Abstract
Carbohydrates are the preferred substrate for contracting skeletal muscles during high-intensity exercise and are also readily utilized during moderate intensity exercise. This use of carbohydrates during physical activity likely played an important role during the survival of early Homo sapiens, and genes and traits regulating physical activity, carbohydrate metabolism, and energy storage have undoubtedly been selected throughout evolution. In contrast to the life of early H. sapiens, modern lifestyles are predominantly sedentary. As a result, intake of excessive amounts of carbohydrates due to the easy and continuous accessibility to modern high-energy food and drinks has not only become unnecessary but also led to metabolic diseases in the face of physical inactivity. A resulting metabolic disease is type 2 diabetes, a complex endocrine disorder characterized by abnormally high concentrations of circulating glucose. This disease now affects millions of people worldwide. Exercise has beneficial effects to help control impaired glucose homeostasis with metabolic disease, and is a well-established tool to prevent and combat type 2 diabetes. This chapter focuses on the effects of exercise on carbohydrate metabolism in skeletal muscle and systemic glucose homeostasis. We will also focus on the molecular mechanisms that mediate the effects of exercise to increase glucose uptake in skeletal muscle. It is now well established that there are different proximal signaling pathways that mediate the effects of exercise and insulin on glucose uptake, and these distinct mechanisms are consistent with the ability of exercise to increase glucose uptake in the face of insulin resistance in people with type 2 diabetes. Ongoing research in this area is aimed at defining the precise mechanism by which exercise increases glucose uptake and insulin sensitivity and the types of exercise necessary for these important health benefits.
1. INTRODUCTION
The unique ability of humans to perform endurance running has likely contributed to the evolution of Homo sapiens from other primates.1 High levels of physical activity were required in order to evade predators as well as to obtain food. To maintain these high levels of physical activity, the working skeletal muscles require increased substrates for generation of adenosine triphosphate (ATP). A major substrate for the working muscles is carbohydrates, with one source being in the muscle itself in the form of glycogen, and another source glucose coming from the blood. The break-down of glycogen from the muscle (glycogenolysis) and the regulation of glucose uptake into the muscle from the blood are highly regulated processes, and in this chapter, current knowledge on these functions will be discussed.
Since carbohydrate utilization promotes human survival, genes and traits regulating carbohydrate metabolism during exercise and energy storage have been selected throughout evolution.2 However, current lifestyles are pre-dominantly sedentary, which coupled with the intake of excessive amounts of carbohydrates, has led to metabolic diseases such as type 2 diabetes. On the other hand, exercise has beneficial effects on carbohydrate metabolism, and as a result, exercise is a well-established tool to prevent and combat type 2 diabetes. The molecular mechanisms that mediate the effects of exercise to increase skeletal muscle glucose uptake and increase insulin sensitivity in healthy people and people with type 2 diabetes will also be discussed.
2. CARBOHYDRATE UTILIZATION DURING REST AND EXERCISE
At rest, the energy used by the human body is predominantly derived from the oxidation of carbohydrates and fats. Blood glucose, plasma-free fatty acids, muscle glycogen, and intramuscular triglycerides are major substrate sources for energy production in skeletal muscles.3,4 The contribution of proteins to the pool of usable energy is very limited, as amino acid oxidation is usually strictly adjusted to the intake of amino acids.
At rest, ingestion of carbohydrates results in insulin release from the pancreas, and the ensuing increase in plasma insulin concentrations has a myriad of metabolic effects. One important effect of insulin is to promote glucose transport into skeletal muscle. Insulin also suppresses fatty acid release from adipose tissue while increasing fat storage by activation of lipoprotein lipase.5,6 Intake of physiologically normal carbohydrate levels has no impact on adipose tissue levels via de novo lipogenesis,7 suggesting that the human body can accommodate intake of relatively large amounts of carbohydrates without a need to store carbohydrates as fat.
The contraction of skeletal muscles during physical exercise results in an increased energy demand for the muscle. The challenge for the working muscle is to increase production of ATP, and several cellular processes function to meet this need. Accordingly, metabolic pathways that oxidize both carbohydrates and fat need to be activated simultaneously.3,4 Intensity, duration, and type of exercise determine the mechanisms through which this extra energy is supplied.
The enzyme ATPase facilitates the breakdown of ATP to ADP + inorganic phosphate (Pi) to generate energy for rapid use; however, only a small amount of ATP is present within the muscle cells.8 An additional but even smaller source of stored energy is creatine phosphate, which can be resynthesized to ATP by the enzyme creatine kinase to replenish depleted ATP levels. Thus, the major sources of energy during exercise are carbohydrates and fats. Sources of carbohydrates for the muscle include blood glucose, muscle glycogen, and liver glycogen.9
Glucose and glycogen are converted to glucose-6-phosphate before they can be used to generate energy. One fate of glucose-6-phosphate is conversion to lactic acid, which results in the formation of three molecules of ATP per glycogen molecule or two molecules of ATP per glucose molecule (anaerobic glycolysis). The ATP generated by anaerobic glycolysis is not large enough to sustain continued muscle activity for long durations. With submaximal exercise, oxygen uptake increases, and within several minutes, a steady state is reached. This steady state indicates that the aerobic processes are supplying the majority of energy required by the contracting muscles. Aerobic generation of ATP from the glucose molecule is many times more efficient than the anaerobic reaction of glycolysis. During the aerobic reaction of glycolysis, glycogen is converted to pyruvic acid, which is then converted to acetyl-CoA and utilized for ATP production in the Krebs cycle within the mitochondria. Although the primary fuels contributing to oxidative metabolism during exercise are fats and carbohydrates, under extreme conditions amino acids can also be used as source of substrate oxidation.9
In the fasted state and during low intensity exercise, the bulk of energy required by the muscle is provided by oxidation of free fatty acids that are predominantly derived from the plasma.10 When exercise increases to a moderate level of intensity (60–70% VO2 peak), the source of fatty acids for oxidation also includes intramuscular triglyceride. Although both sources of fatty acids contribute to the energy needs of the muscle, even when combined they are not sufficient to meet the energy demand. Therefore, during moderate intensity exercise about half of the total energy derived is from oxidation of carbohydrates, coming from both muscle glycogen and blood glucose.11 During high-intensity exercise, the contribution of plasma fatty acid oxidation becomes even less and carbohydrate oxidation provides roughly two-thirds of the total energy need. Carbohydrate metabolism is the preferred source of fuel under these conditions because the rate of ATP production is two times higher than fatty acids.9
3. MUSCLE GLYCOGEN
As noted above, glycogen is an essential fuel for energy production in the contracting skeletal muscles. Glycogen is a branched polymer of glucose with a mixture of α-1,4 and α-1,6 linkages between glucose units. The liver has the highest concentration of stored glycogen; however, skeletal muscle, as a result of its total weight, is the largest reserve of stored glycogen in the body. Intramuscular glycogen is associated with several organelles including the sarcolemma, sarcoplasmic reticulum, mitochondria, and myofibrils.3,12 Granules of glycogen, or glycosomes, are also physically associated with several proteins including glycogen phosphorylase, phosphorylase kinase, glycogen synthase, glycogenin, protein phosphatases, and adenosine monophosphate (AMP)-activated protein kinase (AMPK).3,12 The synthesis of glycogen involves multiples enzymes, and glycogen synthase is the rate-limiting enzyme. The breakdown of glycogen (glycogenolysis) is also controlled by a multienzyme system, and this will be discussed in more detail below.
Glycogen utilization is rapidly initiated at the onset of exercise and increases exponentially with exercise intensity.13 Regulation of glycogenolysis is very sensitive to the metabolic rate of skeletal muscle during exercise.14,15 Glycogen phosphorylase is the enzyme responsible for the rate-limiting step during muscle glycogenolysis.3,16 At rest, glycogen phosphorylase exists primarily in the inactive b form, whereas with the onset of exercise phosphorylase kinase phosphorylates the b form to the active a form.3 Phosphorylase kinase activation results from elevated calcium levels and binding of epinephrine to β-adrenergic receptors on the sarcolemma. Activation of phosphorylase kinase by stimulation of β-adrenergic receptors on the sarcolemma is mediated by cyclic AMP. Elevated epinephrine levels increase glycogen phosphorylase activity and glycogenolysis in perfused rat hind limbs and glycogenolysis in humans during moderate exercise.16,17
Muscle glycogenolysis does not always correlate tightly with levels of phosphorylase kinase a.18 This suggested that posttranslational factors enhance the glycogenolytic rate during various intensities of exercise. Indeed, AMP and adenosine diphosphate (ADP) levels, and increased levels of Pi can all allosterically regulate activity of the a and b forms of phosphorylase kinase.3 With increase exercise duration, there is a decrease in glycogen availability in parallel with decreased phosphorylase activity, while there is increased availability of other substrates for oxidation, such as plasma glucose and free fatty acids.
Muscle fiber type can also be a factor in determining the regulation of muscle glycogenolysis. During moderate intensity exercise, muscle glycogenolysis occurs predominantly in type I muscle fibers. As exercise duration increases or if exercise intensity increases, type I fibers become depleted and increasing amounts of glycogen are degraded in type II muscle fibers. Thus, as exercise intensity increases, recruitment of type II fibers increases accordingly. With short-term exercise at intensities approaching and exceeding VO2max, glycogenolysis occurs in all fibers, but the highest rates are in type II fibers.3
Once muscle glycogen is depleted or near depleted, fatigue sets in, and exercise capacity is compromised.14,15 Although duration and intensity of exercise play a role in regulating glycogen breakdown in muscle, diet history and training status also regulate muscle glycogenolysis during exercise. In general, increased carbohydrate intake is associated with greater muscle glycogen utilization, whereas increased fat intake results in decreased muscle glycogen utilization during exercise.3 This attenuation of muscle glycogenolysis during exercise following the intake of a high-fat diet appears to be dependent on metabolic adaptations resulting from the high-fat diet and independent of muscle glycogen availability, which was similar at the onset of exercise.19 Following exercise, glycogen synthase is activated and muscle glycogen concentrations are increased in the resting muscle.20,21 Despite this increase in resting muscle glycogen levels, muscle glycogenolysis is decreased during dynamic exercise following short-term endurance training.22 This decrease in muscle glycogenolysis is contributing to the well described increases in muscle oxidative capacity.22
4. GLUCOSE TRANSPORT
The other major source of carbohydrate during exercise is circulating blood glucose. Blood glucose concentrations during exercise are controlled by a precise regulatory mechanism, and the source of the circulating glucose is primarily the liver. In the resting state, food consumption also regulates blood glucose concentrations, and the removal of glucose from the circulation in response to both food consumption and physical exercise is a critical factor for the maintenance of normal glycemia in humans.
The transport of glucose into skeletal muscle is essential for tissue homeostasis, and under normal physiologic conditions, the transport process is rate limiting for glucose utilization.23 Transport occurs by facilitated diffusion, and there is an increase in the maximal velocity of transport without an appreciable change in the substrate concentration at which glucose transport is half maximal.24 The transport of glucose utilizes specific carrier proteins called glucose transporters, which are a family of structurally related proteins that are expressed in a tissue-specific manner.25 In skeletal muscle from rodents and humans, GLUT4 is the major isoform expressed, whereas expression of the GLUT1, GLUT5, and GLUT12 isoforms is much lower.26–28 Studies where there was genetic ablation of GLUT4 in the skeletal muscles of mice reveal that GLUT4 is necessary for normal rates of basal, insulin, and exercise-stimulated glucose transport.29,30
The mechanism by which exercise increases glucose transport via the GLUT4 transporter has been an area of intense investigation for many years. Likewise, there has been great interest in understanding the mechanism for the effects of insulin on glucose transport. Early studies using subcellular fractionation of skeletal muscle tissue,31,32 and more recently work using in vivo confocal microscopy,33,34 have clearly established that both exercise and insulin increase glucose transport in skeletal muscle through the translocation of GLUT4 from an intracellular compartment to the sarcolemma and transverse tubules. The GLUT4 translocation process is very complex, involving numerous cellular processes. In skeletal muscle, the movement of transporters occurs by the exocytosis, trafficking, docking, and fusion of GLUT4-containing storage compartment or “vesicles” into the cell-surface membranes. Our knowledge of the composition, specificity, and trafficking of GLUT4 vesicles has increased in recent years, although they are still not fully understood.35 There is good evidence that multiple soluble N-ethylmaleimide attachment protein receptor (SNARE) proteins regulate the docking and fusion of GLUT4-containing vesicles. With stimulation such as exercise, muscle contraction, or insulin, the vesicle-associated SNARE proteins (v-SNARE), including vesicle-associated membrane protein-2 (VAMP-2), bind to the target-membrane SNARE proteins (t-SNARE), which include syntaxin 4 and SNAP23. This complex is thought to facilitate the fusion of GLUT4-containing vesicles into the cell-surface membrane. In studies with syntaxin 4 heterozygous-knockout (KO) mice, syntaxin 4 has been shown to be a major molecule responsible for the regulation of insulin-stimulated GLUT4 redistribution and glucose transport in skeletal muscle.36 The roles of the SNARE proteins in exercise-stimulated GLUT4 translocation are less well understood, although VAMP2 has been shown to translocate to the cell surface in response to exercise.37
When skeletal muscles are stimulated simultaneously by contraction and insulin treatments, there are additive or partially additive effects on glucose transport.24,38 Consistent with these findings, the combination of exercise and insulin can have additive effects on GLUT4 translocation to the sarcolemma.38 These data support the concept that there are different mechanisms leading to the stimulation of muscle glucose transport by exercise and insulin.39
5. EXERCISE SIGNALS REGULATING GLUCOSE TRANSPORT
The intracellular signaling proteins that regulate the increase in GLUT4 translocation and glucose transport in skeletal muscle with exercise have also been an area of intensive investigation during the last 10 years. Since insulin and exercise both stimulate GLUT4 translocation, it has been hypothesized that there may be similar signaling proteins involved in the translocation process. Insulin signaling involves the rapid phosphorylation of the insulin receptor, insulin receptor substrate-1/2 (IRS-1/2) on tyrosine residues, and the activation of phosphatidylinositol 3-kinase (PI3-K).40,41 In contrast, exercise does not result in tyrosine phosphorylation of the insulin receptor and IRS-1, and there is no increase in PI3-K activity.42,43 Additional evidence that exercise can increase glucose transport in the absence of insulin signaling comes from a study investigating mice that lack insulin receptors in skeletal muscle (muscle-specific insulin receptor KO mice; MIRKO).44,45 While these mice have blunted insulin-stimulated glucose transport,45 they have normal exercise-stimulated glucose transport.44 Taken together, these studies reveal that insulin and exercise mediate GLUT4 translocation in skeletal muscle through different proximal signaling mechanisms.
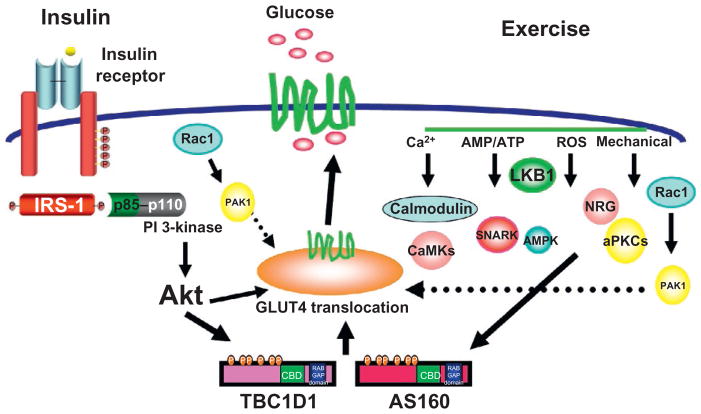
It is well known that a single bout of exercise activates multiple signaling pathways46–48; however, the precise signaling mechanism that mediates exercise-stimulated glucose transport is still not fully understood. Muscle contractile activity results in numerous alterations within the muscle fibers including changes in energy status (i.e., increased AMP/ATP), increases in intracellular Ca2+ concentration, increased reactive oxygen species, and stretching of the muscle fibers. These modifications can activate various signaling cascades, some of which have been implicated in exercise-stimulated glucose transport49,50 (Fig. 1).
Figure 1. Exercise and insulin regulation of glucose transport.
Proposed model for the signaling pathways mediating exercise- and insulin-induced skeletal muscle glucose transport. Insulin is initiated by binding to its cell service receptor leading to a cascade of phosphorylation reactions involving IRS-1, PI 3-kinase, and Akt among other proteins. Exercise works through a proximal signaling mechanism from that is distinct form that of insulin and is less well defined. It is likely that the proximal exercise signaling mechanism has redundancy as number of stimuli have been implicated in this process including changes in intracellular Ca2+, the AMP:ATP ratio, generation of reactive oxygen species, and mechanical stresses. The insulin and exercise signaling pathways are thought to converge at the level of the Rab GAP proteins TBC1D1 and AS160, which allow for the release of the GLUT4-containing vesicles from intracellular stores, translocation to the transverse tubules and sarcolemma, and an increase in glucose uptake. Adapted from Ref. 50.
5.1 AMPK and LKB1
AMPK is a heterotrimeric protein composed of a catalytic α-subunit and regulatory β- and γ-subunits. The α- and β-subunits each exist in two isoforms (α1, α2 and β1, β2), and the γ-subunit exists in three isoforms (γ1, γ2, and γ3). AMPK is activated by phosphorylation by one or more upstream kinases, including LKB1.52–54
AMPK and LKB1 have been widely studied for their potential role in exercise-stimulated glucose transport.55 The initial evidence for a role of AMPK in exercise-stimulated glucose transport came from studies using the AMP-analog, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR).56,57 These studies showed that AICAR increases glucose transport in skeletal muscle,56,57 and similar to muscle contraction, the effects of AICAR are additive with insulin and PI 3-kinase-independent.56,58 Some studies have shown that mice overexpressing a dominant negative AMPK α2 construct in muscle or α1 and α2 KO mice have impaired exercise-stimulated glucose uptake.24,59–63 In contrast, other studies using mouse models with ablated AMPK activity demonstrate that inhibition of AMPK has little or no effect on exercise-induced glucose uptake,62,64,65 or exercise-stimulated glucose uptake in vivo,66 suggesting redundancy in the system. Therefore, it is still controversial whether AMPK is necessary for exercise-stimulated glucose uptake.
The role of LKB1 in exercise-stimulated glucose transport is also not clear. Mice with KO of LKB1 specifically in skeletal muscle have been shown to have a severe blunting of contraction-stimulated glucose transport.51,67 This decrease in glucose transport could be due to decreased activation of AMPK and one or more of the AMPK-related kinases that are substrates of LKB1. One possible LKB1 substrate that may regulate exercise-stimulate glucose transport is the sucrose-nonfermenting AMPK-related kinase (SNARK). Decreased SNARK activity in skeletal muscle was shown to decrease exercise-stimulated glucose transport.68
While contraction-stimulated glucose transport was shown to be impaired in LKB-1 KO mice51,67 and with decreased SNARK activity,68 another recent study showed that glucose uptake during treadmill running was similar, if not higher, in LKB-1 KO mice compared to wild-type controls.69 In yet another study, muscle-specific deletion of LKB1 only partially inhibited exercise-stimulated glucose transport.51 These data suggest that while AMPK, SNARK, and LKB1 may be important in the regulation of exercise-stimulated glucose uptake, this system must have a high degree of redundancy, and it is likely that there are several overlapping signaling systems that can control exercise-stimulated glucose transport in skeletal muscle. This theory is consistent with the importance of carbohydrate utilization during exercise for survival.
5.2 Ca2+/Calmodulin-Dependent Protein Kinases
Skeletal muscle contractile activity requires an increase in intracellular Ca2+ concentrations, and some studies have indicated that Ca2+/calmodulin signaling and Ca2+/calmodulin-dependent protein kinases are critical signals mediating exercise-stimulated glucose transport in skeletal muscle. Incubation of rat skeletal muscle with the Ca2+/calmodulin inhibitor KN-93 decreased contraction-stimulated glucose transport.70 KN-93 also inhibited exercise-induced CaMKII phosphorylation in the absence of AMPK inhibition, suggesting that CaMKs regulate glucose transport independently of AMPK signaling.70,71 These studies also showed that overexpressing a constitutively active CaMKKα in mouse skeletal muscle increased AMPK Thr-172 phosphorylation and skeletal muscle glucose uptake.71 Electroporation of a specific CaMKII inhibitor into mouse tibialis anterior muscle reduced exercise-stimulated glucose uptake by 30%.72 However, a separate study found that increases in Ca2+ concentration in muscle caused very little increase in glucose uptake when the contractile response of the muscle was impaired.73 These data point to an indirect effect of Ca2+ on muscle glucose uptake, and the study of calcium signaling in the regulation of exercise-stimulated glucose transport needs further investigation.
5.3 Downstream Signals Mediating Exercise-Stimulated Glucose Transport
The signaling proteins downstream in the exercise and insulin signaling pathways have been proposed to converge at the Rab GAP proteins Akt substrate of 160 kDa (AS160/TBC1D4) and Tre-2/USP6, BUB2, cdc16 domain family member 1 (TBC1D1). AS160 and TBC1D1 are linked to GLUT4 translocation via the Rab (ras homologous from brain) proteins. Rab proteins are members of the Ras small GTPases superfamily74 and have been shown to be involved in many membrane-trafficking events. Active Rabs recruit various effector proteins that are involved in vesicle budding, tethering, and fusion.49,74,75 In addition to the well-established roles of the Rab proteins, there is evidence that the Rho family GTPase Rac1 is involved in both insulin- and exercise-stimulated GLUT4 translocation.76,77 Mice deficient in Rac1 (Rac1 KO) have decreased insulin-stimulated GLUT4 translocation,71,76 and Rac1 inhibition decreased contraction-stimulated glucose uptake in mouse skeletal muscle.77
5.4 AS160 and TBC1D1
AS160 was initially demonstrated to regulate insulin-stimulated GLUT4 translocation in 3T3LI adipocytes.78–80 AS160 has numerous phosphorylation sites, and Rab GAP activity is controlled by phosphorylation. The best-studied phosphorylation sites are a group of six distinct sites that were identified as substrates for Akt. These are collectively referred to as phospho-Akt-substrate (PAS) sites and both insulin and exercise increase AS160 PAS phosphorylation in skeletal muscle.78,81,82 Prolonged exercise in humans82–84 and rats,78 as well as AICAR, are also known to cause AS160 PAS phosphorylation. Therefore, in addition to Akt, AMPK has been shown to phosphorylate AS160.81 Mutation of four PAS sites significantly inhibits both insulin- and exercise-induced glucose uptake.85 AS160 also contains a calmodulin-binding domain, and mutation of this domain inhibits exercise-, but not insulin-stimulated glucose uptake.86 These data show that both phosphorylation and calmodulin binding on AS160 are involved in the regulation of exercise-stimulated glucose uptake. These data also suggest that while AS160 may serve as a point of convergence for both insulin- and exercise-dependent signaling in the regulation of glucose uptake, other proteins may be involved in this regulation of glucose uptake.
TBC1D1 is another potential molecular link among signaling pathways converging on GLUT4 translocation in skeletal muscle.78,81,87–91 TBC1D1 and AS160 share 47% overall identity and have several comparable structural features. TBC1D1 was first identified in adipocytes in culture but has only very limited expression in this tissue. In contrast, TBC1D1 is highly expressed in skeletal muscle.89 Insulin increases TBC1D1 PAS phosphorylation in skeletal muscle90,92,93 but, unlike AS160, TBC1D1 can regulate insulin-stimulated glucose transport through a PAS-independent mechanism.92 Mutations of TBC1D1 differentially regulate insulin- and exercise-stimulated glucose transport in skeletal muscle.92,93 Thus, TBC1D1 regulates both insulin- and exercise-stimulated glucose transport in muscle, but through distinct phosphorylation sites. Taken together, these data demonstrate that AS160 and TBC1D1 are a point of convergence for the regulation of GLUT4 translocation for insulin- and exercise-stimulated glucose transport in skeletal muscle.
6. INCREASES IN INSULIN SENSITIVITY FOR GLUCOSE TRANSPORT AFTER EXERCISE
The effects of an acute bout of exercise on glucose transport are relatively short-lived, returning to baseline typically in ~30–40 min. However, once the acute effects of exercise per se have disappeared, there is a period characterized by an increased effectiveness of insulin to stimulate glucose transport.94,95 This increase in postexercise insulin sensitivity has been observed up to 48 h after exercise in humans.96 The mechanisms for increased insulin sensitivity are not known. Although decreased muscle glycogen concentrations may play a part in exercise-induced increases in insulin sensitivity, the increased insulin action can occur even after full glycogen repletion.94 The signaling mechanisms mediating the postexercise increase in insulin sensitivity are also not known, but similar to the acute effects of exercise on glucose transport, are not thought to be due to increased activity of the insulin receptor or IRS-1.94,95,97 However, we and others have data suggesting that there is enhanced IRS-2 tyrosine phosphorylation,98,99 Akt phosphorylation44,100,101 and activity,44 Atk substrate of 160 kDa (AS160) phosphorylation,100 and expression of cytoplasmic SHP2101 in the postexercise state.
7. EXERCISE TRAINING: IMPACT ON HEALTHY PEOPLE AND PEOPLE WITH TYPE 2 DIABETES
Regular physical activity leads to numerous adaptations in skeletal muscle which allow the muscle to more efficiently generate ATP and become more resistant to fatigue.102 In regards to carbohydrate metabolism, some of the key adaptations that occur in skeletal muscle with exercise training include enhanced glucose uptake and increased expression of GLUT4.103,104 Trained muscles are also characterized by increased concentrations of glycogen, which is an important factor in the decreased rates of fatigue with prolonged exercise. Exercise training causes muscle fiber type transformation to a more oxidative and perhaps slow phenotype,105–107 and an increase in mitochondrial activity and content.108–110 In addition, exercise training can increase insulin sensitivity and improve overall glucose homeostasis,111–113 which are of particular importance for individuals with metabolic diseases such as type 2 diabetes.
Type 2 diabetes arises from a combination of genetic susceptibility and environmental factors including physical inactivity and poor nutrition.114 Thus, type 2 diabetes typically develops as individuals become more obese and less active, leading to insulin resistance, impaired glucose tolerance, and eventually, the onset of full blown type 2 diabetes. While type 2 diabetes is a multifactorial disease, it is a disease of altered carbohydrate metabolism on many levels. In people with type 2 diabetes, insulin levels are normal or high, but tissues such as liver, skeletal muscle, and adipose tissue become resistant to insulin. The pancreas compensates by producing large amounts of insulin, but this stress can eventually lead to pancreatic failure and the need for exogenous insulin treatment. The hyperinsulinemic state can result in impaired glucose transport into the liver, skeletal muscle, and adipose tissue.115 While type 2 diabetes is usually adult-onset, the number of children and adolescents afflicted by this disease is dramatically increasing. In fact, there are currently 23.6 million people in the United States, which reflects approximately 8% of the population that have diabetes, a number that has doubled over the last 15 years and is continuing to increase at epidemic rates.116
Although these statistics are discouraging, the good news is that regular physical exercise can delay or prevent the onset of type 2 diabetes.117–120 Studies using randomized trials have found that lifestyle interventions, which included ~150 min of physical activity per week, combined with diet-induced weight loss, reduced the risk of type 2 diabetes by 58% in an at-risk population.91,117 Exercise interventions, independent of diet, have also been shown to be effective for the prevention and the progression of type 2 diabetes.118 Exercise training in people with type 2 diabetes can improve blood glucose concentrations, body weight, lipids, blood pressure, cardiovascular disease, mortality, and overall quality of life.121–127 The Look AHEAD study has demonstrated that combined weight loss and physical activity in people with type 2 diabetes causes modest weight loss of approximately 6%, improved glycolated hemoglobin, improved mobility, and improved kidney function but no improvement in cardiovascular disease over a 10-year period.121,123,124,126 However, since the level of fitness was only assessed through year 4 of the study, conclusions on the effects of fitness level on cardiovascular disease cannot be made.121,123,124,126 Increasing physical activity in adults with type 2 diabetes has been shown to result in partial or complete remission of type 2 diabetes in 11.5% of subjects within the first year of intervention, and an additional 7% had partial or complete remission of type 2 diabetes after 4 years of exercise intervention.122 Taken together, all of these data show that the effects of exercise on carbohydrate metabolism have profound effects on metabolic health, and this knowledge is important as we work to address the epidemic of type 2 diabetes.
Acknowledgments
Work in the author’s laboratory was supported by National Institutes of Health Grants R01-AR42238, R01-DK101043, R01-DK099511 (to L.J.G.), and 5P30 DK36836 (Joslin Diabetes Center, DRC). J.D.M. is supported by a mentor-based fellowship from the American Diabetes Association (to L.J.G.). K.I.S. was been supported by an American College of Sports Medicine Research Endowment Grant and Mary K. Iacocca Fellowship at the Joslin Diabetes Center and is currently supported by National Institutes of Health K01-DK105109.
ABBREVIATIONS
- ADP
adenosine diphosphate
- AICAR
aminoimidazole-4-carboxamide ribonucleoside
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- AS160
Akt substrate of 160 kDa
- ATP
adenosine triphosphate
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- GLUT4
glucose transporter type 4
- LKB1
liver kinase B1
- MIRKO
muscle-specific insulin receptor knockout mice
- PAS
phospho-Akt-substrate
- Pi
inorganic phosphate
- Rab
ras homologous from brain
- SNARK
sucrose nonfermenting AMPK-related kinase
References
- 1.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 3.Hargreaves M. The metabolic systems; carbohydrate metabolism. In: Farrell PA, Joyner MJ, Caizozzo VJ, editors. Advanced Exercise Physiology. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. pp. 3–391. [Google Scholar]
- 4.Spriet LL. The metabolic systems; lipid metabolism. In: Farrell PA, Joyner MJ, Caizozzo VJ, editors. Advanced Exercise Physiology. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. pp. 392–407. [Google Scholar]
- 5.Sidossis LS, Stuart CA, Shulman GI, Lopaschuk GD, Wolfe RR. Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry into the mitochondria. J Clin Invest. 1996;98:2244–2250. doi: 10.1172/JCI119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. Am J Clin Nutr. 1998;67:519S–526S. doi: 10.1093/ajcn/67.3.519S. [DOI] [PubMed] [Google Scholar]
- 7.Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jequier E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr. 1988;48:240–247. doi: 10.1093/ajcn/48.2.240. [DOI] [PubMed] [Google Scholar]
- 8.Maughan RJ. Sports Nutrition. Handbook of Sports Medicine and Science. London: Blackwell Science; 2002. [Google Scholar]
- 9.McArdle W, Katch FKV. Essentials of exercise physiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 10.van Baak MA. Physical activity and energy balance. Public Health Nutr. 1999;2:335–339. doi: 10.1017/s1368980099000452. [DOI] [PubMed] [Google Scholar]
- 11.Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 12.Graham TE. Glycogen: an overview of possible regulatory roles of the proteins associated with the granule. Appl Physiol Nutr Metab. 2009;34:488–492. doi: 10.1139/H09-048. [DOI] [PubMed] [Google Scholar]
- 13.van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hultman E, Harris RC. Carbohydrate metabolism. In: Poortmans JR, editor. Principles of Exercise Biochemistry. Basel: Karger; 1988. pp. 78–119. [Google Scholar]
- 16.Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol. 1982;242:E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- 17.Watt MJ, Howlett KF, Febbraio MA, Spriet LL, Hargreaves M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol. 2001;534:269–278. doi: 10.1111/j.1469-7793.2001.t01-1-00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlett RA, Parolin ML, Dyck DJ, et al. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Am J Physiol. 1998;275:R418–R425. doi: 10.1152/ajpregu.1998.275.2.R418. [DOI] [PubMed] [Google Scholar]
- 19.Burke LM, Angus DJ, Cox GR, et al. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J Appl Physiol. 2000;89:2413–2421. doi: 10.1152/jappl.2000.89.6.2413. [DOI] [PubMed] [Google Scholar]
- 20.Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 21.Bergstrom J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210:309–310. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- 22.Chesley A, Heigenhauser GJ, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Physiol. 1996;270:E328–E335. doi: 10.1152/ajpendo.1996.270.2.E328. [DOI] [PubMed] [Google Scholar]
- 23.Kubo K, Foley JE. Rate-limiting steps for insulin-mediated glucose uptake into perfused rat hindlimb. Am J Physiol. 1986;250:E100–E102. doi: 10.1152/ajpendo.1986.250.1.E100. [DOI] [PubMed] [Google Scholar]
- 24.Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985;249:C226–C232. doi: 10.1152/ajpcell.1985.249.3.C226. [DOI] [PubMed] [Google Scholar]
- 25.Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993;268:19161–19164. [PubMed] [Google Scholar]
- 26.Kayano T, Burant CF, Fukumoto H, et al. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6) J Biol Chem. 1990;265:13276–13282. [PubMed] [Google Scholar]
- 27.Klip A, Paquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- 28.Rogers S, Macheda ML, Docherty SE, et al. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab. 2002;282:E733–E738. doi: 10.1152/ajpendo.2002.282.3.E733. [DOI] [PubMed] [Google Scholar]
- 29.Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377:151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- 30.Zisman A, Peroni OD, Abel ED, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 31.Hirshman MF, Wallberg-Henriksson H, Wardzala LJ, Horton ED, Horton ES. Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett. 1988;238:235–239. doi: 10.1016/0014-5793(88)80486-1. [DOI] [PubMed] [Google Scholar]
- 32.Hirshman MF, Goodyear LJ, Wardzala LJ, Horton ED, Horton ES. Identification of an intracelular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990;265:987–991. [PubMed] [Google Scholar]
- 33.Lauritzen HP, Galbo H, Brandauer J, Goodyear LJ, Ploug T. Large GLUT4 vesicles are stationary while locally and reversibly depleted during transient insulin stimulation of skeletal muscle of living mice: imaging analysis of GLUT4-enhanced green fluorescent protein vesicle dynamics. Diabetes. 2008;57:315–324. doi: 10.2337/db06-1578. [DOI] [PubMed] [Google Scholar]
- 34.Lauritzen HP, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes. 2010;59:2134–2144. doi: 10.2337/db10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 36.Yang C, Coker KJ, Kim JK, et al. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest. 2001;107:1311–1318. doi: 10.1172/JCI12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristiansen S, Hargreaves M, Richter EA. Exercise-induced increase in glucose transport, GLUT-4, and VAMP-2 in plasma membrane from human muscle. Am J Physiol. 1996;270:E197–E201. doi: 10.1152/ajpendo.1996.270.1.E197. [DOI] [PubMed] [Google Scholar]
- 38.Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol. 1988;65:909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997;273:E1039–E1051. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- 40.Folli F, Saad MJ, Backer JM, Kahn CR. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992;267:22171–22177. [PubMed] [Google Scholar]
- 41.Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphoproteins and PI 3-kinase activity in rat skeletal muscle. Am J Physiol. 1995;268:E987–E995. doi: 10.1152/ajpendo.1995.268.5.E987. [DOI] [PubMed] [Google Scholar]
- 42.Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 43.Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol. 1989;256:E138–E144. doi: 10.1152/ajpendo.1989.256.1.E138. [DOI] [PubMed] [Google Scholar]
- 44.Wojtaszewski JF, Higaki Y, Hirshman MF, et al. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest. 1999;104:1257–1264. doi: 10.1172/JCI7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knock-out exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 46.Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol. 2005;99:330–337. doi: 10.1152/japplphysiol.00175.2005. [DOI] [PubMed] [Google Scholar]
- 47.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–395. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- 48.Lessard SJ, Rivas DA, Alves-Wagner AB, et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes. 2013;62:2717–2727. doi: 10.2337/db13-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:933–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 50.Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60:145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koh HJ, Arnolds DE, Fujii N, et al. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol. 2006;26:8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardie DG. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc. 2011;70:92–99. doi: 10.1017/S0029665110003915. [DOI] [PubMed] [Google Scholar]
- 53.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 54.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 55.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2014;33C:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 57.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 58.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 59.Abbott MJ, Bogachus LD, Turcotte LP. AMPKalpha2 deficiency uncovers time dependency in the regulation of contraction-induced palmitate and glucose uptake in mouse muscle. J Appl Physiol. 2011;111:125–134. doi: 10.1152/japplphysiol.00807.2010. [DOI] [PubMed] [Google Scholar]
- 60.Jensen TE, Rose AJ, Jorgensen SB, et al. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- 61.Jensen TE, Schjerling P, Viollet B, Wojtaszewski JF, Richter EA. AMPK alpha1 activation is required for stimulation of glucose uptake by twitch contraction, but not by H2O2, in mouse skeletal muscle. PLoS One. 2008;3:e2102. doi: 10.1371/journal.pone.0002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jorgensen SB, Viollet B, Andreelli F, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 63.Lefort N, St Amand E, Morasse S, Cote CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab. 2008;295:E1447–E1454. doi: 10.1152/ajpendo.90362.2008. [DOI] [PubMed] [Google Scholar]
- 64.Fujii N, Hirshman MF, Kane EM, et al. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 65.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 66.Maarbjerg SJ, Jorgensen SB, Rose AJ, et al. Genetic impairment of AMPKalpha2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am J Physiol Endocrinol Metab. 2009;297:E924–E934. doi: 10.1152/ajpendo.90653.2008. [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto K, McCarthy A, Smith D, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koh HJ, Toyoda T, Fujii N, et al. Sucrose nonfermenting AMPK-related kinase (SNARK) mediates contraction-stimulated glucose transport in mouse skeletal muscle. Proc Natl Acad Sci U S A. 2010;107:15541–15546. doi: 10.1073/pnas.1008131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeppesen J, Maarbjerg SJ, Jordy AB, et al. LKB1 regulates lipid oxidation during exercise independently of AMPK. Diabetes. 2013;62:1490–1499. doi: 10.2337/db12-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2 + and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- 71.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2 +/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- 72.Witczak CA, Jessen N, Warro DM, et al. CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1150–E1160. doi: 10.1152/ajpendo.00659.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen TE, Sylow L, Rose AJ, et al. Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmatic reticulum Ca(2 +) release. Mol Metab. 2014;3:742–753. doi: 10.1016/j.molmet.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 75.Kaddai V, Marchand-Brustel Y, Cormont M. Rab proteins in endocytosis and Glut4 trafficking. Acta Physiol (Oxf ) 2008;192:75–88. doi: 10.1111/j.1748-1716.2007.01787.x. [DOI] [PubMed] [Google Scholar]
- 76.Sylow L, Jensen TE, Kleinert M, et al. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes. 2013;62:1865–1875. doi: 10.2337/db12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sylow L, Jensen TE, Kleinert M, et al. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes. 2013;62:1139–1151. doi: 10.2337/db12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- 79.Sano H, Kane S, Sano E, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 80.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology. 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 81.Kramer HF, Witczak CA, Fujii N, et al. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 82.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 83.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes. 2006;55:1776–1782. doi: 10.2337/db05-1419. [DOI] [PubMed] [Google Scholar]
- 84.Sriwijitkamol A, Coletta DK, Wajcberg E, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose–response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 86.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 2007;56:2854–2862. doi: 10.2337/db07-0681. [DOI] [PubMed] [Google Scholar]
- 87.Kane S, Sano H, Liu SC, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692–1697. doi: 10.2337/diabetes.54.6.1692. [DOI] [PubMed] [Google Scholar]
- 89.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor EB, An D, Kramer HF, et al. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Treebak JT, Glund S, Deshmukh A, et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 92.An D, Toyoda T, Taylor EB, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vichaiwong K, Purohit S, An D, et al. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem J. 2010;431:311–320. doi: 10.1042/BJ20101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- 95.Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46(11):1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 96.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 97.Wojtaszewski JF, Hansen BF, Gade J, et al. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 98.Howlett KF, Sakamoto K, Hirshman MF, et al. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes. 2002;51:479–483. doi: 10.2337/diabetes.51.2.479. [DOI] [PubMed] [Google Scholar]
- 99.Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism. 2006;55(8):1046–1052. doi: 10.1016/j.metabol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292(4):E1191–E1200. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- 101.Wadley GD, Konstantopoulos N, Macaulay L, et al. Increased insulin-stimulated Akt pSer473 and cytosolic SHP2 protein abundance in human skeletal muscle following acute exercise and short-term training. J Appl Physiol. 2007;102(4):1624–1631. doi: 10.1152/japplphysiol.00821.2006. [DOI] [PubMed] [Google Scholar]
- 102.Tonkonogi M, Sahlin K. Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev. 2002;30(3):129–137. doi: 10.1097/00003677-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 103.James DE, Kraegen EW, Chisholm DJ. Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Invest. 1985;76:657–666. doi: 10.1172/JCI112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goodyear LJ, Hirshman MF, Valyou PM, Horton ES. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes. 1992;41:1091–1099. doi: 10.2337/diab.41.9.1091. [DOI] [PubMed] [Google Scholar]
- 105.Andersen P, Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand. 1977;99(1):123–125. doi: 10.1111/j.1748-1716.1977.tb10361.x. [DOI] [PubMed] [Google Scholar]
- 106.Fitts RH. Effects of regular exercise training on skeletal muscle contractile function. Am J Phys Med Rehabil. 2003;82(4):320–331. doi: 10.1097/01.PHM.0000059336.40487.9C. [DOI] [PubMed] [Google Scholar]
- 107.Röckl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56(8):2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 108.Holloszy JO. Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 109.Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 110.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Plant Physiol Plant Mol Biol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 111.Bjorntorp P, Fahlen M, Grimby G, et al. Carbohydrate and lipid metabolism in middle-aged physically well trained men. Metabolism. 1972;21:1037–1044. doi: 10.1016/0026-0495(72)90034-0. [DOI] [PubMed] [Google Scholar]
- 112.Ruderman NB, Ganda OP, Johansen K. The effect of physical training on glucose tolerance and plasma lipids in maturity-onset diabetes. Diabetes. 1979;28:89–92. doi: 10.2337/diab.28.1.s89. [DOI] [PubMed] [Google Scholar]
- 113.Ostergard T, Andersen JL, Nyholm B, et al. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;290(5):E998–E1005. doi: 10.1152/ajpendo.00012.2005. [DOI] [PubMed] [Google Scholar]
- 114.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 115.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.National Task Force on the Prevention, Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 117.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 118.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–172. doi: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- 120.Kinney JM, Tucker HN. Energy Metabolism. Tissue Determinants and Cellular Corollaries. New York, NY: Raven Press; 1992. [Google Scholar]
- 121.Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548–2556. doi: 10.2337/dc14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(10):801–809. doi: 10.1016/S2213-8587(14)70156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 126.Rejeski WJ, Bray GA, Chen SH, et al. Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci. 2015;70(3):343–351. doi: 10.1093/gerona/glu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]