Abstract
Purpose
To prolong the release of a heparan sulfate binding peptide, G2-C, using a commercially available contact lens as a delivery vehicle and to demonstrate the ability of the released peptide to block herpes simplex virus-1 (HSV-1) infection using in vitro, ex vivo, and in vivo models of corneal HSV-1 infection.
Methods
Commercially available contact lenses were immersed in peptide solution for 5 days prior to determining the release of the peptide at various time points. Cytotoxicity of the released samples was determined by MTT and cell cycle analysis, and the functional activity of the released samples were assessed by viral entry, and viral spread assay using human corneal epithelial cells (HCE). The ability to suppress infection in human and pig cornea ex vivo and mouse in vivo models were also assessed.
Results
Peptide G2-C was released through the contact lens. Following release for 3 days, the peptide showed significant activity by inhibiting HSV-1 viral entry and spread in HCE cells. Significant suppression of infection was also observed in the ex vivo and in vivo experiments involving corneas.
Conclusions
Extended release of an anti–HS peptide through a commercially available contact lens can generate significant anti–HSV-1 activity and provides a new and effective way to control corneal herpes.
Keywords: virus infection, contact lens, peptide, corneal epithelium
Herpes simplex virus-1 (HSV-1) is a double-stranded DNA virus widely prevalent in humans and estimated to have infected 60% to 90% of the population.1 Primary and recurrent ocular herpetic infections cause corneal scarring, neovascularization, and epithelium and stromal keratitis; in severe cases, these result in blindness.2,3 While the current available topical treatments include the use of trifluorothymidine (TFT) or the use of ganciclovir gels, oral treatments involve the use of steroids, acyclovir and its analogues valacyclovir that are nucleoside analogues. Dual treatments with steroids are also prescribed by clinicians.4 However, some limitations to the above mentioned treatments include the following:
Eye drops constitute ∼90% of ophthalmic medications and have advantages, including ease of administration, safety and efficacy, but this is limited by low residence time due to rapid drainage from the lacrimal ducts resulting in frequent applications5,6;
Increasing cases of resistance to acyclovir, the first line of therapy in HSV infections7,8;
Use of steroids causes ocular complications such as cataract and steroid-induced glaucoma9–11; and
TFT has been shown to pose toxicity at high doses and hence cannot be prescribed for prolonged usage.12,13
While all of these treatments are effective, the limitations pose the requirement of new treatment options for ocular herpes, which may also include improved methods of administration.
The envelop glycoprotein D (gD) of HSV-1 is essential for HSV-1 entry and infection.14,15 To date, the following surface receptors have been identified that bind gD and allow infection to proceed: herpesvirus entry mediator (HVEM), Nectin-1 and ‐2, and 3-O-sulfated heparan sulfate (3-OS-HS), a rare modification of the polysaccharide heparan sulfate (HS).15,16 Heparan sulfate is a highly abundant and ubiquitously expressed cell surface receptor, which is also a key molecule for infection as it is required for attachment, viral surfing, capsid penetration, and its removal is needed during egress of newly generated virions.16–18 Therefore, in our quest to target this very common entry coreceptor for antiviral effects, prior studies in our laboratory identified two 12-mer peptides, called G1 and G2, which showed strong anti–HSV-1 properties.19 The peptides G1 and G2 were specifically selected against HS and 3-OS-HS respectively. The in vivo prophylactic activity of the peptides was confirmed using a corneal infection model.19 In another study from our laboratory, the therapeutic effect of G2 along with acyclovir as a potential dual therapy was also tested.20
The aim of this study was to determine the antiviral efficacy of a relatively more stable variant of G2 peptide, G2-C, delivered via contact lenses for extended antiviral therapy. Herpes simplex virus 1 infects corneal epithelium where HS is normally prevalent during initial infection.20 The parental G2 peptide is known to inhibit the interaction19 of virus with HS, but it must be maintained for extended periods to maintain activity. As a potential alternative, many studies have successfully investigated the use of contact lenses for drug delivery to improve the bioavailability and residence time of topically applied drugs.21 Using a similar strategy, we wanted to deliver the G2-C peptide using a vehicle that would release the peptide over an extended period of time, thereby increasing the residence time of the peptide on the corneal epithelium. Our results demonstrate that a commercially available contact lens loaded with the G2-C peptide solution is able to prolong the release of the peptide and the released peptide is able to block HSV-1 infection and replication in natural target cells as well as in corneal infection models.
Materials and Methods
Peptide Synthesis and Preparation
The peptide G2 (MPRRRRIRRRQK) that was previously described19 was modified by attaching a cysteine residue at the C-terminus of the peptide (MPRRRRIRRRQKC). The peptide was synthesized at the University of Illinois at Chicago Research Resources Center. The purity (>95%) and molecular weight (1802 g/mol) of the peptide was confirmed by high performance liquid chromatography and mass spectrometer respectively. The peptide was dissolved in sterile PBS to obtain concentrations of 10 mg/mL and was stored at −20°C.
Cells, Viruses, and Contact Lenses
The human corneal epithelial (HCE; obtained from Kozaburo Hayashi, National Eye Institute, Bethesda, MD, USA) and the African green monkey kidney (Vero; obtained from Patricia G. Spear, Northwestern University, Chicago, IL, USA) cell lines were used in this study. Vero and HCE cells were cultured in MEM and Dulbecco's modified Eagle's medium (DMEM; Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich Corp., St. Louis, MO, USA) and 1% penicillin/streptomycin (Gibco, Life Technologies), respectively. The viruses HSV-1 (KOS) gL86, HSV-1 (KOS; all obtained from Patricia G. Spear) and HSV-1 (K26GFP; obtained from Prashant Desai, Johns Hopkins University, Baltimore, MD, USA) were used in this study.22 The viruses were propagated and titered on Vero cells and the stocks were stored at −80°C. The contact lenses used in this study were purchased commercially (Cooper Vision Biomedic 55; 1-800 CONTACTS, Orem, UT, USA). Contact lenses were composed of 45% ocufilcon D and 55% of water with a diameter of 14.20, power of −2.5 and base curve of 8.6 mm.
Standard Curve of the Peptide
A standard curve was designed by serially diluting the G2-C peptide or bovine serum albumin (BSA) in sterile PBS (Gibco, Life Technologies). The absorbance of the peptide was calculated at 210 nm using the spectrophotometer (DU 800; Beckman Coulter, Brea, CA, USA).
Diffusion Experiments
In a Franz cell diffusion chamber containing water jacket, PBS was added to the receiver chamber. A contact lens was placed between the receiver chamber and donor chamber, containing 1 mg/mL G2-C peptide solution or equimolar amounts of BSA, as a control. To seal the chamber, a rubber gasket (∼1 mm) was placed on both sides of the contact lens. Constant stirring using magnetic stirrers and constant temperature at 37°C was maintained. At indicated intervals of time, a volume (500 μL) through the sampling arm was removed and replaced with fresh, sterile PBS. Absorbance values at 210 nm were measured using the spectrophotometer and the concentration of the peptide or BSA was interpolated using the standard curve.
Loading Experiments
The contact lenses were washed thoroughly for 5 days in 1 L sterile deionized water. The washed contact lenses were then immersed in 100 μL 10 mg/mL G2-C peptide and placed in water bath (37°C) for 5 days. Sterile PBS was used as a control. After 5 days, dilutions of the peptide were made and the absorbance of the peptide at 210 nm was obtained. Using this absorbance value, the concentration of the peptide was interpolated from the standard curve and subtracted from the initial concentration of the peptide used for loading to determine the amount of peptide retained in the contact lenses.
Release Studies
After loading the peptide with the contact lenses, the lenses were quickly immersed in 1 mL sterile PBS in Eppendorf tubes to desorb any loosely bound peptide. The absorbance and the concentration of the eluted solution was obtained and subtracted from the concentrations obtained in the loading experiments. The lenses were then placed in 1 mL PBS in sterile Eppendorf tubes and were placed in a 37°C water bath. At indicated time, the PBS was removed and replaced with fresh sterile PBS. The absorbance was calculated at 210 nm and using the standard curve the concentrations of the released peptide were obtained. The background obtained from the PBS only samples were subtracted. For all functional assays, the release was carried until day 3 (without changing PBS) and the peptide solution was removed and stored at −20°C.
Cytotoxic Assays
We performed the MTT assay23 on HCE cells. The cells were plated on 96-well cell culture plates and were treated with the PBS and G2-C released samples (dissolved in MEM) for 24 hours. After 24 hours, the cells were carefully washed with PBS and 0.5 mg/mL MTT (Promega, Madison, WI, USA) in MEM were added to the cells. After 4 hours, the color was developed and read on a microplate reader (TECAN, Mannedorf, Switzerland). Cell cycle analysis was also examined to determine the influence of the drug on replication of the cells. Human corneal epithelial cells in a 12-well cell culture plate were treated with the PBS and G2-C released samples. Twenty-four hours after treatment, the cells were harvested, fixed in 100% ethanol (Thermo Fisher Scientific, Waltham, MA, USA), treated with 10 μg/mL RNAse A (Thermo Fisher Scientific), and stained with 50 μg/mL propidium iodide (Fluka; Sigma-Aldrich Corp.) in PBS. Using FACS (LSR Fortessa; BD Biosciences, Franklin Lakes, NJ, USA), the cells were analyzed to determine different stages of the cell cycle.
Entry Assay
Confluent monolayers of HCE cells in a 96-well cell culture plate were pretreated with the PBS and G2-C released samples for 30 minutes at 37°C followed by infection with HSV-1 gL86 virus at a multiplicity of infection (MOI) of 5. Six hours post infection (hpi), the cells were washed with PBS and a solution containing 3 mg/mL β-galactosidase substrate: o-nitrophenyl-β-galactoside (ONPG; Thermo Fisher Scientific) and 0.5% Nonidet P40 (USB Corporation, Cleveland, OH, USA) in PBS were added. The color development was quantified using a microplate plate reader.
Immunofluorescence Imaging
We grew HCE cells in 6-well glass-bottom plates (MatTek Corporation, Ashland, MA, USA) and treated with PBS and G2-C released samples for 30 minutes at 37°C. A high MOI of 50 was used to infect the HCE cells with HSV-1 (KOS) K26-GFP virus22 and the cells were incubated at 4°C to allow viral adsorption. Entry was initiated by incubating the plate at 37°C for 15 minutes and the cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA), permeabilized with 0.01% Triton-X (Thermo Fisher Scientific), and stained with rhodamine-phalloidin (Invitrogen, Thermo Fisher Scientific). We used 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies) as a counterstain. The images were captured under ×63 objectives using an observer microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with a spinning disk (CSU-X1; Yokogawa, Tokyo, Japan). We used digital imaging software (Axiovision; Carl Zeiss Microscopy GmbH) was used to quantify fluorescence intensity.
Virus Spread Assay
Human corneal epithelial cells in a 24-well cell culture plate were grown and pretreated with PBS and G2-C released samples for 30 minutes at 37°C. They were then infected with HSV-1 at 0.00001 MOI for 2 hours at 37°C following which the cells were washed and incubated with MEM containing 1% methyl cellulose (Sigma-Aldrich Corp.). At 72 hours post infection, the cells were fixed with 100% methanol (Thermo Fisher Scientific) and stained with crystal violet. The plaques were visualized and counted manually and the plate was photographed using a digital single-lens reflex camera.
Ex Vivo Infection
All human subject research was reviewed by the University of Illinois at Chicago (UIC) Institutional Review Board and deemed exempt human research, where human tissues were discarded surgical tissue. All animal experiments were reviewed by the UIC Animal Care Committee and the experiments were performed in adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision research. Human cadaver corneas aged 2 to 3 weeks (provided by Ali R. Djallian, University of Illinois at Chicago, Chicago, IL, USA) and freshly donated pig corneas (Pack Parking, Inc., Chicago, IL, USA) were washed with sterile PBS and disinfected for 1 hour at 37°C in MEM media supplemented with 5% antibiotic-antimycotic (Gibco, Life Technologies) and 1% insulin–transferrin–sodium selenite (Sigma-Aldrich Corp.). Viability of cells in these corneas was checked by a protocol described previously.24,25 Briefly, the corneas were immersed in 0.4% trypan blue solution (Sigma-Aldrich Corp.) for 2 to 3 minutes and washed twice with sterile PBS before observing them under the microscope (Axiovert 200; Carl Zeiss Microscopy GmbH). We observed near complete viability of cells. Using a 23-G needle, the cornea was gently scarred in a 3 × 3 grid pattern and was cut into two halves. One half was treated with PBS released sample and the other half was treated with G2-C released sample for 30 minutes at 37°C, following which the halves were infected with HSV-1 (KOS) at 106 plaque forming units (pfu) for 2 hours. The tissues were then washed with PBS and cultured for 24 hours.
Ex Vivo Immunoblotting
At 24 hours post infection, the supernatants from human corneal sections were collected and titered as mentioned below and the corneas were washed with ice cold PBS and the epithelial cell layer from the infected corneal halves were gently scraped off with a sterile surgical scalpel into radio-immunoprecipitation assay (Sigma-Aldrich Corp.) buffer containing protease-phosphatase cocktail inhibitor (Halt; Thermo Fisher Scientific), incubated on ice for 30 minutes, and centrifuged at 16,000g for 15 minutes at 4°C. The supernatants were collected and electrophoresed on a denaturing SDS-polyacrylamide gel (4%–12% Bis-Tris NuPage; Novex). Proteins on the gel were transferred onto a PVDF membrane using a dry blotting system (iBlot; Life Technologies), followed by blocking of nonspecific binding with 5% nonfat milk in Tris-buffered saline (TBS), incubation with primary and then horseradish peroxidase–conjugated secondary antibodies. The membrane was developed using a commercial substrate (Femto-Sensitivity ECL; Thermo Fisher Scientific), and the chemiluminescence was detected with a digital image system (ImageQuant LAS4000; GE Life Sciences). Protein band density quantification was performed using image analysis software (ImageQuant TL; GE Life Sciences), using housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control.
Ex Vivo Virus Secretion Titer
Vero cells in a 12-well tissue culture dish were infected with the supernatants, obtained from the human corneal sections, and diluted in medium (Opti-MEM; Gibco, Life Technologies) for 2 hours. After infection, the cells were washed with PBS and incubated at 37°C with DMEM with 1% methylcellulose to prevent secondary plaque formation. At 72 hours post infection, cells were fixed with 100% methanol and subsequently stained with crystal violet. Plaques were counted manually.
Immunohistochemistry
At 24 hours post infection, pig corneal sections were washed in ice-cold PBS, embedded in a commercial compound (Tissue-Tek O.C.T.; Sakura, Zoeterwoude, The Netherlands), flash frozen in dry ice/ethanol mixture, and stored at −80°C. Cryosections were cut using a cryostat (Minotome; Cole Parmer, Vernon Hills, IL, USA) at 5 μm thickness. The sections were washed twice in tris-buffered saline (Bio-Rad Laboratories, Hercules, CA, USA) with 0.025% Triton X-100 (Thermo Fisher Scientific) and blocked with 1% BSA for 2 hours. Primary HSV-1 antibody (ab9533; Abcam, Cambridge, UK) diluted in TBS with 1% BSA (1:200) was added to the sections and incubated overnight at 4°C. Following two washes in TBS with Triton X-100, the sections were incubated in the dark with anti-mouse IgG-FITC (Sigma-Aldrich Corp.) and diluted in TBS with 1% BSA (1:200) for 1 hour at room temperature. Additional washes followed and the sections were mounted in medium (VectaShield; Vector Laboratories, Burlingame, CA, USA) containing DAPI and imaged under a confocal microscope at ×63 (Zeiss 710; Carl Zeiss Microscopy GmbH).
Mouse Cornea Infection
We housed BALB/C mice (male and female, 3 weeks old) at the University of Illinois at Chicago animal Facility. Mice were anesthetized using ketamine (100 mg/kg) and xylazine (5 mg/kg). The right eye of the mice was prescarred with a 30-G sterile needle in a 3 × 3 grid pattern following which 20 μL released PBS and released G2-C peptide were added. We used PBS as a control. Infection using HSV-1 K26-GFP virus at 5 × 105 pfu followed by another round of treatment with the released PBS and released G2-C was performed. At 24 hours post infection, the mice received another round of treatment.
Mouse Cornea Imaging and Virus Titer
At 48 hours post infection, using a previously described protocol,26 fluorescence images of the mouse eyes were captured using a stereo and zoom microscope (SteREO Discovery.V20; Carl Zeiss Microscopy GmbH). Briefly, proparacaine (5 mL, 0.5%; Bausch & Lomb, Tampa, FL, USA) was applied to the right eye of an anesthetized mouse for 5 minutes. The pupil was constricted with 0.01% carbachol (Miostat Alcon, Fort Worth, TX, USA) for 5 minutes following which the mouse was placed on the stereoscope stage with mouse adaptor (RWD Life Science, San Diego, CA, USA) to acquire images. To determine HSV-1 viral titers, the mouse was euthanized and the right eye was removed and stored overnight at −80°C. Using a mechanical tissue homogenizer, individual mouse eyes were homogenized in medium (Gibco, Life Technologies), centrifuged at 16,000g at 4°C to pellet out the debris, and diluted in medium (Gibco, Life Technologies) before infecting Vero cells.
Statistical Analysis
Statistical software (GraphPad Prism 6; GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the data. Data represents the means of three independent experiments unless otherwise specified and the error bars represent SEM. Statistical significance was determined using Student's t-test and 2-way ANOVA. Asterisks denote significant difference: *P ≤ 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Results
Addition of Cysteine Does Not Change the Antiviral Activity of the Peptide
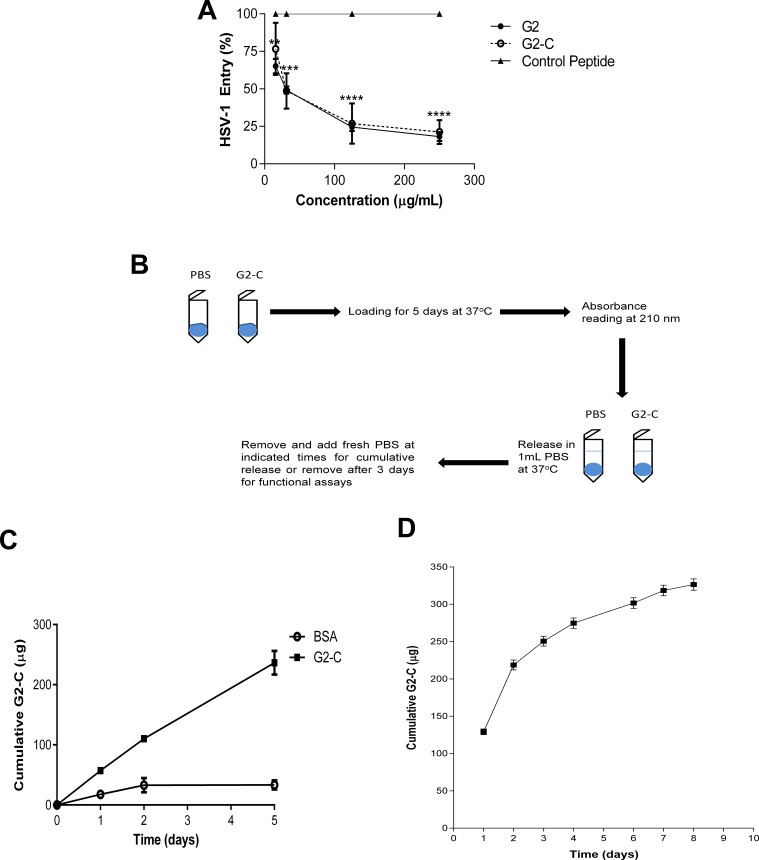
To increase the stability and improve the activity of the G2 peptide, a single cysteine was added to the peptide. The addition of cysteine to the C-terminus of a peptide is one of the common peptide modifications that have been used to increase stability.27,28 The peptides G2 and the G2-C were synthesized with high purity (>95%; see Supplementary Fig. S1) by a protocol described previously.29 We first tested whether the addition of cysteine to the G2 peptide would significantly affect the entry inhibiting activity of the peptide using an HSV-1 viral entry assay. The peptide G2-C blocked HSV-1 entry to the same extent as the parental G2 peptide as observed from the dose-response relationship (Fig. 1A). This assay confirmed that addition of a cysteine did not negatively impact the activity of the G2 peptide. Hence we used the G2-C peptide for the study.
Figure 1.
Peptide G2-C is released from a contact lens. (A) Addition of cysteine does not change the activity of the G2 peptide. Human corneal epithelial cells were pretreated with the indicated treatments and concentrations before being infected with HSV-1 (KOS) gL-86 virus. Six hours post infection, the ONPG substrate was added and the color developed was quantified on a microplate reader at 405 nm. Values are normalized to the control peptide. (B) Schematic of the loading and release experiments conducted with the contact lenses. (C) Cumulative diffusion of BSA and G2-C (μg) over time (days) measured using a Franz cell. (D) Cumulative release of G2-C (in micrograms) over time (in days). All data are presented as mean ± SEM of three independent experiments. Asterisks denote significant difference: **P < 0.01. ***P < 0.001. ****P < 0.0001.
G2-C Releases Through the Contact Lens
Contact lenses have been previously used to deliver small molecules for ocular indications30,31; however, ocular delivery of peptides having anti–HSV-1 properties have not been studied previously, and therefore, we first examined the release of the peptide (Fig. 1B).
In order to determine the release profile of the peptide, we needed to ensure that our peptide is capable to diffuse through a contact lens. Using a Franz-cell, we determined that the peptide is able to diffuse through the contact lens (Fig. 1C). Bovine serum albumin, which is much bigger than the G2-C peptide, was used as a control. Compared with BSA, we observed a significant amount of the peptide being diffused through the contact lens. We then proceeded to determine the release profiles of the peptide. From the dose-response curve, the G2 and the G2-C peptides have similar entry inhibiting activity and these peptides show their maximum inhibiting activity around 250 μg/mL (Fig. 1A). Therefore, we loaded the contact lenses with the G2-C peptide from a concentration of 10 mg/mL. After loading, the average peptide loaded onto the contact lenses was estimated (as mentioned under the Materials and Methods, Loading Experiments section) to be approximately 84%, that is, 840 μg over 95% pure peptide was loaded onto each lens. The G2-C peptide solution was released from the contact lens over a period of several days (Fig. 1D). We also observed that by day 3, approximately 250 μg G2-C peptide was released. Hence, to determine the functional activity of the peptide, the contact lenses loaded with the peptide were allowed to release for 3 days after which the peptide solution was tested for its activity.
Released Peptide Solutions Are Not Toxic
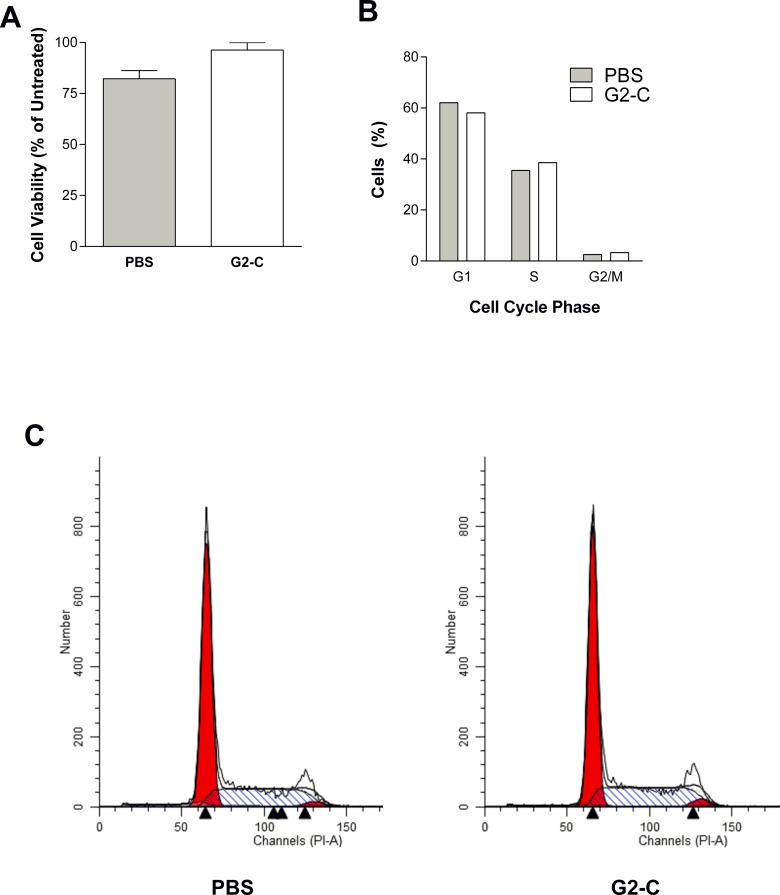
In order to control for nonspecific cytotoxic materials, we employed two assays to determine the toxicity on HCE cells. In the MTT assay, which assesses cell metabolic activity,23 no significant loss of cell viability was observed in both the control and G2-C samples (Fig. 2A). Because HSV interferes with the cell cycle events32 during the course of infection, we wanted to rule out the possibility that the released samples may cause changes in the cell cycle that could indirectly affect HSV-1 infection. Propidium iodide (PI), a fluorescent dye that binds to DNA, was employed to analyze changes in the DNA content using fluorescence-activated cell sorting. No significant changes in the various stages of cell cycle were observed when the cells were treated with the released samples (Figs. 2B, 2C). Taken together, these results indicate that the samples released from the contact lenses were not toxic.
Figure 2.
Peptide G2-C released from contact lens is not toxic. (A) Cell viability using MTT assay was determined on HCE cells treated with PBS and G2-C released from the contact lens. Results are mean ± SEM of three independent experiments. (B–C) The percentage of cells and representative plots of the cell cycle were analyzed on HCE cells treated with PBS and G2-C released from the contact lens by FACS using PI dye.
G2-C Released From the Contact Lens Blocks Infection
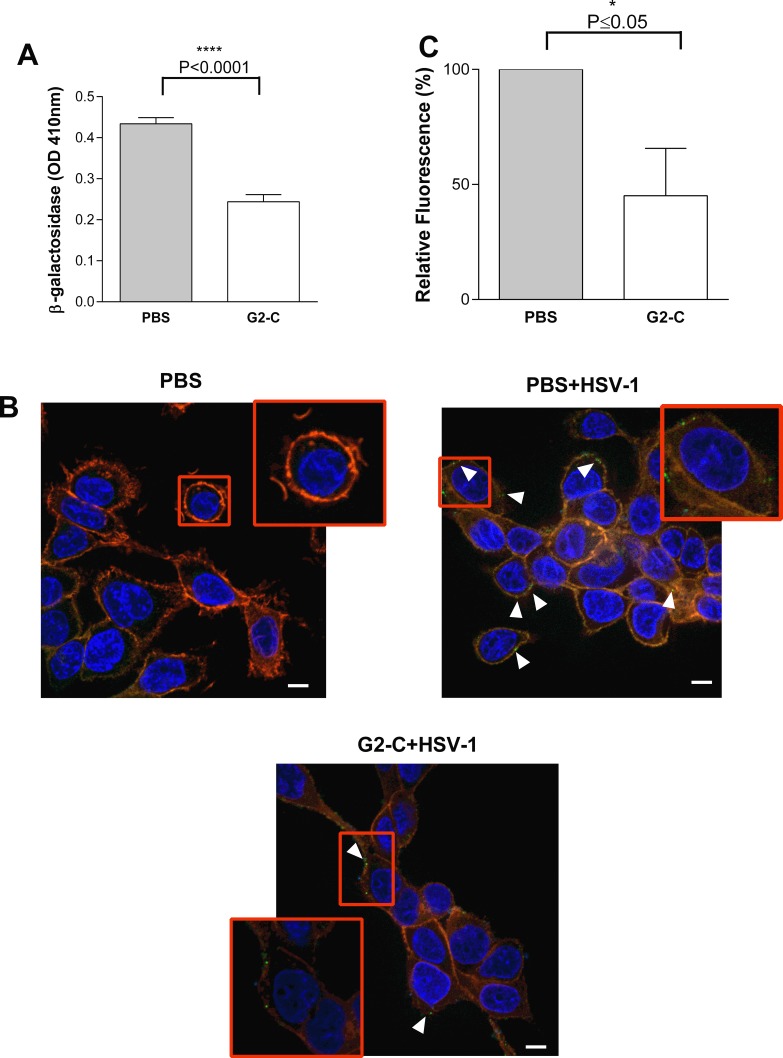
Viral entry is the preliminary step in the life cycle of most viruses, and the G2 peptide blocks this step in the life cycle of HSV-1. Having confirmed that the contact lens released samples are not toxic to the cells, we tested the ability of the released G2-C peptide to inhibit viral entry using a reporter virus.33 A significant reduction (∼50%) of viral entry was observed when the cells were treated with G2-C peptide after release from the contact lenses compared with cells treated with PBS (Fig. 3A). The inhibitory activity of the released peptide was further confirmed by a viral adsorption assay that determines the amount of virus adsorbed on the cell surface using a GFP-tagged HSV-1 virus.22 Human corneal epithelial cells that were pretreated with the G2-C peptide were infected with HSV-1 and showed less virus adsorbed on the cell surface compared with the cells that were pretreated with PBS only (Fig. 3B). Upon quantifying these GFP-tagged HSV-1 virus particles on the cell surface, a reduction of ∼50% in the fluorescence intensity was obtained with cells that were pretreated with the released G2-C peptide. Using two different assays to determine viral entry activity, we concluded that the G2-C peptide released from contact lenses was capable of blocking the entry of HSV-1.
Figure 3.
Released G2-C inhibits entry of HSV-1. (A) Entry assay was performed with the released PBS and G2-C on HCE cells and the β-galactosidase levels were quantified 6 hpi. Data are mean ± SEM of three independent experiments. Asterisks denote significant difference: ****P < 0.0001. (B) Representative immunofluorescence images showing the adsorption of HSV-1 (green) on the surface of HCE cells that were either pretreated with PBS or G2-C released from the contact lens before infecting the cells at 4°C. DAPI (blue) was used as a counterstain and the Rhodamine-phalloidin (red) was used to stain the actin cytoskeleton. Scale bars: 5 μm. (C) The images were quantified for the GFP-tagged virus. Thirty cells were picked at random and the software (MetaMorph; Carl Zeiss Microscopy GmbH) was used for analysis. Asterisks denote significant difference: *P ≤ 0.05.
Released G2-C Blocks Cell-to-Cell Spread
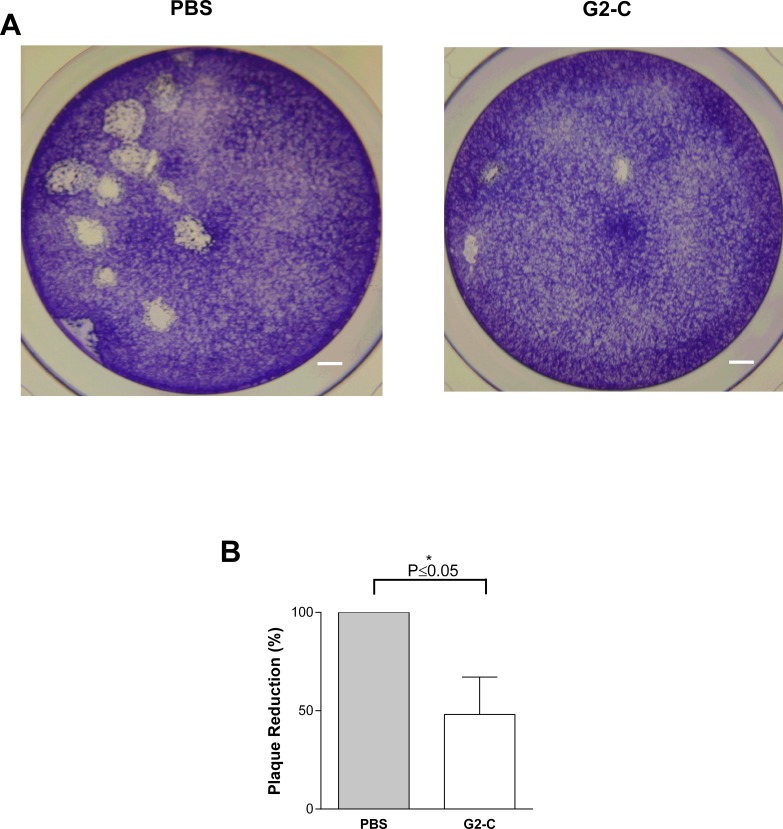
One of the traits of herpesvirus is to spread from one cell to another cell during replication and cause cell death. This mode of transmission is a major route for the virus to spread in tissues. The role of 3-OS-HS in the virus cell-to-cell spread has been established.34 The G2-C peptide is specific to heparan sulfate and expected to inhibit 3-OS-HS–viral binding. Due to this, we sought to determine if the released peptide is capable of blocking cell-to-cell spread. Pretreating HCE cells with G2-C released peptide showed a significant reduction in the number of infected plaques suggesting that the peptide is able to block virus cell-to-cell spread (Fig. 4). The plaque size was also significantly small compared with the PBS released samples. Thus, along with the ability of blocking viral entry, the released peptide can also block cell-to-cell spread.
Figure 4.
Peptide G2-C released from the contact lens blocks viral spread. (A) Representative images of the stained viral plaques (clear portion) on HCE cells that were pretreated with PBS or G2-C released from the contact lenses prior to infection with HSV-1 (KOS). Scale bars: 100 pixels. (B) Quantification of the plaque numbers normalized to PBS. All data are presented as mean ± SEM of three independent experiments. Asterisk denotes significant difference: *P ≤ 0.05.
Released Peptide Suppresses Infection in Ex Vivo Model of HSV-1
Human and pig corneas that are organotypically maintained in culture have been utilized as ex vivo models to study HSV-1 infections.20,35,36 Using the same model, we wanted to demonstrate the inhibitory effect of the released peptide on donated unlabeled human corneas obtained from recent cadavers or transplant patients. We hypothesized that because the peptide acts in blocking the entry of the virus into the epithelial cells, fewer viruses should enter and the eventual viral replication rounds would also decrease upon exposure to released solution containing G2 peptide. To account for cornea-to-cornea variability, random human corneas were chosen and cut into two halves: one half was pretreated with PBS released from contact lens and the other half was pretreated with G2-C released from the contact lens and then infected. At 24 hours post infection, the HSV-1 viral tegument protein VP1637 was observed less in human corneas that were pretreated with the released G2-C peptide compared with the corneas that were pretreated with PBS (Fig. 5A). A significant reduction of viral titers secreted from the infected corneas into culture media was also observed in the corneas pretreated with G2-C compared with the corneas treated with PBS (Fig. 5B). Along the same lines, immunohistochemistry with the pig corneal sections also demonstrated the ability of the released G2-C peptide to suppress HSV-1 infections (Fig. 5C). The representative image of the cornea that received PBS treatment shows the presence of GFP-tagged virus particles (indicated by arrows), whereas virtually no virus was detected in the corneas that received G2-C. As hypothesized, the contact lens released G2-C peptide is efficient in suppressing the viral infection ex vivo.
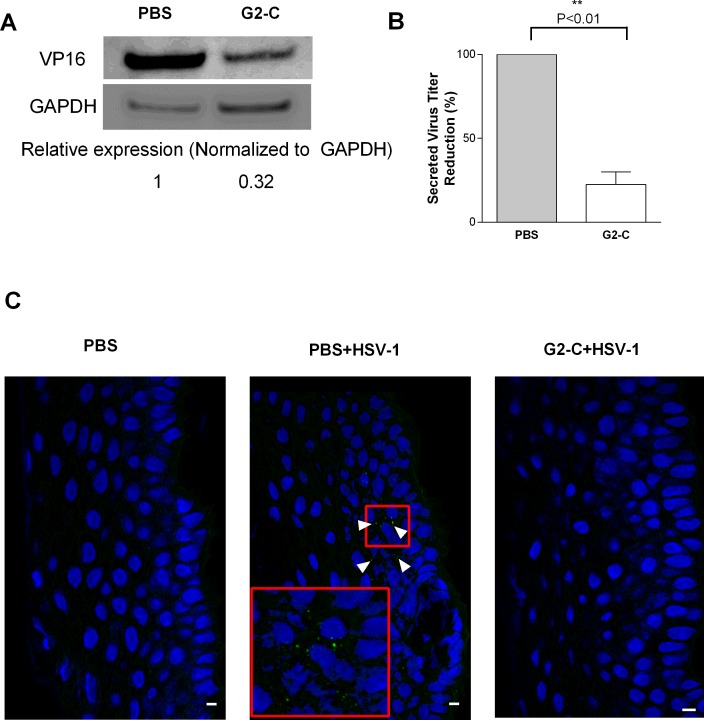
Figure 5.
Peptide G2-C released from the contact lens suppresses HSV-1 infection ex vivo. (A) Immunoblots representing the levels of the viral tegument protein VP16 from human cornea cadavers that were pretreated with the indicated treatments. Below: Band intensities were quantified using image analysis software (GE Healthcare) and were normalized to GAPDH. (B) The supernatant from the cultured human corneas were serially diluted and titered on Vero cells. Plaque numbers are normalized to PBS. Results are mean ± SEM of three independent experiments. Asterisks denote significant difference: **P < 0.01. (C) Representative sections from pig corneas that received the indicated treatments. The arrow indicates the presence of virus (green) on the epithelial cells (blue). Scale bars: 10 μm.
HSV-1 Infection in the Mouse Is Suppressed by G2-C Peptide Released From the Contact Lens
Having confirmed the activity of the peptide in the ex vivo models, we finally determined the ability of the released G2-C peptide to suppress HSV-1 infection in the mouse model. The mouse corneas were treated either with G2-C or the vehicle control and then imaged at 48 hpi for the presence of a GFP-tagged virus, which was used for corneal infection. The representative image of the mouse cornea that received released PBS as a treatment shows the presence of GFP-tagged virus, whereas virtually no virus was detected in the corneas treated with the released G2-C (Fig. 6A). Along the same lines, mouse corneas that received the released G2-C peptide showed significantly less viral titers compared with the mouse corneas that received released PBS (Fig. 6B). These results further strengthen our findings that the G2-C peptide being released from the contact lens retains its activity.
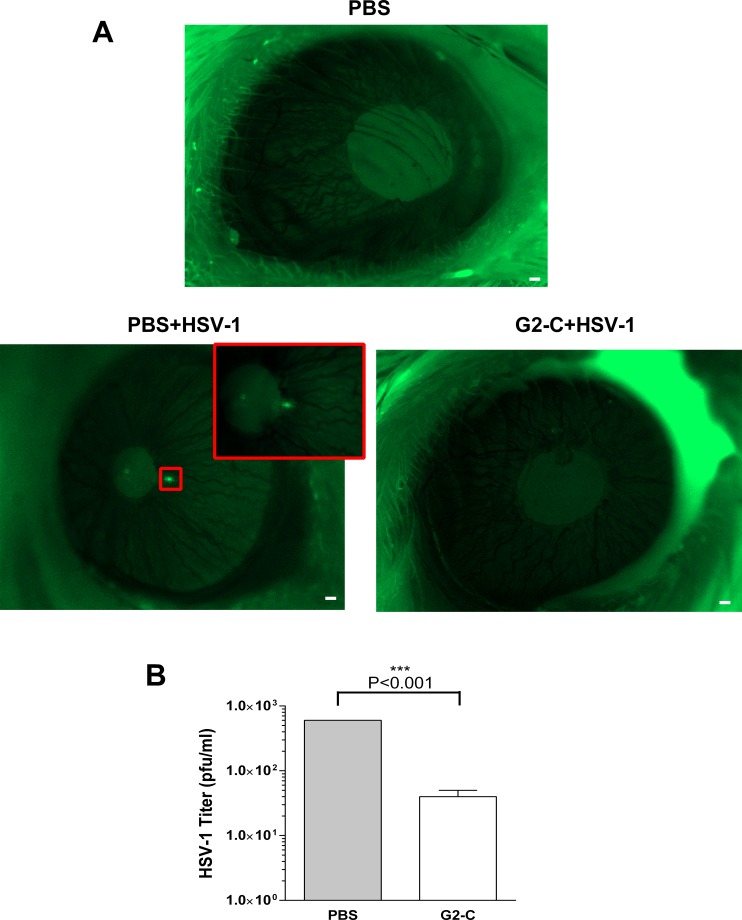
Figure 6.
Peptide G2-C released from the contact lens suppresses HSV-1 in vivo. (A) Representative images of the mice (n = 2 per group) that received the indicated treatments prior to infection with GFP-tagged HSV-1. Forty-eight hours post infection, mice were anesthetized and imaged. Green indicates the virus. Scale bars: 100 μm. (B) The eyes of euthanized mouse were homogenized, serially diluted, and titered on Vero.
Discussion
Our study shows the release of a cysteine attached arginine rich peptide through a commercially available contact lens and characterizes its functional activity by exogenously adding it on the cells and tissues to demonstrate that it inhibits the entry and spread of HSV-1.
Herpes simplex virus 1 infects the cornea that is 500 to 600 μm thick and specifically infects the epithelium cells that are present as the top five to six layers on the cornea. Most of the currently available drugs for HSV-1 infection are nucleoside analogues. With increasing incidences of resistance against ACV and limitations to other treatments, there is a need to identify potential targets and new molecules for antiviral research. Entry into cells is the preliminary step in the lifecycle of HSV-138 and blocking entry would result in lower levels of infections. In pursuit of finding HSV-1 entry inhibitors, previous work from our lab discovered two peptides, G1 and G2, and established their antiviral properties in the in vitro and in vivo models of HSV-1 infection. These peptides are specific to HS or 3-OS-HS that the virus utilizes as one of the receptors for attachment and entry.16,33,39 In the present study, we used a modified form of the G2 peptide by adding a cysteine to the C-terminal. Modification of the peptide did not change the function of the peptide as similar levels in the reduction of HSV-1 entry were observed with the G2 and G2-C peptides (Fig. 1A). Addition of cysteine provides a main advantage in that it can serve as a coupling site for various peptide extensions that can be used to increase the half-life of the peptide and prevent it from degradation.
In addition to the stability-inducing modification, we sought a therapeutic approach that would retain the peptide on the surface of the eye for substantial periods. Contact lenses are used extensively as delivery vehicles for ophthalmic drugs.6 Contact lenses act as reservoirs through which the loaded drug releases slowly providing more local concentration at the target site and increasing the residence time of the drug; hence, we decided to use contact lenses as a delivery vehicle for our peptide. After having confirmed with preliminary experiments that the peptide diffuses from the contact lens (Fig. 1C), we loaded the contact lens with the G2-C peptide solution and determined the release profile over time. Drug solutions released from contact lenses or any delivery vehicle have an initial “burst” of the drug resulting in a very high local concentration of the drug which in some cases are toxic. In our release studies, we saw a burst of the peptide solution released within the first hour (Fig. 1D). While we have shown that the peptide itself doesn't cause any toxicity,19 the peptide solution being released from the contact lenses also do not cause toxicity to the cultured cells (Fig. 2).
The maximum entry blocking activity of G2 and G2-C (Fig. 1A) was approximately 250 μg/mL. Using 10 mg/mL as the loading concentration, we observed that by around the third day, 250 μg peptide released from the contact lenses. To determine whether this released peptide is functionally active, we decided to do a continuous release up to day 3 then use the peptide solution to determine the functional activity by exogenously adding it to cultured cells. However, upon conducting the release we observed only 110 μg peptide released and, hence, we achieved only approximately 50% entry inhibition. This was due to the way the two release studies were conducted. In the release studies for determining the functional activity of the peptide, the peptide with the contact lens is allowed to release in PBS for 3 days without changing the release buffer. This creates a condition where there is no “sink,” meaning that there is a reduction diffusion of the peptide from the contact lens due to reduced concentration gradient and hence a lower amount of peptide was obtained. While in the typical release experiment, fresh buffer is replaced at each time point, there is always a sink condition maintained and the concentration gradient is maintained, allowing for more peptide to be released from the contact lens. Although not unexpected, this variation in the in vitro release rate is not uncommon40 and there is a need for further in vitro–in vivo correlation to determine the most appropriate method to estimate the in vivo activity of this type of device.41,42 It is thought that with local tear fluid volume, local elimination (i.e., lacrimal gland), and cell uptake, the release from contact lens can best be estimated using the methods used, but this will be re-evaluated after further experiments in animals.
Having confirmed that the G2-C peptide was being released, we then moved on to test the functional activity of the released peptide. We determined the ability of the released peptide to block the entry of HSV-1 (Fig. 2A). Because HS has been shown to play a role in one of the major routes of viral transmission, spread from cell-to-cell, we demonstrated the ability of the released G2-C peptide in blocking virus spread (Fig. 3). To further show the antiviral effect of the released peptide, we transitioned from the cell culture models of HSV-1 to a relatively close clinical model: organotypic cultures of human and pig corneas. The released G2-C peptide was capable of suppressing the infection in these corneas further strengthening our finding (Fig. 5). Finally, the activity of the released G2-C peptide in the mouse corneas were also tested and yielded similar findings that the peptide is indeed capable of suppressing HSV-1 infection (Fig. 6). While we observed ∼50% of viral entry and spread being blocked by the released G2-C peptide in the in vitro experiments, the inhibitory activity of the same amount of released G2-C peptide was much more effective in the ex vivo and in vivo experiments. Studies to generate better blocking effects by dose optimization and peptide manipulation will be performed in the future.
Topical delivery of drugs to the corneal epithelial faces several challenges that limit the effectiveness of topically applied drug solutions. Tear turnover causes the drug to be cleared via the nasolacrimal duct leading to significant loss of drug from the corneal surface. Also, the drug tends to be absorbed and distributed to neighboring ocular tissues leading to elimination by the systemic circulation and reducing the efficacy of the drug. Therefore, methods to extend the amount of time that a drug remains on the corneal surface are needed to ensure maximal activity of the drug. These limitations are even further complicated by peptides, which are generally rapidly degraded after administration.
Peptides are increasingly gaining attention as therapeutic molecules due to their high selectivity, ease of manufacture, safety, and efficacy.43,44 They are also being actively investigated as antivirals and vaccine candidates.45,46 This study is an effort to overcome the limitations of topical delivery while taking advantage of the properties of peptide antivirals in blocking HSV-1 infection. While this study uses the released peptide as “eye drops” to test the functional activity of the released peptide in animals, a more appropriate application of this study will be in placing a G2-C loaded contact lens on the eye of the animal and determining the levels of HSV-1 infection. This future work will need to be performed to pave way for further studies at a clinical level.
In conclusion, this is the first study to show that a 12-mer antiviral peptide can be successfully released from a contact lens and that this released peptide solution when added exogenously to cultured cells, organotypic corneas, and the corneas of living mouse can significantly suppress HSV-1 infection. Our study describes a novel way to suppress ocular herpes without the need for multiple rounds of daily or weekly treatments. Future studies will shed more light on the clinical use of contact lenses to control and treat ocular herpes.
Supplementary Material
Acknowledgments
The authors thank Ruth Zelka, MS, for her help with the Zeiss Spinning Disk imaging, Ali Djalilian, MD, for donated human corneas, and Balaji Ganesh, PhD, for his help with the cell cycle using FACS.
Supported by National Institutes of Health Grant AI105573 (DS) and National Eye Institute Grant EY024710 (DS).
Disclosure: D. Jaishankar, None; J.S. Buhrman, None; T. Valyi-Nagy, None; R.A. Gemeinhart, None; D. Shukla, None
References
- 1. Xu F,, Sternberg MR,, Kottiri BJ,, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006; 296: 964–973. [DOI] [PubMed] [Google Scholar]
- 2. Whitley RJ,, Kimberlin DW. Herpes simplex encephalitis: children and adolescents. Semin Pediatr Infect Dis. 2005; 16: 17–23. [DOI] [PubMed] [Google Scholar]
- 3. Farooq AV,, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012; 57: 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001; 20: 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Bourlais CL,, Acar L,, Zia H,, Sado PA,, Needham T,, Leverge R. Ophthalmic drug delivery systems--recent advances. Prog Retin Eye Res. 1998; 17: 33–58. [DOI] [PubMed] [Google Scholar]
- 6. Alvarez-Lorenzo C,, Hiratani H,, Concheiro A. Contact lenses for drug delivery. 2006; 4: 131–151. [Google Scholar]
- 7. Bacon TH,, Levin MJ,, Leary JJ,, Sarisky RT,, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003; 16: 114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chilukuri S,, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003; 21: 311–320. [DOI] [PubMed] [Google Scholar]
- 9. James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007; 23: 403–420. [DOI] [PubMed] [Google Scholar]
- 10. Tripathi RC,, Parapuram SK,, Tripathi BJ,, Zhong Y,, Chalam KV. Corticosteroids and glaucoma risk. Drugs Aging. 1999; 15: 439–450. [DOI] [PubMed] [Google Scholar]
- 11. Urban RC,, Jr, Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1986; 31: 102–110. [DOI] [PubMed] [Google Scholar]
- 12. Imperia PS,, Lazarus HM,, Dunkel EC,, Pavan-Langston D,, Geary PA,, Lass JH. An in vitro study of ophthalmic antiviral agent toxicity on rabbit corneal epithelium. Antiviral Res. 1988; 9: 263–272. [DOI] [PubMed] [Google Scholar]
- 13. Lass JH,, Langston RH,, Foster CS,, Pavan-Langston D. Antiviral medications and corneal wound healing. Antiviral Res. 1984; 4: 143–157. [DOI] [PubMed] [Google Scholar]
- 14. Akhtar J,, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009; 276: 7228–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shukla D,, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001; 108: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spear PG,, Eisenberg RJ,, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000; 275: 1–8. [DOI] [PubMed] [Google Scholar]
- 17. Oh M,, Akhtar J,, Desai P,, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2009; 391: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hadigal SR,, Agelidis AM,, Karasneh GA,, et al. Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nat Commun. 2015; 6: 6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tiwari V,, Liu J,, Valyi-Nagy T,, Shukla D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem. 2011; 286: 25406–25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park PJ,, Antoine TE,, Farooq AV,, Valyi-Nagy T,, Shukla D. An investigative peptide-acyclovir combination to control herpes simplex virus type 1 ocular infection. Invest Ophthalmol Vis Sci. 2013; 54: 6373–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White CJ,, Tieppo A,, Byrne ME. Controlled drug release from contact lenses: a comprehensive review from 1965–present. J Drug Deliv Sci Technol. 2011; 21: 369–384. [Google Scholar]
- 22. Desai P,, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998; 72: 7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 24. Chan KY,, Cho P,, Boost M. Corneal epithelial cell viability of an ex vivo porcine eye model. Clin Exp Optom. 2014; 97: 337–340. [DOI] [PubMed] [Google Scholar]
- 25. Choy EP,, To TS,, Cho P,, Benzie IF,, Choy CK. Viability of porcine corneal epithelium ex vivo and effect of exposure to air: a pilot study for a dry eye model. Cornea. 2004; 23: 715–719. [DOI] [PubMed] [Google Scholar]
- 26. Sarkar J,, Chaudhary S,, Namavari A,, et al. Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2012; 53: 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bultmann H,, Teuton J,, Brandt CR. Addition of a C-terminal cysteine improves the anti-herpes simplex virus activity of a peptide containing the human immunodeficiency virus type 1 TAT protein transduction domain. Antimicrob Agents Chemother. 2007; 51: 1596–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu YL,, Huang J,, Xu J,, et al. Addition of a cysteine to glucagon-like peptide-1 (GLP-1) conjugates GLP-1 to albumin in serum and prolongs GLP-1 action in vivo. Regul Pept. 2010; 164: 83–89. [DOI] [PubMed] [Google Scholar]
- 29. Jaishankar D,, Yakoub AM,, Bogdanov A,, Valyi-Nagy T,, Shukla D. Characterization of a proteolytically stable D-peptide that suppresses herpes simplex virus 1 infection: implications for the development of entry-based antiviral therapy. J Virol. 2015; 89: 1932–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bengani LC,, Hsu KH,, Gause S,, Chauhan A. Contact lenses as a platform for ocular drug delivery. Expert Opin Drug Deliv. 2013; 10: 1483–1496. [DOI] [PubMed] [Google Scholar]
- 31. Souza JG,, Dias K,, Pereira TA,, Bernardi DS,, Lopez RFV. Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol. 2014; 66: 507–530. [DOI] [PubMed] [Google Scholar]
- 32. Flemington EK. Herpesvirus lytic replication and the cell cycle: arresting new developments. J Virol. 2001; 75: 4475–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shukla D,, Liu J,, Blaiklock P,, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999; 99: 13–22. [DOI] [PubMed] [Google Scholar]
- 34. Tiwari V,, O'Donnell C,, Copeland RJ,, Scarlett T,, Liu J,, Shukla D. Soluble 3-O-sulfated heparan sulfate can trigger herpes simplex virus type 1 entry into resistant Chinese hamster ovary (CHO-K1) cells. J Gen Virol. 2007; 88: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 35. Drevets P,, Chucair-Elliott A,, Shrestha P,, Jinkins J,, Karamichos D,, Carr DJ. The use of human cornea organotypic cultures to study herpes simplex virus type 1 (HSV-1)-induced inflammation. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alekseev O,, Tran AH,, Azizkhan-Clifford J. Ex vivo organotypic corneal model of acute epithelial herpes simplex virus type I infection. J Vis Exp. 2012: e3631. [DOI] [PMC free article] [PubMed]
- 37. Heine JW,, Honess RW,, Cassai E,, Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974; 14: 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agelidis AM,, Shukla D. Cell entry mechanisms of HSV: what we have learned in recent years. Future Virology. 2015; 10: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tiwari V,, Tarbutton MS,, Shukla D. Diversity of heparan sulfate and HSV entry: basic understanding and treatment strategies. Molecules. 2015; 20: 2707–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abouelmagd SA,, Sun B,, Chang AC,, Ku YJ,, Yeo Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: are we doing it right? Mol Pharmaceutics. 2015; 12: 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karalis V,, Magklara E,, Shah VP,, Macheras P. From drug delivery systems to drug release, dissolution, IVIVC, BCS, BDDCS, bioequivalence and biowaivers. Pharm Res. 2010; 27: 2018–2029. [DOI] [PubMed] [Google Scholar]
- 42. Shen J,, Burgess DJ. In vitro-in vivo correlation for complex non-oral drug products: where do we stand? J Control Release. 2015; 219: 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Craik DJ,, Fairlie DP,, Liras S,, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013; 81: 136–147. [DOI] [PubMed] [Google Scholar]
- 44. Fosgerau K,, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015; 20: 122–128. [DOI] [PubMed] [Google Scholar]
- 45. Mulder KC,, Lima LA,, Miranda VJ,, Dias SC,, Franco OL. Current scenario of peptide-based drugs: the key roles of cationic antitumor and antiviral peptides. Front Microbiol. 2013; 4: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srivastava R,, Khan AA,, Huang J,, Nesburn AB,, Wechsler SL,, BenMohamed LA. Herpes simplex virus type 1 human asymptomatic CD8+ T-cell epitopes-based vaccine protects against ocular herpes in a “humanized” HLA transgenic rabbit model. Invest Ophthalmol Vis Sci. 2015; 56: 4013–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.