Abstract
Objectives
To determine, using data from a real-world setting, the overall and sex-specific risk of cardiovascular (CV) events in patients with rheumatoid arthritis (RA), with or without comorbid hyperlipidemia, relative to those in a non-RA cohort.
Methods
This retrospective cohort study using claims data from a US commercial health plan (2005–2011) included patients with RA and a matched non-RA cohort. Cox proportional hazards regression model determined the hazard ratio (HR) for CV events (myocardial infarction, stroke, revascularization procedures), using the presence of RA and hyperlipidemia as the independent variables, controlling for other covariates (age, sex, diabetes, and hypertension).
Results
The incidence of CV events per 1000 person-years was 10.19 for the RA cohort and 6.41 for the non-RA cohort (crude rate ratio [RR] =1.59). Within the RA cohort, incidence was 15.54 for patients with hyperlipidemia and 7.05 for patients without hyperlipidemia (crude RR=2.21); in the non-RA cohort, incidence was 10.55 and 3.82 for those with and without hyperlipidemia, respectively (crude RR=2.76). After controlling for covariates, the HR of CV events among RA patients was 1.68 (95% CI: 1.50, 1.87) relative to non-RA patients. After multivariable adjustment, hyperlipidemia conferred a significant risk of CV events in both RA and non-RA patients; the interaction between RA and hyperlipidemia was not significant (p=0.13).
Conclusion
This real-world analysis demonstrates that patients with RA have an increased risk of CV events. Similar to a non-RA cohort, CV event rates were incrementally higher for those patients with hyperlipidemia.
Significance
Cardiovascular disease is an increasingly visible topic of concern in the rheumatoid arthritis community. However, there are only limited data that informs both the absolute and relative rates of CVD events, and the contribution of various risk factors such as hyperlipidemia, compared to non-RA populations
The ‘lipid paradox’ hypothesis in RA suggests that elevated LDL cholesterol has a negligible effect on CVD risk in RA, unlikely in the general population where it is a well-accepted CVD risk factor
The incidence of CVD events in RA patients was 10/1000 patient years, a 1.6 fold greater risk compared to non-RA patients
The contribution of hyperlipidemia to CVD risk was associated with comparable or greater absolute increases in the rate of CV events compared to non RA patients, a finding that does not support the lipid paradox.
Keywords: Rheumatoid arthritis, Inflammation, Hyperlipidemia, Cardiovascular disease
Introduction
Cardiovascular disease (CVD) is the most common cause of mortality in patients with rheumatoid arthritis (RA) [1]. There is an increased risk of CV events in patients diagnosed with RA [2–5]. This risk includes not only traditional CV risk factors but also risk related to inflammation as measured by laboratory studies such as the erythrocyte sedimentation rate and/or C-reactive protein (CRP) [6,7]. Many patients with RA also have hyperlipidemia, another traditional risk factor for CVD [8]. The current literature on the relationship between lipids and CV risk in RA patients is controversial. In the general population, dyslipidemia is a common risk factor for CVD, and multiple studies have shown an increase in cardiovascular risk associated with an increase in serum cholesterol levels [9]. In patients with RA, hyperlipidemia may be affected by inflammation and some RA medications. Untreated RA patients with high levels of inflammation appear to have lower lipid levels [10]. Moreover, previous studies have shown that RA patients treated with many biologics (e.g. tocilizumab, anti-TNF therapy) have a reduced inflammation coinciding with an increase in lipid levels [11–15].
The relative importance of traditional CVD risk factors such as age, sex, diabetes, and hyperlipidemia on CVD event rates in patients with RA versus non-RA patients is not well characterized [8,16]. Some traditional risk factors for CVD events in RA patients may be as common as but of lesser importance than in non-RA patients. For example, male sex has been found to be a risk factor for CVD in both RA and non-RA patient cohorts; however, the association may be stronger in non-RA patients [16]. Additionally, the association and the absolute risk of events in RA patients with CV risk factors such as concomitant hyperlipidemia is not well understood. For example, the TRACE-RA study that sought to attenuate CV risk in RA patients was abandoned due to futility, in part may be related to a relative lack of knowledge of the absolute risk of CVD events in RA patients [17]. The objective of this study was to determine, using data from a real-world setting, the overall and sex-specific risk of cardiovascular (CV) events in patients with RA, comparing those with and without comorbid hyperlipidemia, relative to the corresponding risk in a non-RA cohort.
Methods
We conducted a retrospective cohort analysis that utilized claims data from a commercial health plan database. Patient-level data were collected from the database from January 1, 2005 to March 31, 2011. Our study population consisted of an RA and non-RA cohort. The RA cohort included patients with >2 physician diagnoses of RA (ICD-9: 714.0, 714.2) with >7 days and <365 days between diagnosis. This approach has been shown to have high validity [18]. The non-RA cohort included patients with no RA diagnoses matched 3:1 with the RA cohort on baseline demographics (age, sex, region, index year). All patients were aged >18 years on index date and had 12 months of full medical and pharmacy benefits prior to the index date. The index date was designated as the date on which patients had 1 year of full coverage with medical and pharmacy benefits, having met RA (or non-RA) disease criteria. We excluded patients with prevalent CV events in the pre-index period, defined as 1 year of full coverage with medical and pharmacy benefits prior to the index date, as well as patients with other inflammatory diseases (e.g., systemic lupus erythematosus, inflammatory bowel disease, psoriatic arthritis, etc.) in the pre-index period. The post-index period was defined as patients who were followed from the index date until the earliest of the following: first CV event, end of enrollment, end of study period, or a new RA diagnosis (in the non-RA cohort).
Exposures and outcomes of interest
Our dependent variable was defined as a CV event: ICD-9 diagnosis code on a hospital discharge claim in the primary position for myocardial infarction (MI) (410.xx), ischemic stroke (433.xx, 434.xx, 436, 437.1) or a procedure code for percutaneous coronary intervention/coronary artery bypass graft (PCI/CABG) (ICD-9: 36.xx, Current Procedural Terminology: 33510–33536, 92973, 92980–92998) during post-index follow-up. A composite endpoint of MI/stroke/PCI-CABG was also analyzed. Besides RA, our main independent variable was hyperlipidemia, defined as an ICD-9: 272.xx diagnosis claim or a prescription for an antihyperlipidemic agent during the pre-index period. Baseline covariates included: age, sex, region, diabetes, and hypertension.
Statistical analysis
Incidence rates (IRs) and incidence rate ratios (IRRs) for CV event (MI, ischemic stroke, PCI-CABG and composite) in the post-index period were determined per 1000 person-years for each cohort, stratified by the presence/absence of hyperlipidemia and by sex. The time to first CV event in the RA and non-RA cohort was assessed using Kaplan–Meier curves. Patients in the non-RA cohort were censored if they had evidence of a new diagnosis of RA. Cox proportional hazards regression determined hazard ratios (HR) for CV events using CV events as the dependent variable and RA and hyperlipidemia as the independent variables, controlling for baseline covariates that were determined based upon content expertise. Matching factors including age and sex were included as covariates based upon interest to estimate their relationship with the outcome, although were not expected to be needed to control for confounding given the matched design. An interaction term for RA and hyperlipidemia was used as a covariate to test the hypothesis that hyperlipidemia might have a different effect (i.e. be a weaker risk factor) in RA patients. This analysis used only secondary data and so no explicit patient consent was required. Because the study data was completely de-identified, it was considered as exempt from institutional review board approval. All analyses were conducted in SAS 9.2.
Results
Baseline characteristics and comorbidities of the included subjects are described in Table 1. The RA cohort consisted of 51,130 patients who were matched with non-RA patients. A total of 37.9% of patients in the RA cohort and 38.7% in the non-RA cohort had concomitant hyperlipidemia at baseline (Table 1). Approximately three fourths of both cohorts were female (75.8% in the RA cohort and 75.7% in the non-RA cohort).
Table 1.
Baseline* characteristics of eligible RA and non-RA patients.
| RA N=51,130 |
Non-RA N=154,292 |
P value | |
|---|---|---|---|
|
| |||
| Age, mean (SD) years | 51.8 (12.4) | 51.4 (12.8) | <0.001 |
|
| |||
| Sex | |||
| Male | 12,395 (24.2) | 37,526 (24.3) | 0.717 |
| Female | 38,735 (75.8) | 116,766 (75.7) | |
|
| |||
| Region | |||
| Midwest | 14,154 (27.7) | 42,678 (27.7) | 1.0 |
| Northeast | 4,568 (8.9) | 13,779 (8.9) | |
| South | 23,986 (46.9) | 72,423 (46.9) | |
| West | 8,422 (16.5) | 25,412 (16.5) | |
|
| |||
| Hyperlipidemia | 19,360 (37.9) | 59,709 (38.7) | 0.001 |
|
| |||
| Diabetes | 674 (1.3) | 1,124 (0.7) | <0.001 |
|
| |||
| Hypertension | 2,399 (4.7) | 3,768 (2.4) | <0.001 |
Data are n (%) unless indicated otherwise.
measured in the 6 months prior to the start of follow-up
Figure 1 show the time to a CV event in the RA and non-RA cohorts. At the end of follow-up (mean duration of follow-up 2.3 years), a greater proportion of patients in the RA cohort experienced a CV event than in the non-RA cohort (97.2% vs 95.4%, respectively; p<0.001). The incidence of CV events per 1000 person-years was 10.19 for the RA cohort and 6.41 for the non-RA cohort (crude rate ratio [RR] =1.59) (Table 2). The incidence rate ratios and rate differences in the RA vs non-RA cohorts for each component of the composite CV event endpoint (MI, stroke, and PCI-CABG) are shown in Table 2.
Figure 1.
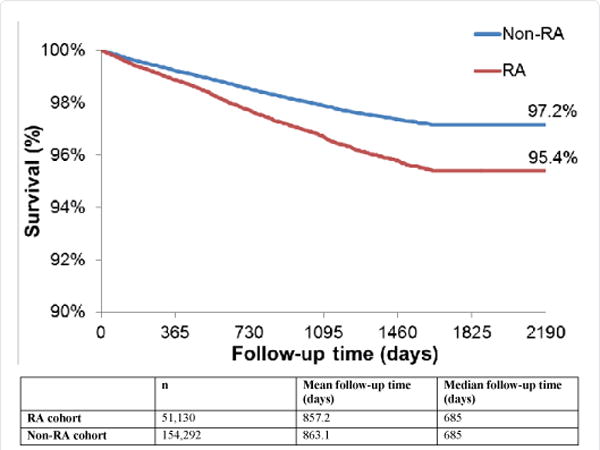
Time to CV event.
*CV events are a composite of MI, stroke, and PCI-CABG events
Table 2.
Cardiovascular event incidence rates in the RA cohort versus Non-RA cohort.
| RA N=51,130 |
Non-RA N=154,292 |
Crude RR (95% CI) | Crude risk difference (95% CI) | |
|---|---|---|---|---|
| CV event IR* Events/total person-years | 10.19 1202/117,944 |
6.41 2311/360,550 |
1.59 (1.48, 1.70) | 3.78 (3.22, 4.35) |
| MI IR Events/total person-years | 3.73 449/120,454 |
2.09 765/365,780 |
1.78 (1.59, 2.00) | 1.64 (1.31, 1.96) |
| Stroke IR Events/total person-years | 5.30 636/120,001 |
3.42 1247/364,442 |
1.55 (1.41, 1.70) | 1.88 (1.47, 2.29) |
| PCI-CABG IR Events/total person-years | 3.98 477/119.899 |
2.44 889/364,471 |
1.63 (1.46, 1.82) | 1.54 (1.19, 1.89) |
CV=cardiovascular; IR=incidence rates; MI=myocardial infarction; PCI=percutaneous coronary intervention; CABG=coronary artery bypass graft; RR=rate ratio
CV outcomes stratified by presence/absence of hyperlipidemia are shown in Figure 2. Within the RA cohort, the incidence of CV events per 1000 person-years was 15.54 for patients with hyperlipidemia and 7.05 for patients without hyperlipidemia (crude RR=2.21). In the non-RA cohort, the incidence of CV events per 1000 person-years was 10.55 and 3.82 for those with and without hyperlipidemia, respectively (crude RR=2.76). The incidence rate ratios and rate differences in patients with hyperlipidemia versus those without hyperlipidemia for each component of the composite CV event endpoint (MI, stroke, and PCI-CABG) are shown in Figure 2 for both the RA and non-RA cohorts. As shown, although the incidence rate ratios (IRRs) associated with having hyperlipidemia were numerically lower for every outcome in the RA cohort compared to the non-RA cohort, the incidence rate differences (IRDs) were numerically higher in the RA cohort.
Figure 2.
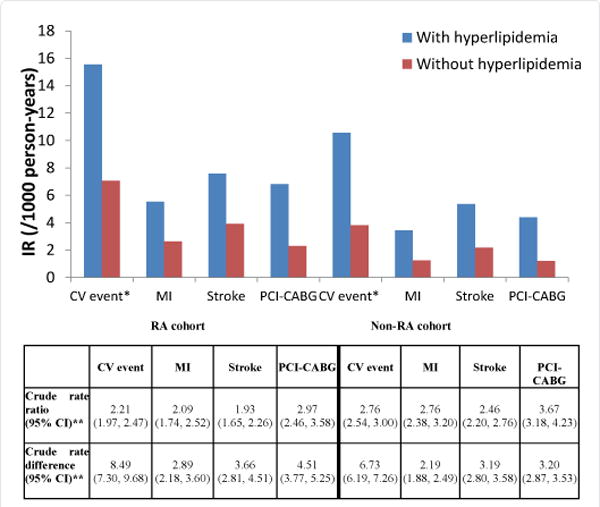
CV Event Incidence Rates, Incidence Rate Ratios, and Incidence Rate Differences for Hyperlipidemia versus No Hyperlipidemia, Stratified by Having RA or Not Having RA.
*CV event IRs are a composite of MI, stroke and PCI-CABG events
**rate ratios and rate differences compare the risk between having hyperlipidemia and not having hyperlipidemia within the RA cohort and within the non-RA cohort
CV=cardiovascular; IR=incidence rates; MI=myocardial infarction; PCI-CABG=percutaneous coronary intervention/coronary artery bypass graft
Figure 3 shows CV outcomes stratified by sex and hyperlipidemia. Within both the RA and non-RA cohorts, the presence of hyperlipidemia was associated with a higher absolute incidence of CV events among both men and women. The incidence of CV events associated with RA without hyperlipidemia (12.85 in men, and 5.58 in women) was comparable to non-RA patients with hyperlipidemia (14.74 and 8.66, respectively). The IRRs and IRDs in men and women with hyperlipidemia versus those without hyperlipidemia are shown in Figure 3 for the RA and non-RA cohorts. Like the results presented in Figure 2, the IRRs for having concomitant hyperlipidemia were numerically smaller in RA patients than in non-RA patients. However, the incidence rate differences were numerically greater in RA patients than non-RA patients.
Figure 3.
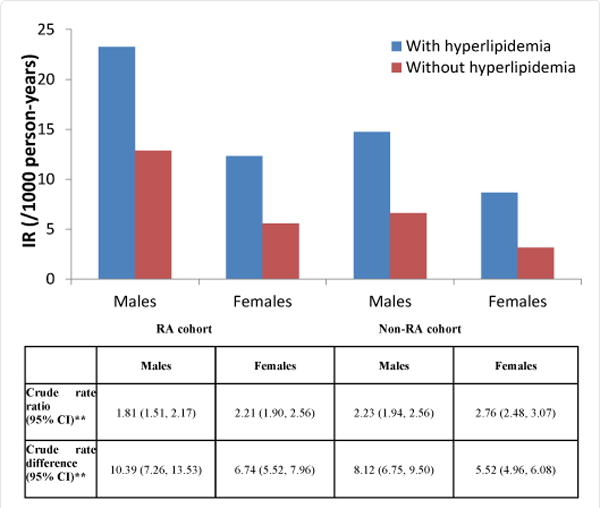
Sex-specific Incidence Rates, Incidence Rate Ratios, and Incidence Rate Differences of CV Events* for Hyperlipidemia in RA and Non-RA Cohorts.
*CV event IRs are a composite of MI, stroke, and PCI-CABG events
**rate ratios and rate differences compare the risk between having hyperlipidemia and not having hyperlipidemia within the RA cohort and within the non-RA cohort
CV=cardiovascular; IR=incidence rate; MI=myocardial infarction; PCI-CABG=percutaneous coronary intervention/coronary artery bypass graft
Table 3 shows the risk of CV events after multivariable adjustment. After controlling for covariates, the HR of CV events for patients with RA was 1.68 (95% CI: 1.50, 1.87) relative to non-RA patients. The presence of hyperlipidemia conferred a significant risk of CV events (HR=1.47, 95% CI: 1.35, 1.60; p, 0.0001). The interaction p value between RA and hyperlipidemia was not significant (p=0.13). The presence of diabetes increased CVD risk by 2.6 fold, greater than that associated with RA, and the 95% confidence intervals of these two risk estimates were non-overlapping.
Table 3.
Multivariable-adjusted risk of CV events*.
| Parameter | Hazard ratio | 95% CI |
|---|---|---|
| Rheumatoid arthritis | 1.68 | 1.50, 1.87 |
| Hyperlipidemia | 1.47 | 1.35, 1.60 |
| Interaction term between RA and hyperlipidemia | 0.90 | 0.78, 1.03 |
| Diabetes | 2.64 | 1.95, 3.56 |
| Age group | ||
| 31–40 vs 18–30 | 1.73 | 0.91, 3.31 |
| 41–50 vs 18–30 | 4.69 | 2.57, 8.55 |
| 51–60 vs 18–30 | 9.88 | 5.45, 17.90 |
| 61–64 vs 18–30 | 17.27 | 9.49, 31.44 |
| ≥65 vs 18–30 | 39.62 | 21.86, 71.81 |
| Male sex | 1.82 | 1.70, 1.94 |
| Region | ||
| Midwest vs South | 1.09 | 1.01, 1.18 |
| Northeast vs South | 1.00 | 0.89, 1.13 |
| West vs South | 0.85 | 0.77, 0.94 |
| Hypertension | 2.00 | 1.77, 2.26 |
CV event IRs are a composite of MI, stroke, and PCI-CABG events
CV=cardiovascular; IR=incidence rate; MI=myocardial infarction; PCI-CABG=percutaneous coronary intervention/coronary artery bypass graft
Discussion
Inflammation associated with RA has been shown to be an important contributor to the increased risk of CV events in patients with RA [15]. Hyperlipidemia is a common comorbidity in RA patients, whether occurring independently or as an effect of RA medications or other disease-related factors. This study examined the interaction of RA and CVD with and without hyperlipidemia. As shown by others [19], we found that patients with RA have an ~1.7-fold increased risk of CV events versus those without RA. Importantly, hyperlipidemia seemed to confer the same incremental risk for CV events in RA patients as for non-RA patients. This finding was robust in the main analysis, and after stratifying by age/sex, and following multivariable adjustment. Given that diabetes was a stronger risk factor for CVD events than was RA, these results might suggest that RA should not be considered a CVD risk equivalent to diabetes with respect to management of hyperlipidemia. We also found that magnitude of the effect of having RA on the absolute risk of CVD events was not large, informing the feasibility of current and future CVD studies in RA patients.
In both the RA and non-RA cohorts, CV event rates were incrementally higher for those patients with hyperlipidemia. Our analysis revealed that the incidence rate ratios of CV events in patients with RA (with hyperlipidemia versus without hyperlipidemia) were numerically lower than the non-RA patients. However, the incidence rate differences of CV events in patients with RA (with hyperlipidemia – without hyperlipidemia) were numerically greater than in the non-RA cohort. This yields the conclusion that the choice of risk scale, ratio versus difference, is important in interpreting the importance of a risk factor such as hyperlipidemia, because the ‘base’ event rate is not the same between RA and non-RA patients. The lack of statistical significance of the interaction term in the multivariable model suggests that hyperlipidemia confers the same incremental risk for CV events in RA patients as for non-RA patients. In total, these results do not provide much support for the lipid paradox, the hypothesis that hyperlipidemia has a different clinical “meaning” in RA patients compared to non-RA patients [20].
Based upon our multivariable models, we evaluated the association between a variety of covariates and CVD risk. We found that RA was a weaker risk factor (HR = 1.7) compared to diabetes (HR = 2.6). This data adds information to the controversy as to whether RA should be considered a CHD risk equivalent with respect to management of hyperlipidemia [21]. Based upon our findings, it does not appear warranted to consider RA as a CHD risk equivalent.
Within our study, we examined the absolute incidence of CVD event in RA patients versus non-RA patients. Despite an elevated risk for CVD events in RA patients, the absolute incremental risk for CVD events in RA was only about 3.78 (~ 4) per 1000 people per year. This translates to an extra one event per 265 patients (1000/3.78), which is relatively low. Even for people with RA and hyperlipidemia, the absolute risk difference is only 5.0, which translates to 1 ‘extra’ event for every 200 RA patients with hyperlipidemia compared to non-RA patients with hyperlipidemia. This makes CVD prevention trials, or CVD outcomes trials, in RA patients challenging from a feasibility perspective. Indeed, one relatively high profile UK trial, TRACE-RA was abandoned for futility, in large part for this reason [17]. A number of ongoing large cardiovascular safety studies (e.g. tocilizumab vs. anti-TNF, tofacitinib vs. anti-TNF) may encounter similar challenges with low event rates, even though they have tried to select for high CVD risk patients [22,23].
We recognize that our study has a number of limitations. Claims data are not collected for research, resulting in the potential for misclassification. For example, some patients might have been misclassified as having RA who had another type of arthritis. Indeed, the positive predictive value of claims-based definitions for RA range from 67% or lower to as high as 95% or greater, depending on the definition used. Claims data also are relatively insensitive to identify CV-related risk factors such as obesity and smoking. Additionally, no information was available about lipid laboratory test results, and our hyperlipidemia classification was based on physician diagnoses and medications. Our analysis intentionally included and adjusted for only a limited number of comorbidities that could contribute to CVD risk in RA patients. Such a concise list was chosen because it is believed that many conditions that we could adjust for (e.g. glucocorticoid use) singly or as part of a composite comorbidity index may be a “downstream effect” of having RA; thus, additional adjustment would represent ‘overadjustment’ and therefore would be undesirable. Finally, the generalizability of these results deserves mention. Individuals in this cohort were commercially insured and likely younger and more healthy than RA patients in the U.S. who are uninsured, enrolled in Medicare, or disabled and receiving Medicaid services. The lipid paradox might, for example, be more relevant in a cachectic, less well-managed RA patient population.
In conclusion, these results confirm the recognized increased CV risk in RA patients and extend those findings to demonstrate that hyperlipidemia in RA patients confers a similarly excess CV event risk compared to non-RA patients. Diabetes is already known to impart a substantial CVD risk; based on our findings that the magnitude of CVD risk was greater for patients with DM compared to those with RA, RA does not appear to be a CHD risk equivalent to diabetes with regard to hyperlipidemia management. Given these conclusions, reducing hyperlipidemia-related CVD risk in RA patients would appear to have a substantial public health impact, although the number needed to treat (NNT) with statin or other lipid lower agents in an RA population may be larger than previously considered given the relatively low absolute risk associated with RA and hyperlipidemia. Finally, although it is often underutilized, mitigating the increased CV risk in patients with RA by appropriate lipid testing and intervention should be as or more compelling than for the general population [18,19].
Acknowledgments
This study was sponsored by Bristol-Myers Squibb. Dr. Curtis receives partial salary support from NIH (AR064172).
Disclosures
JC: received grant/research support from Roche/Genentech, UCB, Centocor, CORRONA, Amgen, Pfizer, Bristol-Myers Squibb, Crescendo and Abbott; has been a consultant for Roche/Genentech, UCB, Centocor, CORRONA, Amgen, Pfizer, Bristol-Myers Squibb, Crescendo and Abbott. AN: Stocks/stock options and employee (Bristol-Myers Squibb).
References
- 1.Sokka T, Pincus T. Ascendancy of weekly low-dose methotrexate in usual care of rheumatoid arthritis from 1980 to 2004 at two sites in Finland and the United States. Rheumatology (Oxford) 2008;47:1543–1547. doi: 10.1093/rheumatology/ken316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 5.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 6.Del Rincon I, Williams K, Stern MP, Freeman GL, O’Leary DH, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 7.Goodson NJ, Symmons DP, Scott DG, Bunn D, Lunt M, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 8.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172. [PubMed] [Google Scholar]
- 9.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 10.Myasoedova E, Crowson CS, Kremers HM, Fitz-Gibbon PD, Therneau TM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310–1314. doi: 10.1136/ard.2009.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1503–1507. doi: 10.1136/ard.2006.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner G, Urowitz MB. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin Arthritis Rheum. 2009;38:372–381. doi: 10.1016/j.semarthrit.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Schimmel EK, Yazici Y. Increased lipid levels but unchanged atherogenic index in rheumatoid arthritis patients treated with biologic disease modifying antirheumatic drugs: published experience. Clin Exp Rheumatol. 2009;27:446–451. [PubMed] [Google Scholar]
- 14.Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of TNF-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:947–955. doi: 10.1007/s10067-010-1405-7. [DOI] [PubMed] [Google Scholar]
- 15.Daïen CI, Duny Y, Barnetche T, Daurès JP, Combe B, et al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–868. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 17.www.dgoh.nhs.uk/tracera/Default.aspx/Home
- 18.Chung CP, Rohan P, Krishnaswami S, McPheeters ML. A systematic review of validated methods for identifying patients with rheumatoid arthritis using administrative or claims data. Vaccine. 2013;31:K41–61. doi: 10.1016/j.vaccine.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 19.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 20.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a.full.pdf
- 22.https://clinicaltrials.gov/ct2/show/NCT01331837
- 23.https://clinicaltrials.gov/ct2/show/NCT02092467


