Abstract
Background
The American Academy of Pediatrics and other organizations recommend several screening tests as part of preventive care. The proportion of children who are appropriately screened and who receive follow-up care is low.
Objective
To conduct a systematic review of the evidence for practice-based interventions to increase the proportion of patients receiving recommended screening and follow-up services in pediatric primary care.
Data source
Medline database of journal citations.
Study eligibility criteria, participants, and interventions
We developed a strategy to search Medline to identify relevant articles. We selected search terms to capture categories of conditions (e.g., developmental disabilities, obesity), screening tests, specific interventions (e.g., quality improvement initiatives, electronic records enhancements), and primary care. We searched references of selected articles and reviewed articles suggested by experts. We included all studies with a distinct, primary care-based intervention and post-intervention screening data, and studies that focused on children and young adults (≤21 years of age). We excluded studies of newborn screening.
Study appraisal and synthesis methods
Abstracts were screened by 2 reviewers and articles with relevant abstracts received full text review and evaluated for inclusion critieria. A structured tool was used to abstract data from selected articles. Because of heterogeneous interventions and outcomes, we did not attempt a meta-analysis.
Results
From 2547 returned titles and abstracts, 23 articles were reviewed. Nine were pre-post comparisons, 5 were randomized trials, 3 were post-intervention comparisons with a control group, 3 were post-intervention cross-sectional analyses only, and 3 reported time series data. Of 14 articles with pre-intervention or control group data and significance testing, 12 reported increases in the proportion of patients appropriately screened. Interventions were heterogeneous and often multifaceted, and several types of interventions, such as provider/staff training, electronic medical record templates/prompts, and learning collaboratives, appeared effective in improving screening quality. Few articles described interventions to track screening results or referral completion for those with abnormal tests. Data were often limited by single-site, non-randomized design.
Conclusions
Several feasible, practice- and provider-level interventions appear to increase the quality of screening in pediatric primary care. Evidence for interventions to improve follow-up of screening tests is scant. Future research should focus on which specific interventions are most effective, whether effects are sustained over time, and what interventions improve follow-up of abnormal screening tests.
MeSH key words: Mass Screening, Preventive Health Services, Physician’s Practice Patterns, Quality of Health Care
Introduction
Prevention of mortality and morbidity secondary to many conditions depends on effective screening and referral procedures in pediatric primary care.1 For many conditions, such as iron-deficiency anemia, autistic spectrum disorder, and vision and hearing problems, early detection from broad-based, primary screening with timely follow-up care enables children with these conditions to receive treatment that affects long-term health outcomes. The American Academy of Pediatrics, Bright Futures, and other organizations recommend screening procedures for several specific conditions.2, 3
Although many children receive some screening via public health or school-based mechanisms, most screening beyond the newborn period occurs within the context of the primary care office at well-child visits. Even with clear, readily-accessible recommendations, quality of screening in primary care is sub-optimal,4 leaving children at risk when conditions are not identified. Reasons for this quality gap include lack of knowledge of recommendations,5, 6 presumed patient refusal,5 lack of time,6 lack of office staff support,6 inadequate reimbursement,7 and inadequate referral resources for those found to have a problem detected through screening.7
Several interventions have potential to improve screening in primary care settings8 and have been studied to some extent in adults.9 However, which practice-level interventions are most effective for improving screening in pediatric primary care is not known. Interventions in pediatrics may have a different impact compared to adult populations, for several reasons.10 First, children generally seek health care and make decisions through a proxy, usually a parent. Second, children undergo more rapid developmental changes, and screening recommendations change with each well-child visit. Third, most conditions for which children are screened are not thought of as potentially life-threatening, in contrast to cancer screening in adults, which may affect the importance providers and parents place on screening in children. Examining interventions that improve receipt of recommended screening in pediatrics may help physicians and policymakers identify changes most likely to benefit a broader population and may inform a research agenda to address questions about how to improve the quality of screening in pediatric practices.
We undertook this systematic review as part of a larger project to examine evidence regarding six core objectives of the Maternal and Child Health Bureau11 for care for children with special health care needs. Previously, we reviewed the evidence regarding receipt of family-centered care12 and services to transition to adult providers;13 having a medical home;14 and having adequate health insurance coverage.15 We now review evidence for the objective that all children are screened early and continuously for special health care needs. Because high-quality screening in primary care is necessary for objective, we focused our review on office-based interventions to increase the proportion of children receiving recommended screening. Our specific research question was, what is the evidence for interventions to improve such screening in primary care settings? As a secondary objective, we also examined interventions to improve follow-up or referral completion, once screening tests identified concerns.
Methods
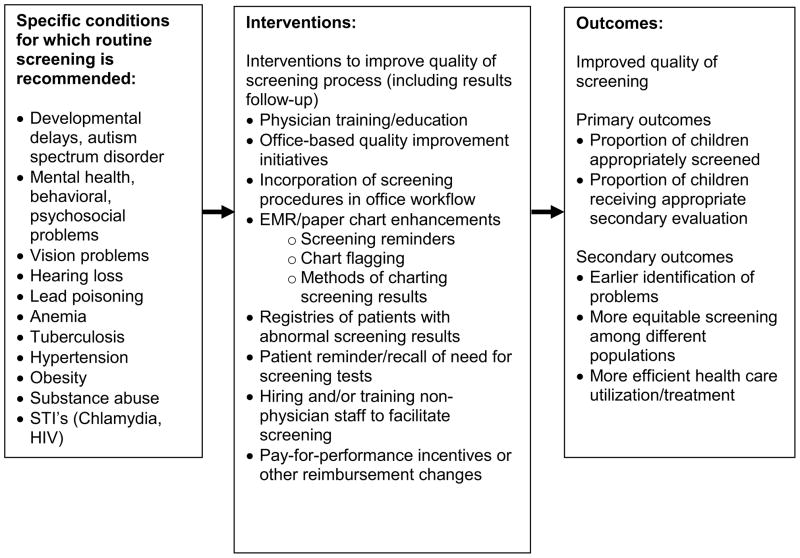
To guide our search strategy (Table 1), we constructed a logic model16 (Figure 1) that depicts the health conditions for which screening tests are recommended, interventions, and outcomes of interest. In developing and refining the model, we held a conference with relevant experts, including policymakers, family advocates, and researchers in the field of improving care for children with special health care needs. The purpose of this panel was to guide the systematic reviews around the MCHB core objectives, and the panel discussed and made recommendations for our logic model and search strategy.
Table 1.
Specific search terms to identify articles testing practice-based interventions to increase the quality of screening in pediatric practices*
| Screening/specific disorders | Setting | Interventions/outcomes |
|---|---|---|
|
| ||
| Mass screening Population surveillance Preventive health services Child development Developmental disabilities Language disorders Child behavior disorders Cerebral palsy Autistic disorder Mental retardation Vision disorders Hearing loss Lead poisoning Anemia Iron deficiency Hypertension Obesity Depression Tuberculosis Sexually transmitted infections |
Primary health care Community health centers Managed care programs Group practice |
Physician’s Practice Patterns Child Health Services Medical Records Systems, Computerized Decision Support Systems, Clinical Information Systems Education, Medical Education, Medical, Continuing Insurance, Health, Reimbursement Total Quality Management Quality Assurance, Health Care Referral and Consultation Primary Prevention Healthcare Disparities Health Care Costs Quality of Health Care Outcome Assessment Process Assessment |
In PubMed, language was limited to “English” and population was limited to “All child: 0–18 years”
Figure 1.
Logic Model for Core Objective: Practice-based interventions to improve screening
Screening tests
To select the screening tests and corresponding specific conditions for inclusion in our search, we reviewed recommendations for preventive care screening from Bright Futures/American Academy of Pediatrics, the US Preventive Services Task Force, and the Centers for Disease Control. We selected screening tests for conditions such as developmental delay, mental health conditions, vision problems, hearing problems, lead poisoning, anemia, hypertension, sexually transmitted infections, and obesity. We did not include conditions detected by newborn screening or prenatal screening, since testing procedures and much of the follow-up occurs not in primary care but in hospitals and in conjunction with state public health authorities.
Interventions
We chose search terms to capture primary care interventions designed to improve receipt of recommended screening and follow up. Specific activities were derived from a review of the literature of interventions to improve quality of other functions of primary care practices (e.g., vaccination) and recommendations from our expert panel.
Interventions included practice-level initiatives such as provider/staff education sessions and materials, quality improvement initiatives, and improvements in office workflow. Our search included interventions to improve patient identification for screening, particularly changes that led to automated identification, such as chart flagging, electronic medical record (EMR) reminders, and patient registries. We also searched for interventions that involved pay-for-performance initiatives targeted toward screening.
Outcomes
Our primary outcomes were the proportion of children appropriately screened, and proportion of children with abnormal screening results who received follow-up care. Appropriateness of screening was determined by the individual studies. Because follow-up care can vary among patients due to family preferences and available referral options, we broadly defined follow up care as any action by the provider that would advance a plan for additional screening, evaluation or treatment prompted by an abnormal result. This definition included discussing abnormal results with parents and patients, retesting patients, and referring to specialists or community resources for further treatment or evaluation. We also included search terms to capture secondary outcomes derived from the Institute of Medicine domains of healthcare quality.17
Database search
We conducted a systematic search of Medline (Jan 1961–Aug 2010) for titles and abstracts relevant to our research question. We queried for articles containing MeSH terms in each of the columns in Table 1, i.e., containing terms that represented a condition, a setting, and an outcome/intervention. We also reviewed bibliographies of selected articles, as well as bibliographies of review articles related to our search. For the bibliography reviews, when we found a potentially relevant title that was missed during the previous search, we obtained the article’s Medical Subject Heading (MeSH) terms from the Medline citation to determine why the article was missed. We then refined the search to include omitted MeSH terms, reran the search and reviewed the additional abstracts. We limited our search to English-language articles studying children and youth aged 0–18 years.
Selection of articles
Two reviewers (JV and AAK) screened titles and abstracts for inclusion in the group of articles for full-text review. Abstracts were selected if the study examined a recommended screening practice and the study was performed in a primary care setting in the United States. Some returned studies included both adults and adolescents, and we included articles if >50% of participants were under age 21 years. Abstracts that lacked detail to make this determination also underwent full-text review. If the abstract was not appropriate for inclusion in the review but possibly referenced relevant articles, the full-text version was obtained and the bibliography scanned. The reviewers met to resolve discrepancies by discussion and mutual agreement. Each reviewer then abstracted a subset of articles using a structured form to report interventions, populations, settings, and outcomes. After abstraction, reviewers finalized the list of articles to be included in the review through discussion and agreement. Reviewers overlapped on a random selection of approximately 20% of abstracted articles. Abstractions were qualitatively reviewed to assess for agreement, and abstracted screening rates and descriptions of the interventions were verified through a second review of the full text articles. We did not contact authors of the studies for further details. No formal assessment of study quality was done using standardized tools, but we grouped studies using a hierarchy of study design quality (e.g., RCTs, designs with control groups, and uncontrolled studies) and reported elements of potential bias in our description of the studies.
Specific categories of excluded studies
We excluded studies to validate screening tools and studies that documented poor-quality screening or follow-up without interventions. We also excluded studies that assessed only feasibility of screening in primary care practices without specific attention to long-term, generalizable changes within the practice (e.g., studies where the intervention was limited to research assistants performing screening procedures). We excluded articles that lacked explicit outcomes related screening or follow-up care.
Results
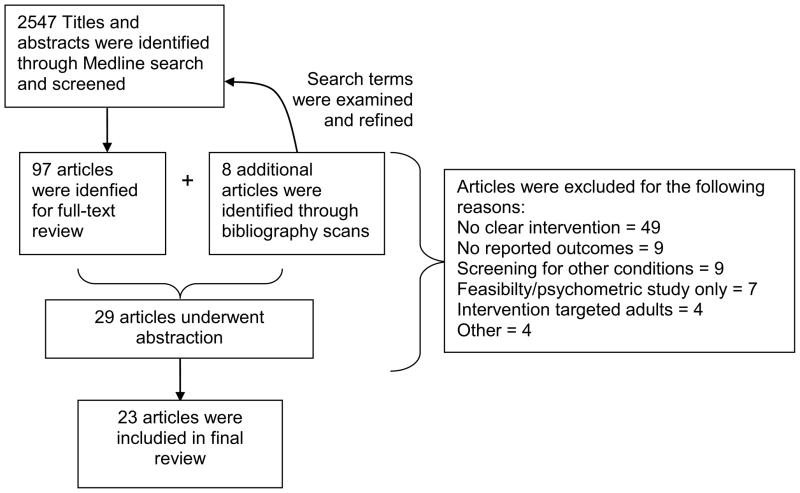
The final search strategy identified 2547 titles (Figure 2). After reviewing titles and abstracts, 105 articles underwent full-text review. Eight articles that underwent full-text review were initially identified from bibliographies of selected articles. Reviewers completed data abstraction for 29 of the 105 full-text articles. Of these 29 articles, 23 met criteria for inclusion in the final review (Table 2). Common reasons for exclusion were because no intervention was tested, proportion of patients screened was not measured, or the patient population was primarily adult-aged. The included 23 articles were 5 randomized controlled trials and 18 observational studies. Among the randomized trials, the practice was usually the unit of randomization. Among the observational studies, 9 used pre-post designs, 3 were post-intervention comparisons with a concurrent control group, 3 reported findings using time-series design where the outcome was measured at regular intervals after the intervention was initiated, and 3 were post-intervention, cross-sectional analyses with no comparison group. The diversity of interventions and outcomes prevented any meta-analysis.
Figure 2.
Flow of titles, abstract and articles included in review
Table 2.
Interventions to improve screening and follow-up of abnormal screening tests in pediatric primary care, by type of study design
| Author, year, design | Condition(s) being screened and screening test(s) | Pre-Intervention or control group screening (%of patients screened, unless otherwise specified) | Post-Intervention or experimental group screening (% of patients screened, unless otherwise specified) | Significance testing (p-value unless otherwise specified) | Nature of the intervention, setting/population, and other comments about the study |
|---|---|---|---|---|---|
| Randomized Controlled Trials | |||||
| 1. Margolis PA, et al. (2004) RCT20 |
Lead poisoning, anemia, and tuberculosis: Serum lead level: Intervention Control |
23% 18% |
68% 30% |
<0.05 |
Intervention: Process improvement methods (aka “knowledge translation”) to improve office systems around preventive care services.
Other comments: Data were collected pre- and post-intervention for both control and experimental group practices. Tuberculosis screening was PPD, Mantoux test, or risk assessment |
| Hematocrit: Intervention Control |
65% 64% |
79% 71% |
<0.05 | ||
| Tuberculosis screening: Intervention Control |
34% 30% |
54% 32% |
<0.05 | ||
| 2. Minkovitz CS, et al. (2003) RCT35 |
Developmental problems: Parent-reported developmental assessment |
41–43% | 82–84% | <0.001 |
Intervention: Healthy Steps (HS) program a. Co-located developmental specialists to enhance well-child visits; also conducted home visits, provided telephone information line for parents about development, written materials, parent groups, linkages to community resources Setting/population: 15 practices randomized in 14 states; experimental n=2021 patients, control n=1716 patients; post-intervention data were collected for children aged 30–33 months. Other comments: Parents reported any developmental screening questions (not specifically whether a formal tool was used) |
| 3. Scholes D, et al. (2006) RCT27 |
Chlamydia infection: Urine Chlamydia screening |
Practice-level intervention: 37.5% | 39.6% | 0.31 |
Intervention: Practice and patient-level interventions
|
| EMR reminder: 40.8% | 42.6% | 0.27 | |||
| 4. Shafer MA, et al. (2002) RCT23 |
Chlamydia infection: Urine Chlamydia screening |
21% | 65% | <0.001 |
Intervention: Quality improvement initiative within managed care network
|
| 5. Tebb KP, et al. (2009) RCT29 |
Chlamydia infection: Urine Chlamydia screening Intervention Control |
26% 32% |
42% 30% |
<0.001 |
Intervention: Quality improvement initiative within managed care network
Other comments: Data were collected pre- and post-intervention for both control and experimental group practices. |
| Pre-post intervention design | |||||
| 6. Adams WG et al. (2003) Pre-post37 |
Developmental problems, anemia, lead poisoning, hearing and vision problems: Language development |
65.1% | 70.0% |
Relative risk (95% confidence interval): 1.07 (0.97–1.09) |
Intervention: EMR template with prompts to improve preventive care services
Other comments: Pre-intervention group had paper charts with well-child visit templates; sample for specific tests varied because some tests are recommended only for a subset based on age. |
| Behavior/social development | 26.4% | 65.7% | 1.16 (1.04–1.28) | ||
| Motor development | 63.8% | 73.9% | 2.49 (2.00–3.10) | ||
| Hematocrit | 82.5% | 85.3% | 1.03 (0.91–1.17) | ||
| Serum lead level | 66.7% | 79.1% | 1.19 (0.99–1.43) | ||
| Vision | 42.9% | 50.0% | 1.17 (0.80–1.70) | ||
| Hearing | 33.3% | 48.3% | 1.45 (0.92–2.28) | ||
| 7. Applegate H, et al. (2003) Pre-post33 |
Behavior, developmental and emotional problems: Discussion about behavior, developmental or emotional problems (# items discussed per visit) |
1.6 items | 10.4 items per visit after Stage 1; 9.9 items per visit after Stage 2 |
Intervention: Provider education and support tools to implement Pediatric Symptom Checklist (PSC); intervention was 2 stages
Other comments: No significance testing reported |
|
| Intervention for behavior and emotional problems (# of interventions per visit) | 0 interventions | 0.125 interventions per visit after Stage 1; 1.9 interventions per visit after Stage 2 | |||
| 8. Block B, et al. (1996) Pre-post40 |
Follow up of elevated lead levels: Follow up plan in chart |
32% | 100% |
Intervention: Nurse-led protocol to follow up abnormally elevated lead levels--
Other comments: No significance testing reported |
|
| Follow up serum lead level done | 9% | 65% | |||
| Parent education about reducing exposure, if persistently high levels | Not measured | 28% | |||
| 9. Bordley WC, et al. (2001) Pre-post22 |
Anemia, lead poisoning, tuberculosis: Hematocrit |
45% | 67% | 0.001 |
Intervention: Quality improvement intervention to improve preventive care:
Other comments: Lead and tuberculosis screening was risk assessment and laboratory/skin testing, if indicated |
| Lead screening | 12% | 48% | 0.001 | ||
| Tuberculosis screening | 50% | 52% | NS | ||
| 10. Dunlop AL, et al. (2007) Pre-post32 |
Obesity: BMI percentile documented in chart |
12% | 15% after Stage 1 28% after Stage 2 |
NS <0.05 |
Intervention: Provider training and support tools for obesity. 2 staged intervention:
|
| Nutrition and activity history | 50% | 56% after Stage 1 81% after Stage 2 |
NS <0.05 |
||
| Nutrition and activity counseling | 33% | 35% after Stage 1 47% after Stage 2 |
NS <0.05 |
||
| 11. Lannon CM, et al. (2008) Pre-post21 |
Developmental problems PEDS or ASQ |
30% (received any developmental screening) | 45% (using structured tool (e.g., ASQ)) | NS |
Intervention: Bright Futures Training Intervention Project: learning collaborative/quality improvement initiative to improve preventive care services
Other comments: No participating practices used formal developmental screening tools pre-intervention. |
| 12. Polacsek M, et al. (2009) Pre-post25 |
Obesity: BMI documented in chart Screening with previsit, self-administered tool to assess patient’s behavior around nutrition and physical activity |
38% Not measured |
94% 82% |
<0.001 <0.001 |
Intervention: Learning collaborative
|
| 13. Shaw JS, et al. (2006) Pre-post19 |
Lead poisoning, anemia, tuberculosis, hypertension: Lead screening |
72% | 85% | 0.001 |
Interventions: State-wide learning collaborative with 4 1-day learning sessions
Other comments: Tuberculosis and lead screening were risk assessment and laboratory/skin testing, if indicated. |
| Hematocrit | 70% | 74% | NS | ||
| Vision screening | 62% | 75% | 0.013 | ||
| Tuberculosis screening | 18% | 39% | 0.001 | ||
| Blood pressure | 85% | 82% | NS | ||
| 14. Young PC, et al. (2006) Pre-post18 |
Anemia, vision problems, hypertension, obesity: Hematocrit |
49% | 57% | 0.36 |
Intervention: Learning collaborative
|
| Vision screening | 46% | 75% | 0.007 | ||
| BP screening | 59% | 74% | 0.010 | ||
| BMI recorded | 32% | 45% | 0.078 | ||
| Post intervention with and without a control group | |||||
| 15. Gioia PC. (2001) Post intervention without control group38 |
Lead poisoning: Serum lead level |
Not measured | 81% |
Intervention: EMR with point-of-care reminders displayed on screen Population/setting: Single practice in New York; n=208 patients; children born in 1998 |
|
| 16. Hartmann EE, et al. (2006) Post-intervention without control group34 |
Vision disorders: monocular visual acuity and stereopsis 3 year olds |
Not measured | 70–85% |
Intervention: Vision screening with specific tools for assessing monocular visual acuity and stereopsis.
|
|
| 4 year olds | Not measured | 93–94% | |||
| 17. Hull PC, et al. (2008) Post-intervention with concurrent control group39 |
Lead poisoning, anemia, hearing, vision: “Laboratory testing” (serum lead level and hematocrit) |
74% | 100% | <0.001 |
Intervention: Nurse-led protocol
|
| Hearing | 12% | 100% | <0.001 | ||
| Vision | 23% | 100% | <0.001 | ||
| 18. Niederman LG, et al. (2007) Post-intervention with concurrent control group36 |
Anemia and lead poisoning: Hematocrit |
77% | 73% | NS |
Intervention: Healthy Steps (HS) program implemented in a resident continuity clinic. Population/setting: One academic practice in Illinois; experimental n=71, control n=192 patients; children aged at least 18 months Other comments: Control group were patients in the practice but not enrolled in HS |
| Serum lead level | 64% | 67% | NS | ||
| 19. Ozer EM, et al. (2005) Post-intervention with concurrent control group31 |
Adolescent health risk behaviors: Adolescent health screening questionnaire |
Not measured | 80% | NA |
Intervention: Provider training, patient questionnaire, and prompts to facilitate communication about adolescent risk behaviors 2 stage intervention:
Other comments: Control practices’ screening did not differ over study period |
| Provider asked about alcohol use during visit | 67% | 82% after Stage 1 83% after Stage 2 |
<0.01 <0.001 |
||
| Provider counseled on alcohol use during visit | 59% | 77% after Stage 1 81% after Stage 2 |
<0.01 <0.001 |
||
| 20. Schonwald A, et al. (2009) Post intervention without cuncurrent control group30 |
Behavior and development problems: PEDS |
Not measured | 61% |
Intervention: Implementation of developmental screening using PEDS
Other comments: Use of structured developmental assessments was not routine pre-intervention; authors reported an increase in developmental concerns identified post-intervention (21% vs. 26%, p=0.05); proportion of children referred for developmental concerns did not change post intervention (10% vs 11%). |
|
| Time Series | |||||
| 21. Earls M, et al. (2006) Time series28 |
Developmental problems: ASQ |
24% | 62% at year 2; 76% at year 5 |
Intervention: Quality improvement initiative to improve child development services:
Other comments: No significance testing reported |
|
| 22. King TM, et al. (2010) Time series24 |
Development problems: PEDS or ASQ |
Not measured | 67% at 1 month; 85% at 9 months |
Intervention: Provider and staff education, physician champion identification
Other comments: Post-intervention screening varied among practices (33–100%); no significance testing reported |
|
| 23. Pomietto M, et al. (2009) Time series26 |
Obesity: BMI and weight classification documented in chart |
Not measured | 49% at 1 month; 94% at 9 months |
Intervention: Learning collaborative, combined with community and policy-level interventions.
Other comments: No significance testing reported |
|
Abbreviations:
HS – Healthy Steps
LC – Learning collaborative
BMI – Body mass index
BP – Blood pressure
QI – Quality improvement
HMO – Health maintenance organization
PEDS – Parents’ evaluation of developmental status
EMR – Electronic medical record
EPSDT – Early periodic screening, diagnosis and treatment
ASQ – Ages and stages questionnaire
AAP – American Academy of Pediatrics
RCT – Randomized controlled trial
Types of interventions
The studies described several different types of interventions. The most common interventions were 1) changes to office systems, usually part of a formal quality improvement program such as a learning collaborative, 2) physician and staff education, sometimes facilitated by a “physician champion” of a specific screening test, 3) electronic medical record enhancements (e.g., prompts), and 4) distribution of additional tools for physicians to use when screening or counseling patients. Many studies combined intervention types. In some studies where several practices were enrolled in a quality improvement initiative, specific changes were chosen by each practice. In several studies, quality of preventive care screening was measured along with other preventive care outcomes (e.g., immunizations, preventive care visit attendance, etc).
Twelve articles from ten separate studies18–29 used interventions based largely on learning collaborative methods, including plan-do-study-act cycles and facilitated contact with other intervention practices. Typically, small teams of practitioners and staff from intervention practices addressed barriers related to office system design, provider and staff knowledge gaps, and workflow. Specific changes included chart flagging or routine chart review by non-physician staff to identify patients behind in testing. For some studies, multiple practices participated, multiple screening tests and other preventive care elements were targeted for improvement, and practices were at liberty to choose from several recommended changes those they deemed most likely to work in their practice. Thus, the specific changes associated with the global intervention varied among individual practices. Post-intervention screening ranged from 39–94% of patients screened appropriately. Improvement from baseline varied widely, from 0–80%. Improvement tended to be greater if pre-intervention screening was low or non-existent and if the focus of the intervention was narrowed to specific screening tests or a specific area, such as the study reported by King et al. from a learning collaborative on developmental screening and services.24
Five articles 30–34 described interventions to implement screening using provider training and/or tools for facilitating conversations with parents, such as provider sheets to prompt screening questions or patient questionnaires. These interventions focused on screening for obesity, developmental or mental health problems, or adolescent risky behaviors. Post-intervention screening ranged from 28% (for BMI calculations)32 to 94% (vision screening).34
Two articles35, 36 examined associations between implementing the Healthy Steps program and screening. Healthy Steps is designed for first-time parents and provides co-located developmental specialists to enhance well-child visits.35 Parents also receive home visits, telephone access for developmental questions, written materials, and linkages to community resources. Screening of patients enrolled in Healthy Steps was compared to screening of same-aged patients not enrolled in Healthy Steps (e.g., second-born children) after implementation. Screening for lead poisoning and anemia did not markedly change, but developmental screening doubled, from 41–43% to 82–84%.
Three studies27, 37, 38 examined the effect of EMR enhancements, such as EMR templates and reminders, with varying results. With EMR templates to prompt providers to elicit developmental concerns, screening improved to 65–73% of patients for various areas of development, significant increases from baseline.37 EMR reminders enabled near universal screening (99%) of patients if providers were able to obtain lead levels at the visit, but only 41% for patients required by insurance to have levels drawn off-site.38 For Chlamydia screening, reminders had no effect compared to patient charts without reminders.27
In two studies,39, 40 a nurse and a nurse practitioner were employed to identify and track patients in need of screening. Both interventions involved protocols for identifying and tracking which patients were due for testing or follow up of abnormal tests. Hull et al. found that a nurse-driven protocol to identify and screen patients was highly effective and achieved essentially universal screening in one practice.39 Block et al. found that a similar intervention achieved improved documentation of a follow up plan for elevated lead levels, but smaller improvements for follow-up testing and parent education.40
Interventions to increase follow up of abnormal screening results
We found little evidence about interventions to improve post-visit follow-up or referral completion, once screening tests identified concerns. As mentioned, Block et al.40 examined the effect of a nurse-driven protocol to increase retesting and parent education for abnormal lead levels. Retesting increased to 65% of those with abnormal levels, and 32% of families with persistently high levels received education. Two other studies31, 33 examined discussion with patients and parents following screening tests for behavior problems or risky behaviors. Both studies found that patient/provider handouts facilitated discussion of problems detected using formal assessment tools. Schonwald et al.30 demonstrated that referrals for developmental evaluation remained the same, despite increases in use of formal screening tools.
Discussion
Three key findings emerged from this review of interventions to improve the quality of preventive care screening in pediatric primary care settings. First, most studies reported improved quality of screening post-intervention, usually a modest improvement, although differences were variable across and within studies. Second, because of variable findings, heterogeneous interventions, and relatively few studies with control groups, we could not discern whether a particular type or form of intervention is superior for improving screening. However, we saw patterns where successful interventions tended to emphasize collaborative learning, office-systems changes, and tracking progress over time. Third, we found few interventions that aimed to improve follow-up of abnormal screening results, which offers opportunities for further investigation.
From the articles reviewed, we found screening in pediatric offices generally improved after interventions were implemented. In studies where pre- and post-intervention outcomes with statistical testing were reported, over 80% of interventions demonstrated improvement in at least one area of screening. However, results varied, ranging from no change to an 8-fold increase in the proportion of children screened, and many studies could not control for secular trend with their study designs. The magnitude of the impact of interventions seemed greater when pre-intervention screening was low, and multi-faceted interventions implemented through a learning collaborative structure appeared to be, of all intervention types, more robustly studied and relatively effective. Otherwise, this review identified little regarding the patterns of variable effects or reasons for them, including type of screening or type of intervention. In addition, results varied among practices implementing similar interventions; even when an intervention was introduced in multiple practices as a single study, effects typically varied from practice to practice. No study objectively measured contextual factors (e.g., practice’s motivation to change, staff capacity for the intervention), although some studies included qualitative discussion on contextual reasons for variability in findings across practices (e.g., physician champion left the practice).
With the exception of four studies, fewer than 85% of patients were appropriately screened post-intervention, with most studies reporting post-intervention screening between 50–75%. This finding, which mirrors findings in adult studies,41 suggests that some patients miss screening despite often intensive office-based improvements. Studies in our review that examined characteristics of patients who were not screened found various associations with less screening, including non-English speaking parents, parents who did not have time to complete the screening tool before seeing the physician, and having to go off-site to complete screening tests.30, 37, 38 Furthermore, this finding suggests a “ceiling effect” similar to that found with interventions to increase rates of vaccine coverage and well-child visit attendance.42, 43
The quality of the studies varied, with many using non-randomized study designs, a limited number of practice sites, and with little account for context of the practices receiving intervention. However, five articles reported on randomized trials with consistent positive effects. Most studies were pre-post designs without randomization, and some lacked comparison groups, making it difficult to assess the effect of natural trends over time. Most studies involved multiple practices, but seven studies used only one practice site, limiting the ability to draw conclusions about how broader-based improvement efforts would increase the quality of screening. Because office staff motivation and technological savvy can play a large role in the success of interventions,44 practices differing in these contextual factors would likely have different results.
Most interventions were multifaceted, involving several alterations in office workflow, physician and staff education, and changes in staff time allocation. While multifaceted interventions generally had more success, as did interventions tailored to best fit specific practices, no systematic approach examined which elements provide the greatest benefit, or why the same intervention performed better in some practices than others. Findings from such a systematic approach could be used to design more efficient interventions and advance the field of quality improvement research.
Few studies examined the quality of follow-up care, and few interventions contained elements specifically targeting follow-up of abnormal tests. However, the few studies that did have follow-up as an outcome found 35–65% of patients did not receive follow up care after an abnormal screening result. This finding indicates the need to include outcomes related to follow-up in studies of screening, and that measuring screening alone may overestimate changes in identification and treatment of conditions.
We found no studies testing the effects of performance incentives or physician feedback. This strategy has been studied more in adult settings for screening9, 45 and in pediatrics for immunizations, attendance at well-child visits, and management of chronic conditions.46 Another review of adult cancer screening interventions focused on motivating patients and reducing barriers to care.47 These reviews found variable effects among similar interventions, with most interventions associated with some increase in screening.
The review has several limitations. Many quality improvement interventions do not reach publication, which could have limited identification of informative studies. The search terms used may not have captured all relevant studies, particularly studies examining quality of follow-up care, for which search terms were difficult to define. Many studies tested heterogeneous interventions that were modified for each practice; some interventions were multifaceted so that practices could choose specific elements to implement. This “cafeteria” approach makes comparing interventions in separate studies difficult and may limit reliability and generalizability. However, tailoring the intervention to the context of the practice likely increased the chance of the desired effect, and is more representative of how it would be applied in actual practice.
Conclusion
Although the quality of studies varied, we found a moderate level of evidence that interventions are effective in improving screening in pediatric practices. This review also reveals several avenues for future study that will guide policy makers and practitioners in what specific interventions provide the most value.
Interventions reviewed here appeared to have ceiling effects, which invites the question, given the broad aims of pediatric primary care, what should be the goals for screening, and is there a point of diminishing return where a practice’s extra efforts exceed the value of the gain? Policies around reimbursement based on screening performance should match the right amount of effort to achieve the right rate. Also, improving screening rates from a high baseline will likely require different interventions; near-perfect screening may not be achievable without a large degree of automation and standardization and multiple layers of double-checks performed by non-clinicians or through electronic mechanisms. Lastly, when aiming for high proportions of children appropriately screened, defining the right denominator becomes increasingly important and worth measuring accurately and thoughtfully. A denominator measured by well child visits, versus empanelled patients, might drive different interventions with ultimately different outcomes.
No single type of intervention arose as consistently more effective in increasing screening quality, and few studies addressed the critical issue of assuring adequate follow-up. This review did not identify specific interventions that work better than others, however multi-faceted, practice-tailored interventions with ongoing outcome assessment seemed to be effective, and most comprehensively evaluated. Policies supporting such interventions broadly will likely lead to earlier detection and more effective treatment for a large population of children. Quality improvement activities are now required for maintenance of board certification, and many local health systems and payers ask or require practices to participate. Medical societies, such as the American Academy of Pediatrics, can help provide infrastructure to encourage efforts by individual practices.
This review leaves several additional questions: Which components of interventions add to effectiveness, and which are ineffective? What interventions improve follow-up care? How sustainable are the effects of these interventions? Are different interventions more effective for different types of screening procedures (e.g., questionnaires versus blood draws)? How is practice context best measured, and how is it associated with the success of interventions? Such future avenues for research will help refine interventions to move toward effective, efficient screening in primary care pediatrics.
Acknowledgments
Funding: Maternal and Child Health Bureau, cooperative agreement # 5 U53MC04473-03-00
We are grateful to the Maternal and Child Health Bureau, who provided funding for this study (Cooperative agreement # 5 U53MC04473-03-00).
Abbreviations
- MCHB
Maternal and Child Health Bureau
- HS
Healthy Steps
- LC
Learning collaborative
- BMI
Body mass index
- BP
Blood pressure
- QI
Quality improvement
- HMO
Health maintenance organization
- PEDS
Parents’ evaluation of developmental status
- EMR
Electronic medical record
- EPSDT
Early periodic screening, diagnosis and treatment
- ASQ
Ages and stages questionnaire
- AAP
American Academy of Pediatrics
- RCT
Randomized controlled trial
Footnotes
No conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young PC. Prevention: a new focus for the country but old stuff for pediatricians. Acad Pediatr. 2010 Nov-Dec;10(6):367–368. doi: 10.1016/j.acap.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Practice and Ambulatory Medicine and Bright Futures Steering Committee. Recommendations for Preventive Pediatric Health Care. Pediatrics. 2007;120:1376. [Google Scholar]
- 3.AHRQ Publication No 10-05145. Agency for Healthcare Research and Quality; Rockville, MD: Sep, 2010. [Accessed October 26, 2011]. Guide to Clinical Preventive Services, 2010–2011. http://www.ahrq.gov/clinic/pocketgd.htm. [Google Scholar]
- 4.Mangione-Smith R. Bridging the quality chasm for children: need for valid, comprehensive measurement tools. Arch Pediatr Adolesc Med. 2007 Sep;161(9):909–910. doi: 10.1001/archpedi.161.9.909. [DOI] [PubMed] [Google Scholar]
- 5.Minniear TD, Gilmore B, Arnold SR, Flynn PM, Knapp KM, Gaur AH. Implementation of and barriers to routine HIV screening for adolescents. Pediatrics. 2009 Oct;124(4):1076–1084. doi: 10.1542/peds.2009-0237. [DOI] [PubMed] [Google Scholar]
- 6.Honigfeld L, McKay K. Barriers to enhancing practice-based developmental services. J Dev Behav Pediatr. 2006 Feb;27(1 Suppl):S30–33. doi: 10.1097/00004703-200602001-00009. discussion S34–37, S50-32. [DOI] [PubMed] [Google Scholar]
- 7.Ayres CG, Griffith HM. Perceived barriers to and facilitators of the implementation of priority clinical preventive services guidelines. Am J Manag Care. 2007 Mar;13(3):150–155. [PubMed] [Google Scholar]
- 8.Klabunde CN, Lanier D, Breslau ES, et al. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007 Aug;22(8):1195–1205. doi: 10.1007/s11606-007-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulscher ME, Wensing M, Grol RP, van der Weijden T, van Weel C. Interventions to improve the delivery of preventive services in primary care. American Journal of Public Health. 1999 May;89(5):737–746. doi: 10.2105/ajph.89.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest CB, Simpson L, Clancy C. Child health services research. Challenges and opportunities. JAMA. 1997 Jun 11;277(22):1787–1793. [PubMed] [Google Scholar]
- 11.Maternal and Child Health Bureau. [Accessed March 1, 2011];Achieving and measuring success: A national agenda for children with special health care needs. http://mchb.hrsa.gov/programs/specialneeds/measuresuccess.htm.
- 12.Kuhlthau KA, Bloom S, Van Cleave J, et al. Evidence for family-centered care for children with special health care needs: a systematic review. Acad Pediatr. Mar-Apr;11(2):136–143. e138. doi: 10.1016/j.acap.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Bloom S, Kuhlthau K, Van Cleave J, Knapp A, Newacheck P, Perrin J. Health Care Transition for Youth with Special Health Care Needs. Journal of Adolescent Health. doi: 10.1016/j.jadohealth.2012.01.007. In press. [DOI] [PubMed] [Google Scholar]
- 14.Homer CJ, Klatka K, Romm D, et al. A review of the evidence for the medical home for children with special health care needs. Pediatrics. 2008 Oct;122(4):e922–937. doi: 10.1542/peds.2007-3762. [DOI] [PubMed] [Google Scholar]
- 15.Newacheck PW, Houtrow AJ, Romm DL, et al. The future of health insurance for children with special health care needs. Pediatrics. 2009 May;123(5):e940–947. doi: 10.1542/peds.2008-2921. [DOI] [PubMed] [Google Scholar]
- 16.W.K. Kellogg Foundation. [Accessed April 21, 2010];Logic Model Development Guide. 2004 Jan; http://www.wkkf.org/knowledge-center/resources/2010/Logic-Model-Development-Guide.aspx.
- 17.Committee on Quality of Health Care in America, Institute of Medicine. . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. [PubMed] [Google Scholar]
- 18.Young PC, Glade GB, Stoddard GJ, Norlin C. Evaluation of a learning collaborative to improve the delivery of preventive services by pediatric practices. Pediatrics. 2006 May;117(5):1469–1476. doi: 10.1542/peds.2005-2210. [DOI] [PubMed] [Google Scholar]
- 19.Shaw JS, Wasserman RC, Barry S, et al. Statewide quality improvement outreach improves preventive services for young children. Pediatrics. 2006 Oct;118(4):e1039–1047. doi: 10.1542/peds.2005-2699. [DOI] [PubMed] [Google Scholar]
- 20.Margolis PA, Lannon CM, Stuart JM, Fried BJ, Keyes-Elstein L, Moore DE., Jr Practice based education to improve delivery systems for prevention in primary care: randomised trial. BMJ. 2004 Feb 14;328(7436):388. doi: 10.1136/bmj.38009.706319.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lannon CM, Flower K, Duncan P, Moore KS, Stuart J, Bassewitz J. The Bright Futures Training Intervention Project: implementing systems to support preventive and developmental services in practice. Pediatrics. 2008 Jul;122(1):e163–171. doi: 10.1542/peds.2007-2700. [DOI] [PubMed] [Google Scholar]
- 22.Bordley WC, Margolis PA, Stuart J, Lannon C, Keyes L. Improving preventive service delivery through office systems. Pediatrics. 2001 Sep;108(3):E41. doi: 10.1542/peds.108.3.e41. [DOI] [PubMed] [Google Scholar]
- 23.Shafer MA, Tebb KP, Pantell RH, et al. Effect of a clinical practice improvement intervention on Chlamydial screening among adolescent girls. JAMA. 2002 Dec 11;288(22):2846–2852. doi: 10.1001/jama.288.22.2846. [DOI] [PubMed] [Google Scholar]
- 24.King TM, Tandon SD, Macias MM, et al. Implementing developmental screening and referrals: lessons learned from a national project. Pediatrics. 2010 Feb;125(2):350–360. doi: 10.1542/peds.2009-0388. [DOI] [PubMed] [Google Scholar]
- 25.Polacsek M, Orr J, Letourneau L, et al. Impact of a primary care intervention on physician practice and patient and family behavior: keep ME Healthy---the Maine Youth Overweight Collaborative. Pediatrics. 2009 Jun;123( Suppl 5):S258–266. doi: 10.1542/peds.2008-2780C. [DOI] [PubMed] [Google Scholar]
- 26.Pomietto M, Docter AD, Van Borkulo N, Alfonsi L, Krieger J, Liu LL. Small steps to health: building sustainable partnerships in pediatric obesity care. Pediatrics. 2009 Jun;123( Suppl 5):S308–316. doi: 10.1542/peds.2008-2780J. [DOI] [PubMed] [Google Scholar]
- 27.Scholes D, Grothaus L, McClure J, et al. A randomized trial of strategies to increase chlamydia screening in young women. Prev Med. 2006 Oct;43(4):343–350. doi: 10.1016/j.ypmed.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Earls MF, Hay SS. Setting the stage for success: implementation of developmental and behavioral screening and surveillance in primary care practice--the North Carolina Assuring Better Child Health and Development (ABCD) Project. Pediatrics. 2006 Jul;118(1):e183–188. doi: 10.1542/peds.2006-0475. [DOI] [PubMed] [Google Scholar]
- 29.Tebb KP, Wibbelsman C, Neuhaus JM, Shafer MA. Screening for asymptomatic Chlamydia infections among sexually active adolescent girls during pediatric urgent care. Arch Pediatr Adolesc Med. 2009 Jun;163(6):559–564. doi: 10.1001/archpediatrics.2008.570. [DOI] [PubMed] [Google Scholar]
- 30.Schonwald A, Huntington N, Chan E, Risko W, Bridgemohan C. Routine developmental screening implemented in urban primary care settings: more evidence of feasibility and effectiveness. Pediatrics. 2009 Feb;123(2):660–668. doi: 10.1542/peds.2007-2798. [DOI] [PubMed] [Google Scholar]
- 31.Ozer EM, Adams SH, Lustig JL, et al. Increasing the screening and counseling of adolescents for risky health behaviors: a primary care intervention. Pediatrics. 2005 Apr;115(4):960–968. doi: 10.1542/peds.2004-0520. [DOI] [PubMed] [Google Scholar]
- 32.Dunlop AL, Leroy Z, Trowbridge FL, Kibbe DL. Improving providers’ assessment and management of childhood overweight: results of an intervention. Ambul Pediatr. 2007 Nov-Dec;7(6):453–457. doi: 10.1016/j.ambp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Applegate H, Kelley ML, Applegate BW, Jayasinghe IK, Venters CL. Clinical case study: pediatric residents’ discussions of and interventions for children’s behavioral and emotional problems. J Pediatr Psychol. 2003 Jul-Aug;28(5):315–321. doi: 10.1093/jpepsy/jsg021. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann EE, Bradford GE, Chaplin PK, et al. Project Universal Preschool Vision Screening: a demonstration project. Pediatrics. 2006 Feb;117(2):e226–237. doi: 10.1542/peds.2004-2809. [DOI] [PubMed] [Google Scholar]
- 35.Minkovitz CS, Hughart N, Strobino D, et al. A practice-based intervention to enhance quality of care in the first 3 years of life: the Healthy Steps for Young Children Program. JAMA. 2003 Dec 17;290(23):3081–3091. doi: 10.1001/jama.290.23.3081. [DOI] [PubMed] [Google Scholar]
- 36.Niederman LG, Schwartz A, Connell KJ, Silverman K. Healthy Steps for Young Children program in pediatric residency training: impact on primary care outcomes. Pediatrics. 2007 Sep;120(3):e596–603. doi: 10.1542/peds.2005-3090. [DOI] [PubMed] [Google Scholar]
- 37.Adams WG, Mann AM, Bauchner H. Use of an electronic medical record improves the quality of urban pediatric primary care. Pediatrics. 2003 Mar;111(3):626–632. doi: 10.1542/peds.111.3.626. [DOI] [PubMed] [Google Scholar]
- 38.Gioia PC. Quality improvement in pediatric well care with an electronic record. Proc AMIA Symp. 2001:209–213. [PMC free article] [PubMed] [Google Scholar]
- 39.Hull PC, Husaini BA, Tropez-Sims S, Reece M, Emerson J, Levine R. EPSDT preventive services in a low-income pediatric population: impact of a nursing protocol. Clin Pediatr (Phila) 2008 Mar;47(2):137–142. doi: 10.1177/0009922807306167. [DOI] [PubMed] [Google Scholar]
- 40.Block B, Szekely K, Escobar M. Difficulties in evaluating abnormal lead screening results in children. Journal of the American Board of Family Practice. 1996 Nov-Dec;9(6):405–410. [PubMed] [Google Scholar]
- 41.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. May 18;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 42.Hambidge SJ, Davidson AJ, Phibbs SL, et al. Strategies to improve immunization rates and well-child care in a disadvantaged population: a cluster randomized controlled trial. Arch Pediatr Adolesc Med. 2004 Feb;158(2):162–169. doi: 10.1001/archpedi.158.2.162. [DOI] [PubMed] [Google Scholar]
- 43.Baker AM, McCarthy B, Gurley VF, Yood MU. Influenza immunization in a managed care organization. J Gen Intern Med. 1998 Jul;13(7):469–475. doi: 10.1046/j.1525-1497.1998.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feifer C, Nemeth L, Nietert PJ, et al. Different paths to high-quality care: three archetypes of top-performing practice sites. Ann Fam Med. 2007 May-Jun;5(3):233–241. doi: 10.1370/afm.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabatino SA, Habarta N, Baron RC, et al. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008 Jul;35(1 Suppl):S67–74. doi: 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Chien AT, Conti RM, Pollack HA. A pediatric-focused review of the performance incentive literature. Current Opinion in Pediatrics. 2007 Dec;19(6):719–725. doi: 10.1097/MOP.0b013e3282f1eb70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008 Jul;35(1 Suppl):S34–55. doi: 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]