forward scatter and high-order aberrations that degrade vision.
Corneal haze can be recorded subjectively by observation with a slit-lamp, although more objective methods have been described. Clinical instruments for measurement of haze have included custom modified slit-lamps,3,5,6 clinical confocal microscopes,2,7 and Scheimpflug cameras.8–19 Spatial resolution needed to identify the source of the backscattered light varies among instruments. Some slit-illumination instruments can resolve only approximately thirds of the full corneal thickness,3 while the depth of field with confocal microscopes has been reported from 4 to 26 μm.20
The use of commercial instruments to measure scatter typically is more convenient than developing custom instruments. They are readily available and generally do not require custom modifications or additional parts. However, standardization of these devices typically is not a part of their clinical operation and must be included in a protocol for comparative and longitudinal studies.
The rotating Scheimpflug anterior segment camera (Pentacam; Oculus Optikgeräte, GmbH, Wetzlar, Germany) was designed to capture images of the anterior segment, and to reconstruct anatomic features and measure biometric variables. Its images of the cornea have been used to assess backscatter from the cornea, but we do not know how well corneal haze reported by this instrument (as “densitometry”) agrees with haze measured by other instruments, and whether instruments based on different optical principles can be used interchangeably. We describe standardization and use of this Scheimpflug camera for measuring corneal haze and compare haze measurements to those from a clinical confocal microscope (ConfoScan 4; Nidek Technologies, Fremont, CA, USA).
Methods
Participants
We examined 59 corneas from 35 patients with Fuchs' endothelial corneal dystrophy with the Scheimpflug camera and clinical confocal microscope. Patient age ranged from 44 to 89 years (68 ± 11 years, mean ± SD). Severity of disease in each cornea was graded on a scale of 1 to 6 by the appearance of guttae and stromal edema as described previously (modified Krachmer scale).21,22 Each eye was classified as belonging to one of three groups, mild (grades 1 and 2, 18 eyes of 12 patients), moderate (grades 3 and 4, 19 eyes of 15 patients), and advanced (grades 5 and 6, 22 eyes of 18 patients). Patients had no other ocular abnormalities other than Fuchs' endothelial dystrophy or previous uncomplicated cataract surgery with posterior chamber IOL implantation. In some patients, only one eye was included in the study because the other eye had received a corneal transplant or had another ocular condition that would have interfered with the measurement of scatter. We also examined 15 normal corneas from 9 participants with the Scheimpflug camera and confocal microscope in the same way as the Fuchs' patients were examined. Age of normal participants ranged from 54 to 75 years (63 ± 8 years). Participants were examined before the study and eyes were excluded if they had guttae, cataract, anterior segment surgery, or other abnormalities that could affect corneal backscatter. In three normal participants one eye was excluded because of recent cataract surgery. For the purposes of grading, participants with normal eyes were classified as grade 0. Scheimpflug camera measurements of backscatter of 22 of the corneas with Fuchs' dystrophy and 3 of the normal corneas were reported in a previous clinical study.23 All participants gave written consent to participate after discussion of the possible benefits and consequences of the study. This study was approved by the Institutional Review Board at Mayo Clinic and conformed to the tenets of the Declaration of Helsinki.
Corneal Backscatter, Scheimpflug Camera, and Confocal Microscope
Participants were seated at the chinrest in front of the Scheimpflug camera and after the camera was initially aligned manually, the standard automatic recording system completed the fine alignment and captured 25 cross-sectional images through the cornea and anterior chamber in 1 second. On each measurement, the slit was rotated about the central axis and images were captured at evenly spaced angles. The standard software provided with the instrument reconstructed the 3-dimensional inner and outer surfaces of the cornea and calculated the average brightness in predefined regions of the cornea. For the purposes of this study, we used average image brightness in three cylindrical volumes of tissue, each with a 2-mm diameter centered on the corneal apex and heights that included the anterior 120 μm, posterior 60 μm, and midcornea between these anterior and posterior regions (Fig. 1). The mean image brightness (called “densitometry” in the instrument's software) was expressed as a percent (0–100) of the maximum brightness recordable by the image system. The gain of the camera was fixed by the manufacturer. Typically, one to three sets of rotational scans were recorded and backscatter was determined from one scan with the highest quality and least motion artifacts.
Figure 1.
Corneal volumes selected by the Pentacam Scheimpflug camera for determining backscatter in this study. Mean image brightness was determined in each of three cylindrical volumes, with 2-mm diameter centered on the apex of the cornea. The anterior cylindrical volume included the anterior 120 μm of cornea, the posterior included the posterior 60 μm, and the midcornea included the region between these boundaries.
Participants then were examined with the confocal microscope by using the same method described previously.2,24 Before each exam, proparacaine was instilled in the conjunctival fornix to anesthetize the cornea. A drop of viscous coupling solution (GenTeal Gel; Novartis Ophthalmics, East Hannover, NJ, USA) was placed on the ×40 objective lens of the microscope with the z-ring adapter (to record depth of the frame and stabilize the cornea) and the z-ring and coupling solution were brought into contact with the center of the cornea. The focal plane was aligned manually with the endothelium, advanced 30 to 50 μm posterior to the endothelium, and the operator initiated a scan. The focal plane was scanned under software control through the thickness of the cornea, starting deep to the endothelium and extending anterior to the epithelium. The scan position then was reset for the next scan. The step distance between frames was 4 μm and the instrument recorded 350 frames, typically including two passes through the cornea. The illumination brightness remained fixed for all scans. The mean image brightness of each frame was calculated by custom software in a 300 × 300-pixel area (168 μm × 168 μm) at the center of the image. One pass through the cornea with minimal movement artifacts was selected and video frames with specific landmarks, such as the epithelial and endothelial surfaces, were identified visually in the scan. The mean image brightness was determined in all frames from 20 μm anterior to the epithelial surface to 120 μm deep to the anterior surface (anterior cornea), from 60 μm anterior to the endothelial surface to 20 μm posterior to the endothelial surface (posterior cornea), and from all frames in the corneal stroma between these boundaries (midcornea). Boundaries were determined from the position of the epithelial and endothelial surfaces, based on appearance in the video frames, and the distance recorded through the z-ring. Two experienced observers (KMK and JWM) selected all frames that represented boundary features and the mean frame at each boundary was used to identify the surface. The mean number of images included in the average backscatter from each layer was 33 ± 3, 92 ± 11, and 19 ± 2 in the anterior, middle, and poster layers, respectively. Including the 20-μm regions anterior and posterior to the epithelial and endothelial surfaces assured that we included the entire peak associated with each surface. In this study, we use the term “backscatter” to refer to all light returned from the cornea and recorded by the image system of either instrument including scattered light and reflected light.
Standardization of Image Brightness
Image brightness from both instruments was standardized in two steps. First, image brightness was adjusted for daily variation in sensitivity of the Scheimpflug camera or confocal microscope from measurements of a scatter standard. Before each subject was examined with the Scheimpflug camera, images of a polymethyl methacrylate (PMMA) contact lens that was embedded with titanium oxide24 were recorded. The backscatter from this lens is constant and image brightness should only vary as the illumination and sensitivity of the instrument varies. Image brightness from the cornea was adjusted according to differences in brightness of this lens from its brightness on a reference day.2 Similarly, image brightness of a solution of Amco Clear (GSF Chemicals, Columbus, OH, USA) in a spherical container was scanned with the confocal microscope before each patient examination.2 Brightness of the corneal confocal images was adjusted to compensate for changes in brightness of the standard relative to its brightness on a reference day.
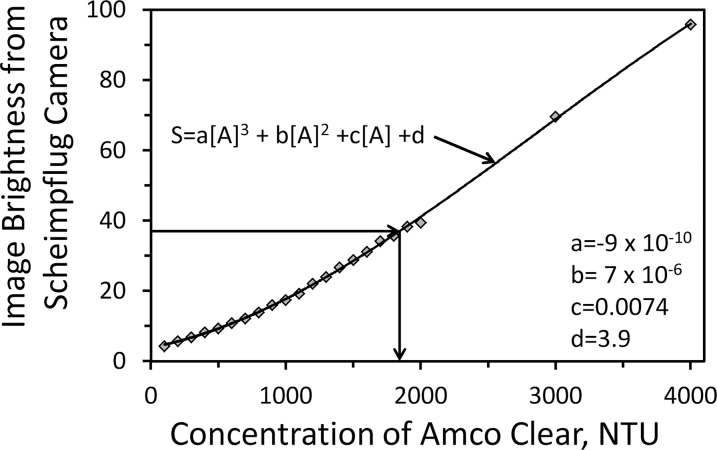
Second, the corneal image brightness was expressed as the equivalent concentration of a standard scatter substance (Amco Clear) that gave the same brightness as the image.2,7 A stock solution of Amco Clear, with concentration specified by the manufacturer as 4000 nephelometric turbidity units (NTU), was diluted to samples with a range of concentrations from 100 to 4000 NTU. Each of these was injected into the vault of a PMMA contact lens, with a base curve radius of approximately 8 mm, and was measured by the Scheimpflug camera. Images were recorded with the same settings as were used for measurements in corneas and brightness at a fixed depth in the solution was determined for each dilution. A third-order polynomial was fitted to the image brightness from the scattering solutions between concentrations from 100 to 4000 NTU, and this curve was used to determine the concentration of the standard solution that gave the same image brightness as the cornea. Units of brightness expressed as concentration were referred to as “Scatter Units” (SU) and corresponded to the concentration of Amco Clear in NTU that gave the same image brightness.
The confocal microscope was standardized in a similar way from scans through a spherical bulb that contained the scattering solutions.2 The relationship between concentration of scattering solution and image brightness was linear, as described previously,2 and was used to determine image brightness from the confocal microscope in scatter units.
Statistical Analysis
The study was powered to have an 80% chance of finding a correlation with a minimum coefficient of r = 0.32 (r2 = 0.10) between instruments and between backscatter and disease severity. This required 70 corneas if such a correlation existed (α = 0.05, β = 0.20), and measurement of 74 corneas provided a slightly higher power. The relationships between image brightness measured from the Scheimpflug camera and the confocal microscope were illustrated by using Pearson regression. Significances of correlations were determined by using generalized estimating equation (GEE) models to account for possible correlation between fellow eyes of individual subjects. Significances of differences between means of the three severity groups and the normal corneas (group 0) were determined by using GEE models and were adjusted for 3 comparisons by using the Bonferroni method. When means were not significantly different, minimum detectable differences were estimated based on an 80% chance of finding a difference if the difference in fact existed (α = 0.05, β = 0.20). This estimate was adjusted for three comparisons and the coefficient of variation used in the estimate was adjusted for the GEE analysis. Limits of agreement, the mean difference ±2 SDs of the difference between instruments, were calculated for each region.25
Results
Image brightness of the scattering solutions recorded by the Scheimpflug camera increased as an upward curve, which was more pronounced at concentrations below 1500 NTU. The fitted third order polynomial used to convert image brightness from corneas to scatter units is illustrated in Figure 2.
Figure 2.
Brightness of Amco Clear in Scheimpflug images. Image brightness of Amco Clear in a contact lens (S) increased as the concentration of Amco Clear (A) increased. Data were fitted to a third-order polynomial, which was used to determine the concentration of Amco Clear that gave the same scatter as the region of interest as indicated by the right-directed and down-directed arrows. Backscatter in SU refers to the concentration of Amco Clear that produced the same image brightness as the cornea.
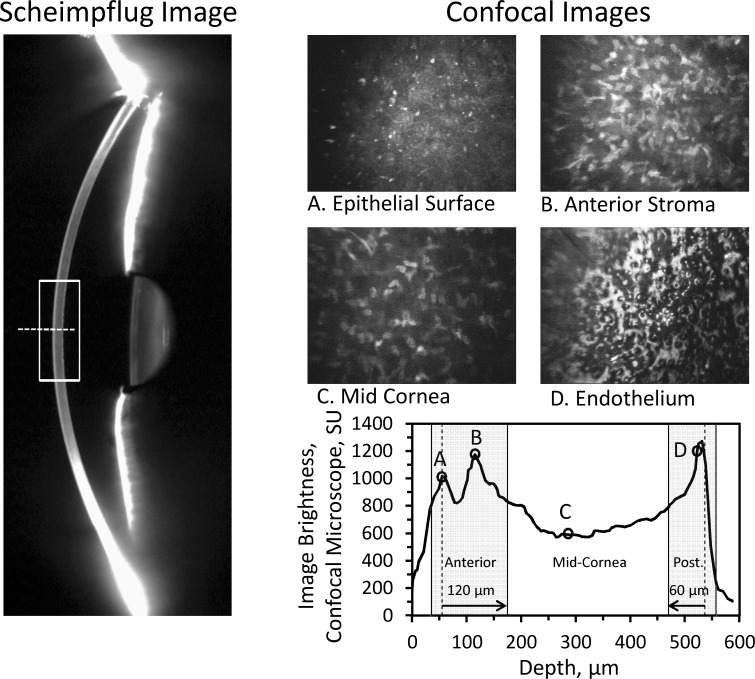
A sample image from a cornea with Fuchs' endothelial dystrophy recorded by the Scheimpflug camera, and the image brightness profile and sample images from the confocal microscope (Fig. 3) illustrate the regions assessed by confocal microscopy. Image brightness from video frames recorded by the confocal microscope in the regions identified in Figure 3 were averaged to determine backscatter from the anterior, mid‐, and posterior cornea. Mean corneal thickness measured by the Scheimpflug camera was 569 ± 52 μm (mean ± SD) and mean thickness measured by the confocal microscope was 570 ± 37 μm. These were not significantly different from each other (P = 0.79, minimum detectable difference = 14 μm).
Figure 3.
Sample images from a patient with Fuchs' endothelial dystrophy. The Scheimpflug image (left) shows one slit image. Backscatter was determined in the region identified by the rectangle. The measurement region of the confocal microscope was scanned axially approximately along the broken line. The image brightness profile through the cornea from the confocal microscope (lower right) shows brightness peaks at the epithelial surface (A), the stromal surface (B), and the endothelial surface with guttae (D), and a region of relatively uniform brightness at the midcornea (C). Representative images from these regions show characteristic structures (upper right). The mean image brightness was calculated from all video frames in the regions represented by the anterior and posterior shaded areas (Anterior and Post.) and the midcornea as described in the text. These regions corresponded to the anterior 120-μm, midcornea, and posterior 60-μm regions automatically selected by the Scheimpflug camera.
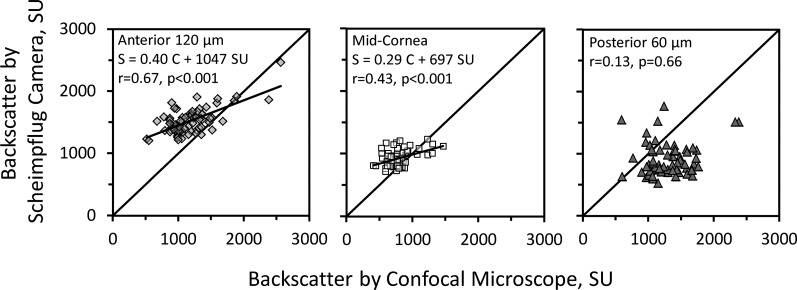
Backscatter measured by the Scheimpflug camera in the anterior 120 μm of the cornea increased with increasing backscatter measured by the confocal microscope (r = 0.67, P < 0.001) in the same region, although the regression line had a slope of only 0.40 Scheimpflug scatter units per confocal scatter unit (Fig. 4). Backscatter from the midcornea was lower than it was in the anterior cornea and the relationship between backscatter measured by the Scheimpflug camera and confocal microscope was similar to the relationship in the anterior cornea, although it was somewhat weaker (r = 0.43, P < 0.001). The regression line through scatter from the midcornea had a slope of 0.29, almost parallel to the regression line through scatter from the anterior cornea. In the posterior 60 μm, there was no significant relationship between backscatter from the Scheimpflug camera and confocal microscope (r = 0.13, P = 0.66).
Figure 4.
Relationships between backscatter (in SU) from the Scheimpflug camera (S) and confocal microscope (C) in all participants. In the anterior (left) and midcornea (center), measurements from the two instruments were correlated, whereas they were not correlated in the posterior cornea (right). The regression lines in the anterior and midcornea were approximately parallel to each other, but were offset and had slopes equal to 0.40 and 0.29, considerably less than 1. The diagonal line in each graph represents the identity line.
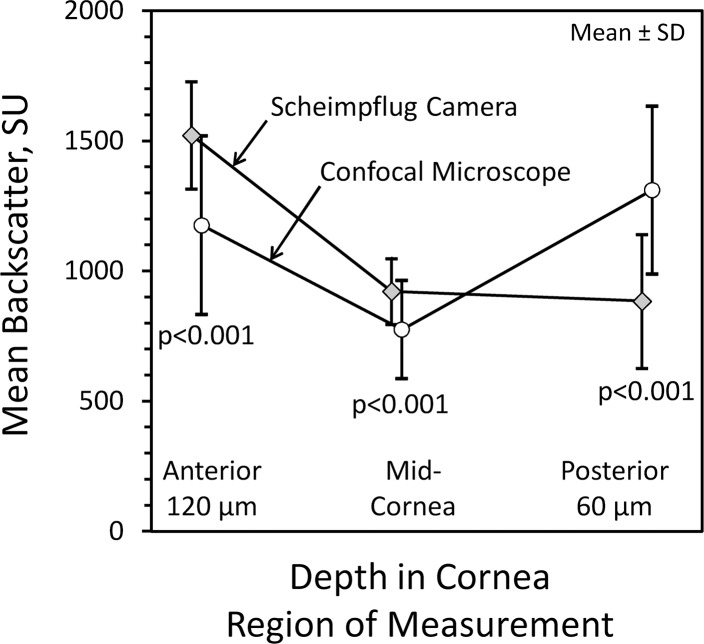
In the anterior 120 μm and midcornea, mean backscatter from all corneas measured by the Scheimpflug camera (1522 ± 206 and 921 ± 127 SU, respectively) was greater than mean backscatter measured by the confocal microscope (1177 ± 343 and 775 ± 189 SU, respectively, P < 0.001, Fig. 5). In the posterior cornea, this difference was reversed; mean backscatter from the Scheimpflug camera (884 ± 257 SU) was less than backscatter measured by using the confocal microscope (1312 ± 323 SU, P < 0.001). Limits of agreement in the anterior 120 μm, midcornea, and posterior 60 μm were −165 to 856, −206 to 499, and −1200 to 344 SU, respectively.
Figure 5.
Mean corneal backscatter (in SU) from the Scheimpflug camera and confocal microscope in three regions of the cornea. Mean backscatter measured by confocal microscopy was less than that measured by Scheimpflug photography in the anterior and midcornea. However, the mean backscatter was higher in the posterior cornea by confocal microscopy compared to Scheimpflug photography. The higher backscatter in confocal microscopy in this region is likely associated with specular reflection from the endothelium.
Backscatter in the anterior 120 μm measured by both instruments increased as severity of the disease increased (Fig. 6), consistent with our previous work.23 Anterior backscatter measured by the Scheimpflug camera indicated a slightly stronger correlation (r = 0.55, P < 0.001) with grade of severity than did backscatter measured using the confocal microscope (r = 0.49, P = 0.003). In neither instrument was the relationship strong enough to be able to identify the grade of disease based on the backscatter. For example, each grade of severity included patients with backscatter near the mean of all patients across all grades. In the posterior 60 μm of the cornea, backscatter from the Scheimpflug camera also was associated with increasing grade of severity (r = 0.65, P < 0.001; Fig. 6). However, backscatter measured by using the confocal microscope was weakly correlated inversely with grade of severity (r = −0.14, P = 0.047).
Figure 6.
Corneal backscatter as a function of severity of Fuchs' dystrophy. Backscatter (in SU) in the anterior cornea measured by both instruments increased with severity of the disease. In the midcornea, the relationship with disease severity was slightly weaker than in the anterior cornea by Scheimpflug photography and not significant by confocal microscopy. In the posterior cornea, when measured by Scheimpflug photography, backscatter increased with disease severity, but when measured by confocal microscopy backscatter trended weakly downward. In regression equations, S = backscatter from Scheimpflug camera, C = backscatter from confocal microscope, Gr = grade of severity of Fuchs' endothelial dystrophy (grades 1–6) and normal (grade 0).
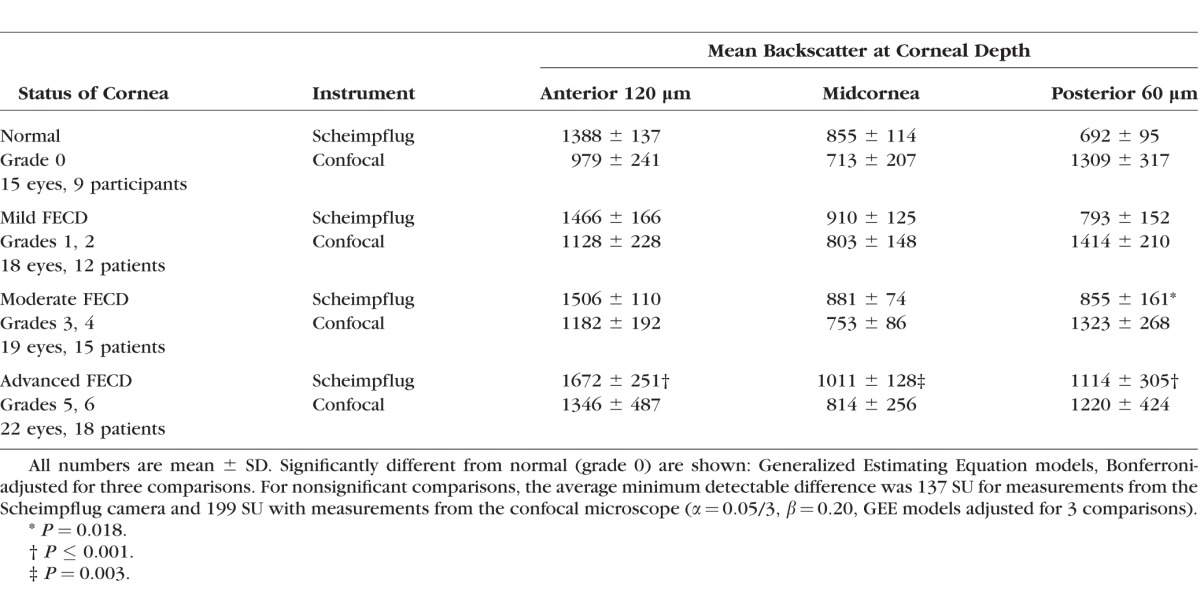
The Scheimpflug camera detected significant differences between mean backscatter from the anterior 120 μm, midcornea, and posterior 60 μm in advanced Fuchs' dystrophy (grades 5, 6) compared to normal (see Table). The Scheimpflug camera also detected a significant difference in the posterior 60 μm in patients with moderate Fuchs' dystrophy (grades 3, 4). Mean backscatter measured by the confocal microscope in the anterior 120 μm, midcornea, and posterior 60 μm were not significantly elevated in any of three severity groups compared to normal (see Table).
Table.
Backscatter in SU in Normal Participants and Patients With Fuchs' Endothelial Corneal Dystrophy (FECD) Measured by Scheimpflug Photography and Confocal Microscopy
Discussion
Corneal backscatter can be an objective measure of corneal health and can be indirectly related to vision in eyes with corneal disease.1,26,27 Scheimpflug imaging offers a simple method for assessing corneal backscatter, but this study showed that the data acquired by Scheimpflug imaging cannot be interchanged with those from confocal microscopy.
In spite of standardizing measurements from the Scheimpflug camera and the confocal microscope to the same scattering solution (Amco Clear), the two instruments demonstrated different response characteristics to increasing corneal backscatter in our sample of Fuchs' dystrophy patients (Figs. 4, 5), and the discrepancy between instruments changed with depth in the cornea. In the anterior and midcornea, backscatter measured by the Scheimpflug camera was higher in most corneas than backscatter measured by the confocal microscope (Fig. 4). In contrast, in the corneas with the highest haze by confocal microscopy, backscatter measured from the Scheimpflug camera was lower than that measured from the confocal microscope. Slopes of the regression lines were ≤0.40 Scheimpflug scatter units per confocal scatter unit, considerably less than a slope of 1, which would be expected if measurements from the two instruments were interchangeable. The relationship between backscatter measured by the two instruments from the deepest layer of the cornea, which includes the endothelium, was different from that in the anterior and middle layers; backscatter measured by the Scheimpflug camera was less than that measured by the confocal microscope and in this region there was no correlation between the instruments.
The lack of agreement between the two instruments can be in part attributed to differences in their optical properties.17 Image brightness from the confocal microscope includes light from specular reflection as well as scatter because the angle between the optical axis of the eye and the illumination is equal to the angle with the measurement pathway. In the Scheimpflug camera, the illumination path is along the optical axis of the eye approximately centered at the apex, whereas the measurement path is at an angle to this axis. Because of this arrangement, the Scheimpflug camera should detect little specular reflected light (Fig. 7) and image brightness should be dominated by backscattered light, whereas image brightness from the confocal microscope is dominated by reflected light. As a result, in the posterior cornea the reflective surface of the endothelium produced a higher corneal backscatter by confocal microscopy than by Scheimpflug photography. In contrast, because the epithelial surface was coupled to the confocal objective with a viscous solution that reduces reflectivity, a similar dominant specular reflection was not apparent in the anterior cornea resulting in a lower backscatter compared to that from the Scheimpflug camera.
Figure 7.
Optical design of the Scheimpflug camera and the confocal microscope. At specular surfaces, such as the endothelium, the specular reflection of the illumination from the Scheimpflug camera is directed back at the light source and image brightness is dominated by backscatter. In the confocal microscope, the specular reflection from the light source is directed into the detection path, because the optical axis of the illuminator and detector are at equal angles to the axis perpendicular to the surface, and image brightness is dominated by specular reflection. This reflection appears as a large peak at the endothelial surface with the confocal microscope and is not seen in the Scheimpflug camera. Instrument lenses and the cornea are not drawn to scale.
The relative size of the sample area across the cornea also may contribute to differences between instruments. The Scheimpflug camera samples the cornea from a disc-shaped area 2 mm diameter, whereas the confocal microscope samples from a square area less than 0.17 mm on each side. Thus, focal areas of high backscatter may influence the confocal microscope more than the Scheimpflug camera and if there are local variations in backscatter, the confocal microscope would produce higher variation from scan to scan depending on the location of the scan relative to the focal scatter. These variations would be averaged in measurements from the Scheimpflug camera. Measurement of backscatter from multiple confocal scans to cover the same area as measured by the Scheimpflug camera might produce a better correlation between instruments. However, this would require a systematic series of scans across the 2-mm disc covered by the Scheimpflug camera, which would be time consuming and difficult considering that the ConfoScan was designed to scan along the central axis of the eye. In this study, our comparison is limited to one scan from each instrument as it was designed to operate and as each would be used generally clinically.
Regardless of the relationship between backscatter measured by the two instruments, backscatter from the anterior 120 μm estimated by each instrument was correlated with the subjective grade of severity of Fuchs' dystrophy (Fig. 6), consistent with previous studies,23,28 although the correlation was stronger with measurements from the Scheimpflug camera. The correlation was significant with both instruments, but measurements from neither instrument could be used to assess the grade of disease in individual patients based on corneal backscatter alone. Backscatter from midcornea and posterior 60 μm reported by the Scheimpflug camera also increased and was correlated with severity of disease, although backscatter measured in the midcornea with the confocal microscope was not correlated with disease severity, and in the posterior 60 μm there was a weak downward trend with severity. This contradictory response may be explained by the relative amount of specular reflection from the endothelium. As illustrated in Figure 7, the confocal image brightness from the endothelium should be high when the endothelial surface is smooth as it is in a normal cornea. As the area of guttae, which are poorly-reflective and appear dark in confocal images, increases with disease severity, the brightness of the endothelial boundary decreases, as noted in Figure 6. In contrast, the rough surface of guttae scatter light at nonspecular angles and the backscatter from this region should increase in the Scheimpflug camera. Confocal microscopes that have different optical properties than the ConfoScan 4, such as the Heidelberg HRT laser confocal microscope with the Rostock corneal module (Heidelberg Engineering, Heidelberg, Germany), may have a different response at the endothelial surface than we measured and the response will depend on the optical design of the instrument. The relationships between backscatter measured by other contemporary confocal microscopes and the Scheimpflug camera have not been tested but should be examined before using these devices interchangeably to study corneal backscatter.
An advantage of Scheimpflug cameras over conventional slit-based microscopes is the clear focus through the entire depth illuminated by the slit. This allows precise discrimination of structures of the entire anterior segment, and with the rotating camera, the anterior segment can be reconstructed in three dimensions. Although spatial relationships are distorted with depth because of differences in magnification across the image, methods for spatial correction of biometry have been described29 and are used in modern commercial instruments. The high quality images of the slit illuminator through the cornea makes Scheimpflug cameras an excellent instrument for assessing backscattered light from the cornea.23 Traditionally, this method has been called “densitometry,” which may originate from measurement of density of photographic images from early Scheimpflug cameras.8 In the current form of the camera, this term can be somewhat misleading, because scattered light, not optical density of the tissue, affects image brightness.
Most investigators have reported image brightness from their measurements with Scheimpflug cameras in arbitrary units on a scale of brightness from 0 to 100, with 0 meaning no scattered light (dark image) and 100 meaning full brightness (saturated white image).9–14,16–18,30 Backscatter measured in the central normal cornea by the Pentacam Scheimpflug camera has been reported from as low as 10 to over 30,11,16 and this variation might be explained partially by differences in sensitivities of particular instruments or differences in the population sampled, although the latter is unlikely. Such variation could be minimized by standardizing image brightness and measuring the sensitivity of the instrument immediately before examining the cornea, steps that also are essential for comparing data across studies or in long-term prospective studies. This problem has been recognized by a few investigators who have attempted to standardize image brightness of the crystalline lens by normalizing to the brightness of the cornea, a presumably stable region in images.15,31 We and other investigators have standardized image brightness in confocal microscopy2,32 and Scheimpflug photography33,34 to measurements of scattering solutions or suspensions of microspheres, which solves the problem of variation and reporting backscatter in common units. It also linearizes the nonlinear response of the Scheimpflug camera to brightness as illustrated in Figure 2 and noted by others.33
Compared to confocal microscopy, the Scheimpflug camera has some advantages for assessing corneal haze. Contemporary commercial Scheimpflug cameras are noncontact and easy to align, and image acquisition is rapid. Image brightness is dominated by scatter rather than specular reflection, and the camera assesses the entire cornea in contrast to sampling an area of less than 0.5 mm2 as sampled by confocal microscopy. Nevertheless, confocal microscopes have higher spatial resolution enabling more accurate determination of the structures that contribute to haze. The tradeoff between the high spatial resolution but limited area of measurement with the confocal microscope and the low depth resolution but large transverse sample area of the Scheimpflug camera must be considered when planning a study. Anterior corneal haze measured by both instruments is correlated with severity of Fuchs' endothelial dystrophy, but measurements from neither can be used to identify the grade of severity. The Scheimpflug camera also is able to detect the correlation between disease severity and haze from the mid- and posterior cornea, whereas these changes seem to be masked by reflection in measurements from the confocal microscope. In our sample this instrument also was able to detect changes in corneal haze in corneas with advanced Fuchs' endothelial dystrophy compared to normal in all regions of the cornea, whereas these changes were not detected by the confocal microscope. Thus, the Scheimpflug camera is superior to the confocal microscope when studying changes in backscatter from the mid- and posterior cornea and its image brightness in the anterior cornea correlates better with disease severity. The choice of which instrument should be used in a study of corneal haze should be based on the need to resolve and identify structures in the cornea, the importance of scatter versus reflected light, and the region of cornea of interest to the study.
Acknowledgments
Supported by Research to Prevent Blindness, New York, New York, United States (an unrestricted department grant and SVP as Olga Keith Weiss Special Scholar), Dr. Werner Jackstaedt-Foundation, Wuppertal, Germany (Research Fellowship to KW), Mayo Clinic Center for Translational Science Activities (Grant No. UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, Bethesda, MD, USA), and Mayo Foundation, Rochester, Minnesota, United States.
Presented in part as a poster at the annual meeting of the Association for Research in Vision and Ophthalmology, Denver, Colorado, United States, May 4, 2015.
Disclosure: J.W. McLaren, None; K. Wacker, None; K.M. Kane, None; S.V. Patel, None
References
- 1. Baratz KH,, McLaren JW,, Maguire LJ,, Patel SV. Corneal haze determined by confocal microscopy 2 years after Descemet stripping with endothelial keratoplasty for Fuchs' corneal dystrophy. Arch Ophthalmol. 2012; 130: 868–874. [DOI] [PubMed] [Google Scholar]
- 2. McLaren JW,, Bourne WM,, Patel SV. Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci. 2010; 51: 5610–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel SV,, Winter EJ,, McLaren JW,, Bourne WM. Objective measurement of backscattered light from the anterior and posterior cornea in vivo. Invest Ophthalmol Vis Sci. 2007; 48: 166–172. [DOI] [PubMed] [Google Scholar]
- 4. Hindman HB,, McCally RL,, Myrowitz E,, et al. Evaluation of deep lamellar endothelial keratoplasty surgery using scatterometry and wavefront analyses. Ophthalmology. 2007; 114: 2006–2012. [DOI] [PubMed] [Google Scholar]
- 5. McCally RL,, Connolly PJ,, Jain S,, Azar DT. Objective measurements of haze following phototherapeutic excimer laser ablation of cornea. SPIE. 1994; 161–165.
- 6. McCally RL,, Hochheimer BF,, Chamon W,, Azar DT. Simple device for objective measurements of haze following excimer laser ablation of cornea. SPIE. 1993; 2126: 20–25. [Google Scholar]
- 7. Hillenaar T,, Cals RHH,, Eilers PHC,, Wubbels RJ,, van Cleynenbreugel H,, Remeijer L. Normative database for corneal backscatter analysis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2011; 52: 7274–7281. [DOI] [PubMed] [Google Scholar]
- 8. Smith GT,, Brown NA,, Shun-Shin GA. Light scatter from the central human cornea. Eye. 1990; 4: 584–588. [DOI] [PubMed] [Google Scholar]
- 9. Arnalich-Montiel F,, Hernandez-Verdejo JL,, Oblanca N,, Munoz-Negrete FJ,, De Miguel MP. Comparison of corneal haze and visual outcome in primary DSAEK versus DSAEK following failed DMEK. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 2575–2584. [DOI] [PubMed] [Google Scholar]
- 10. Bhatt UK,, Fares U,, Rahman I,, Said DG,, Maharajan SV,, Dua HS. Outcomes of deep anterior lamellar keratoplasty following successful and failed ‘big bubble.’ Br J Ophthalmol. 2012; 96: 564–569. [DOI] [PubMed] [Google Scholar]
- 11. Elflein HM,, Hofherr T,, Berisha-Ramadani F,, et al. Measuring corneal clouding in patients suffering from mucopolysaccharidosis with the Pentacam densitometry programme. Br J Ophthalmol. 2013; 97: 829–833. [DOI] [PubMed] [Google Scholar]
- 12. Fares U,, Otri AM,, Al-Aqaba MA,, Faraj L,, Dua HS. Wavefront-optimized excimer laser in situ keratomileusis for myopia and myopic astigmatism: refractive outcomes and corneal densitometry. J Cataract Refract Surg. 2012; 38: 2131–2138. [DOI] [PubMed] [Google Scholar]
- 13. Greenstein SA,, Fry KL,, Bhatt J,, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010; 36: 2105–2114. [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez R,, Lopez I,, Villa-Collar C,, Gonzalez-Meijome JM. Corneal transparency after cross-linking for keratoconus: 1-year follow-up. J Refract Surg. 2012; 28: 781–786. [DOI] [PubMed] [Google Scholar]
- 15. Laser H,, Berndt W,, Leyendecker M,, Kojima M,, Hockwin O,, Cheyne A. Comparison between Topcon SL-45 and SL-45B with different correction methods for factors influencing Scheimpflug examination. Ophthalmic Res. 1990; 22 (suppl 1): 9–17. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda J,, Hieda O,, Kinoshita S. Quantification of corneal opacity after refractive corneal surgery using the anterior segment analyzer [in Japanese] Nippon Ganka Gakkai Zasshi. 2007; 111: 447–453. [PubMed] [Google Scholar]
- 17. Ní Dhubhghaill S,, Rozema JJ,, Jongenelen S,, Ruiz Hidalgo I,, Zakaria N,, Tassignon MJ. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014; 55: 162–168. [DOI] [PubMed] [Google Scholar]
- 18. Takacs AI,, Mihaltz K,, Nagy ZZ. Corneal density with the Pentacam after photorefractive keratectomy. J Refract Surg. 2011; 27: 269–277. [DOI] [PubMed] [Google Scholar]
- 19. Uchino Y,, Shimmura S,, Yamaguchi T,, et al. Comparison of corneal thickness and haze in DSAEK and penetrating keratoplasty. Cornea. 2011; 30: 287–290. [DOI] [PubMed] [Google Scholar]
- 20. McLaren JW,, Bourne WM,, Patel SV. Automated assessment of keratocyte density in stromal images from the ConfoScan 4 confocal microscope. Invest Ophthalmol Vis Sci. 2010; 51: 1918–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Repp DJ,, Hodge DO,, Baratz KH,, McLaren JW,, Patel SV. Fuchs' endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology. 2013; 120: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krachmer JH,, Purcell JJ,, Jr,, Young CW,, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol. 1978; 96: 2036–2039. [DOI] [PubMed] [Google Scholar]
- 23. Wacker K,, McLaren JW,, Amin SR,, Baratz KH,, Patel SV. Corneal high-order aberrations and backscatter in Fuchs' endothelial corneal dystrophy. Ophthalmology. 2015; 122: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLaren JW,, Nau CB,, Patel SV,, Bourne WM. Measuring corneal thickness with the ConfoScan 4 and Z-Ring adapter. Eye Contact Lens. 2007; 33: 185–190. [DOI] [PubMed] [Google Scholar]
- 25. Bland JM,, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 327: 307–310. [PubMed] [Google Scholar]
- 26. Patel SV,, Baratz KH,, Hodge DO,, Maguire LJ,, McLaren JW. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009; 127: 153–160. [DOI] [PubMed] [Google Scholar]
- 27. Patel SV,, McLaren JW,, Hodge DO,, Baratz KH. Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2008; 145: 97–105. [DOI] [PubMed] [Google Scholar]
- 28. Amin SR,, Baratz KH,, McLaren JW,, Patel SV. Corneal abnormalities early in the course of Fuchs' endothelial dystrophy. Ophthalmology. 2014; 121: 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kampfer T,, Wegener A,, Dragomirescu V,, Hockwin O. Improved biometry of the anterior eye segment. Ophthalmic Res. 1989; 21: 239–248. [DOI] [PubMed] [Google Scholar]
- 30. van de Pol C,, Soya K,, Hwang DG. Objective assessment of transient corneal haze and its relation to visual performance after photorefractive keratectomy. Am J Ophthalmol. 2001; 132: 204–210. [DOI] [PubMed] [Google Scholar]
- 31. Hockwin O,, Dragomirescu V,, Laser H. Measurements of lens transparency or its disturbances by densitometric image analysis of Scheimpflug photographs. Graefes Arch Clin Exp Ophthalmol. 1982; 219: 255–262. [DOI] [PubMed] [Google Scholar]
- 32. Hillenaar T,, Sicam VADP,, Vermeer KA,, et al. Wide-range calibration of corneal backscatter analysis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2011; 52: 2136–2146. [DOI] [PubMed] [Google Scholar]
- 33. Qian W,, Soderberg P,, Chen E,, Philipson B. Universal opacity standard for Scheimpflug photography. Ophthalmic Res. 2000; 32: 292–298. [DOI] [PubMed] [Google Scholar]
- 34. Soya K,, Amano S,, Oshika T. Quantification of simulated corneal haze by measuring back-scattered light. Ophthalmic Res. 2002; 34: 380–388. [DOI] [PubMed] [Google Scholar]