Abstract
Objective
To determine the association of the genes that encode α-, β-, and γ-synuclein (SNCA, SNCB, and SNCG, respectively) with diffuse Lewy body disease (DLBD).
Design
Case-control study.
Subjects
A total of 172 patients with DLBD consistent with a clinical diagnosis of Parkinson disease dementia/dementia with Lewy bodies and 350 clinically and 97 pathologically normal controls.
Interventions
Sequencing of SNCA, SNCB, and SNCG and genotyping of single-nucleotide polymorphisms performed on an Applied Biosystems capillary sequencer and a Sequenom MassArray pLEX platform, respectively. Associations were determined using χ2 or Fisher exact tests.
Results
Initial sequencing studies of the coding regions of each gene in 89 patients with DLBD did not detect any pathogenic substitutions. Nevertheless, genotyping of known polymorphic variability in sequence-conserved regions detected several single-nucleotide polymorphisms in the SNCA and SNCG genes that were significantly associated with disease (P=.05 to <.001). Significant association was also observed for 3 single-nucleotide polymorphisms located in SNCB when comparing DLBD cases and pathologically confirmed normal controls (P=.03-.01); however, this association was not significant for the clinical controls alone or the combined clinical and pathological controls (P>.05). After correction for multiple testing, only 1 single-nucleotide polymorphism in SNCG (rs3750823) remained significant in all of the analyses (P=.05-.009).
Conclusion
These findings suggest that variants in all 3 members of the synuclein gene family, particularly SNCA and SNCG, affect the risk of developing DLBD and warrant further investigation in larger, pathologically defined data sets as well as clinically diagnosed Parkinson disease/dementia with Lewy bodies case-control series.
Dementia with Lewy Bodies (DLB) is the second most common form of dementia after Alzheimer disease in elderly white populations.1 Clinical features of DLB include dementia, parkinsonism, visual hallucinations, and fluctuations in cognition.2 Pathologically, DLB is associated with Lewy body and Lewy neuritic pathology in limbic cortices (transitional Lewy body disease) and in the neocortex3 (diffuse Lewy body disease [DLBD]), often with concomitant amyloid pathology.1 In pathologically confirmed cases of Parkinson disease (PD), Lewy pathology is primarily found in the brainstem (brainstem Lewy body disease)4 and may be more widespread in PD dementia. In multiple-system atrophy, glial cytoplasmic inclusions in the basal ganglia are pathological hallmarks.5 The major protein component of both Lewy body and glial cytoplasmic inclusions is α-synuclein, encoded by the SNCA gene (OMIM 163890). Polymorphic variability in the SNCA gene and rare missense and multiplication mutations have been unequivocally implicated in PD, PD dementia, and multiple-system atrophy.6-9
SNCA has 2 paralogous genes, β-synuclein (SNCB; OMIM 602569)) and γ-synuclein (SNCG; OMIM 602998), with which it shares a highly conserved N-terminal domain.10 In transgenic mice, overexpression of human α-synuclein leads to the formation of neuronal aggregates reminiscent of Lewy bodies,11 and wild-type β-synuclein has been suggested to protect against α-synuclein toxicity based on in vitro (inhibition of aggregation and fibril formation)12 and in vivo (reduced aggregation and Lewy body formation) evidence.13 Coding substitutions in SNCB (p.V70M and p.P123H) were detected in 2 unrelated patients with DLB, 1 of whom had a positive family history of DLB.14 γ-Synuclein antibodies have identified axonal spheroid-like lesions in the hippocampal dentate molecular layer of patients with PD and DLB.15 In addition, overexpression of γ-synuclein was recently shown to induce a neurodegenerative phenotype in mice.16
Herein, we investigate the roles of SNCA, SNCB, and SNCG in DLBD by performing (1) a comprehensive sequencing of the 3 genes in pathologically defined DLBD cases and (2) a case-control association in an autopsy series of cases with DLBD compared with pathologically and clinically normal controls.
METHODS
Diagnosis of DLBD was established in accordance with published criteria.17-19 Cases acquired prior to 1998 were evaluated with ubiquitin and tau double immunohistochemistry,20 while for those acquired subsequently, immunohistochemistry for α-synuclein was used.21 The institutional review board of each site approved the study, and brain autopsy material was collected with signed informed consent from each patient or next of kin. All cases and control subjects were from North America and of European descent. Control individuals were free of personal or familial history suggestive of parkinsonism or dementia, and the pathologically confirmed subset did not display any significant neuropathological abnormality.
Genomic DNA was extracted from frozen cerebellar tissue or venous blood using standard protocols. A subset of 89 patients with DLBD (mean [SD] age, 77.3[6.7] years; male: female ratio, 1:2.3) randomly selected from the case series, were included in the sequencing study. Primer pairs for coding regions of SNCA (exons 2-6), SNCB (exons 1-5), and SNCG (exons 1-5) were used and are available on request. Polymerase chain reaction products were purified from unincorporated nucleotides using Agencourt bead technology (Beverly, Massachusetts) with Biomek FX automation (Beckman Coulter, Fullerton, California). Sequence analysis was performed as previously described.22
For the association study, we included samples from 172 cases with DLBD without SNCA multiplications (mean [SD] age, 77.6[9.0] years; male:female ratio, 1:0.79), 97 pathologically normal controls (mean [SD] age, 74.5[14.7] years; male: female ratio, 1:0.73), and 350 clinically normal controls (mean [SD] age, 71.8[11.0] years; male:female ratio, 1:0.81). Detailed clinical information is available for 137 DLBD cases (80%). This major subset presented a median IV Braak stage (inter-quartile range [IQR], III-V), a median of 42 senile plaques counts (IQR, 39-48), a median of 2 neurofibrillary tangles count (IQR, 0-5), and a median of 8 Lewy body count (IQR, 6-13). In addition, 65% carry the APOE ε4 allele (18% homozygote). These pathological features result in 79 samples with high likelihood of DLB/PD dementia, 31 with intermediate likelihood, and 27 with low likelihood according to the Consortium on Dementia with Lewy Bodies criteria.2
Highly conserved regions (conservation score, >200) were identified across SNCA, SNCB, and SNCG (coding regions ±10 kilobase [kb]) using the phastConst software embedded in the UCSC Genome Browser (http://genome.ucsc.edu) based on the National Center for Biotechnology Information March 2006 assembly.23 Thirty-one single-nucleotide polymorphisms (SNPs) within these conserved regions were selected in SNCA (15 SNPs), SNCB (7 SNPs), and SNCG (9 SNPs). Genotyping of SNPs was performed on a Sequenom MassArray iPLEX platform (San Diego, California); all primer sequences are available on request. For each variant, genotyping error was assessed by deviation from Hardy-Weinberg equilibrium expectation. Association between individual SNP genotypes and DLBD was determined using χ2 or Fisher exact tests. Linkage disequilibrium between markers and the appropriate level of statistical correction for the multiple testing were assessed using SNPSpD.24
RESULTS
Sequencing analysis of the coding regions of SNCA, SNCB, and SNCG in 89 DLBD cases did not identify any pathogenic mutations or the c.G208A (p.V70M) and c.C368A (p.P123H) substitutions previously described in SNCB. Two polymorphic coding substitutions, p.A65A (rs760113) in exon 3 and p.E110V (rs9864) in exon 4 of SNCG, were identified. Both rs760113 and rs9864 were genotyped in subsequent association analyses.
Of the 31 variants assessed in the synuclein gene family, only 1 SNP in the 5′ flanking region of SNCG (rs3750823) was significantly associated with DLBD after correction for multiple tests (Pcorrected=.009, .05, and .03 for pathological, clinical, and combined controls, respectively) (Table; allelic analyses are provided in the eTable; www.archneurol.com). Additional variants located in the 5′ region of the SNCG gene showed trends toward an association with DLBD when compared with pathological, clinical, or combined controls; of the 2 coding variants identified in SNCG, rs760113 was marginally associated with DLBD before correction for multiple testing, whereas rs9864 was not (Table). Similar evidence of association was observed for several variants within SNCA; however, with the exception of rs10155475 when compared with pathologically normal controls (Pcorrected=.05), these associations did not stand correction for multiple testing (P=.002). Variants with SNCB showed the least evidence of association with DLBD. Two SNPs located upstream of the SNCB and 1 in intron 5 (rs4868670, rs1352303, and rs11739753) were also indicative of genetic association when compared with pathologically normal controls (Table). However, in contrast to findings in SNCA and SNCG, evidence of SNCB association with DLBD was not confirmed in the larger clinical control group or the 2 control groups combined.
Table.
Genotype Frequencies and P Values for SNCA, SNCB, and SNCG
| No. |
||||||||
|---|---|---|---|---|---|---|---|---|
| SNP by Gene | Genomic Position | Genotype | DLBD Cases (n = 172) |
Pathological Controls (n = 97) |
Clinical Controls (n = 350) |
P Valuea |
||
| Pathological | Clinical | Combined | ||||||
| SNCA | ||||||||
| rs356218 | chr4:90856033 | GG/GA/AA | 80/74/16 | 48/34/11 | 170/142/37 | .25 | .50 | .37 |
| rs17180453 | chr4:90872157 | CC/CT/TT | 151/21/0 | 87/9/1 | 296/51/3 | .13 | .15 | .19 |
| rs3775423 | chr4:90876514 | CC/CT/TT | 145/26/1 | 68/25/4 | 304/42/4 | .002 (.052) | .25 | .26 |
| rs3796661 | chr4:90906530 | CC/CT/TT | 162/10/0 | 87/9/1 | 333/15/2 | .08 | .21 | .27 |
| rs356186 | chr4:90924387 | GG/GA/AA | 122/43/2 | 61/28/2 | 226/103/21 | .27 | .006 (.15) | .01 (.26) |
| rs3775439 | chr4:90928764 | GG/GA/AA | 121/40/2 | 66/28/3 | 252/90/7 | .17 | .47 | .33 |
| rs9995651 | chr4:90935200 | CC/CG/GG | 158/14/0 | 84/11/0 | 324/26/0 | .36 | .77 | .94 |
| rs2583959 | chr4:90940660 | CC/CG/GG | 97/64/10 | 55/32/10 | 204/125/21 | .16 | .70 | .51 |
| rs10155475 | chr4:90957181 | GG/GC/CC | 52/79/39 | 43/45/9 | 132/162/56 | .002 (.05) | .03 (.78) | .006 (.15) |
| rs6532191 | chr4:90964953 | TT/TC/CC | 62/74/29 | 26/47/24 | 108/163/79 | .05 | .09 | .047 (>.99) |
| rs1372518 | chr4:90976317 | CC/CA/AA | 113/43/4 | 48/38/2 | 218/107/25 | .009 (.22) | .02 (.39) | .01 (.30) |
| rs3756063 | chr4:90976417 | CC/CG/GG | 66/75/29 | 26/39/23 | 107/164/79 | .05 | .04 (>.99) | .02 (.63) |
| rs1372520 | chr4:90976528 | CC/CT/TT | 121/44/4 | 58/35/4 | 219/106/25 | .04 (>.99) | .009 (.23) | .009 (.22) |
| rs2619361 | chr4:90976758 | CC/CA/AA | 95/64/10 | 45/32/10 | 204/126/20 | .11 | .65 | .6 |
| rs2583988 | chr4:90979851 | CC/CT/TT | 100/61/10 | 55/32/10 | 204/125/20 | .18 | .95 | .69 |
|
| ||||||||
| SNCB | ||||||||
| rs11739753 | chr5:175982314 | CC/CT/TT | 46/80/46 | 37/45/15 | 91/167/92 | .01 (.34) | .79 | .45 |
| rs13160179 | chr5:175983321 | TT/TC/CC | 115/53/4 | 64/27/6 | 250/93/7 | .10 | .28 | .30 |
| rs6886116 | chr5:175990834 | AA/AC/CC | 171/0/0 | 95/2/0 | 350/0/0 | .06 | >.99 | .38 |
| rs12518180 | chr5:175992222 | CC/CT/TT | 103/60/9 | 48/42/7 | 210/117/23 | .1 | .52 | .46 |
| rs10866701 | chr5:175992943 | CC/CT/TT | 86/73/11 | 51/36/10 | 199/128/23 | .19 | .15 | .15 |
| rs1352303 | chr5:175993452 | GG/GA/AA | 50/77/45 | 37/44/15 | 86/172/92 | .03 (.73) | .24 | .41 |
| rs4868670 | chr5:175994856 | TT/TC/CC | 49/77/46 | 36/46/15 | 85/172/93 | .02 (.62) | .26 | .36 |
|
| ||||||||
| SNCG | ||||||||
| rs12416136 | chr10:88703584 | AA/AC/CC | 41/98/30 | 26/43/23 | 99/173/78 | .07 | .06 | .04 (>.99) |
| rs10887683 | chr10:88706557 | AA/AG/GG | 38/89/25 | 25/47/23 | 102/172/76 | .1 | .05 (>.99) | .04 (.98) |
| rs3750823 | chr10:88707134 | TT/TC/CC | 37/96/31 | 35/31/21 | 74/165/111 | <.001 (.009) | .002 (.05) | .001 (.03) |
| rs1800373 | chr10:88708416 | CC/CA/AA | 54/83/25 | 30/48/18 | 92/172/86 | .48 | .01 (.30) | .02 (.62) |
| rs760112 | chr10:88709549 | GG/GA/AA | 108/58/6 | 52/40/5 | 209/122/19 | .13 | .29 | .22 |
| rs760113 | chr10:88709769 | CC/CG/GG | 107/58/6 | 49/42/6 | 203/127/20 | .05 (>.99) | .19 | .11 |
| rs9864 | chr10:88712378 | AA/AT/TT | 106/58/6 | 49/44/4 | 202/128/20 | .06 | .19 | .13 |
| rs10232 | chr10:88712845 | CC/CT/TT | 154/18/0 | 85/12/0 | 312/37/1 | .63 | .48 | .52 |
| rs7096355 | chr10:88712970 | TT/TG/GG | 152/14/1 | 79/4/0 | 305/44/1 | .21 | .13 | .23 |
Abbreviations: chr, chromosome; DLBD, diffuse Lewy body disease; SNP, single-nucleotide polymorphism.
Significant P values are given in bold, and corrected values for multiple testing using SNPSpD24 are given in parentheses.
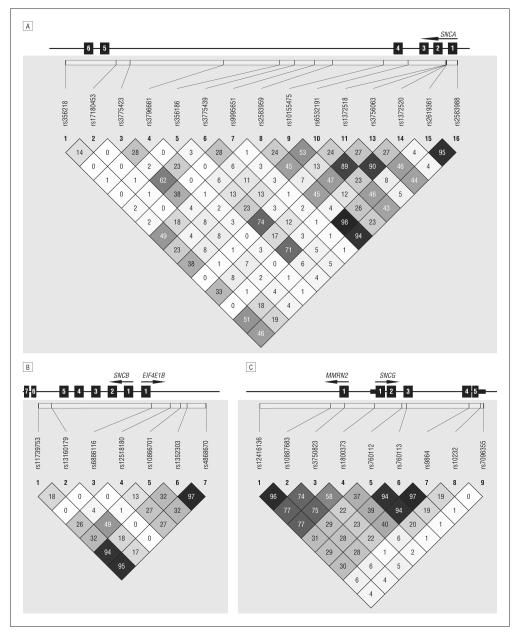
Figure 1.
Linkage disequilibrium patterns for SNCA (A), SNCB (B), and SNCG (C). Values are given as r2. Numbered boxes represent exons.
COMMENT
This is the first study to assess genetic variability in all synuclein paralogues, SNCA, SNCB, and SNCG, in pathologically proven DLBD samples. Nevertheless, the number of autopsy-confirmed control subjects was few (n=97) and underpowered to observe associations of more than modest effect in this pilot study. To help rectify this shortfall, we used a second series of clinical control subjects (n=350). Significant association between the SNCG locus (rs3750823) and DLBD was identified in the pathological series, and then replicated using a larger clinical control group. Genotype distributions for several other variants within the SNCG gene were also indicative of association including rs12416136, rs10887683, and rs1800373. SNCG was previously examined for association with PD in populations from Germany (25 patients; 55 controls) and the United Kingdom (262 patients; 179 controls) with inconclusive results, although rs3750823 was not included in the analysis.25,26 The significantly associated SNP in SNCG (rs3750823) is locatedapproximately1.2 Kb upstream from the gene in exon 1 of the MMRN2 gene and results in a nonsynonymous amino acid substitution; however, the pattern of linkage disequilibrium around rs3750823 spans the 5′end of SNCG (Figure 1).
Our sequencing study did not identify any coding mutation in SNCG, suggesting that the responsible mutation is located in intronic or regulatory regions that we postulate may affect gene expression. Recently, a study in mice showed that overexpression of mouse γ-synuclein leads to its aggregation, neuronal loss, motor deficits, and premature death.16 The SNCG-MMRN2 region of chromosome 10 is homologous with the SNCA-MMRN1 region of chromosome 4, for which similar evidence of genetic association to PD is well established. MMRN homologues encode large, disulfide-linked proteins expressed by megakaryocytes and endothelial cells, with a role in hemostasis.27
There is also evidence of an association between SNCA variability and DLBD (Table). Linkage disequilibrium between most of the SNPs analyzed is moderately low (r2 < 0.5) and suggests that multiple rather than few risk haplotypes are associated with disease (Figure 1). Nevertheless, the results obtained did not remain significant after correction for multiple testing. Sample size, given the number of tests, may be the issue. Despite this, the association appears to be reproducible in pathologically confirmed and clinically normal controls. The trends observed are reminiscent of past associations identified in PD,7,28-31 later confirmed in larger sample series and through meta-analyses (http://www.pdgene.org/). Marginal associations were identified in SNCB when compared with pathologically normal controls. Since this association was not identified with the larger clinical control group, this may be a spurious association owing to the small sample size of the pathological controls. Only 2 studies have examined evidence of association of SNCB and PD with equivocal results,32,33 and coding mutations have not been found in patients with PD.34,35 The 2 SNCB coding substitutions previously described in patients with DLB (p.V70M and p.P123H) have not been proven to segregate with familial disease and were not identified in our sequencing study. Although their pathogenicity remains unproven, they are not a common cause of DLB/DLBD.
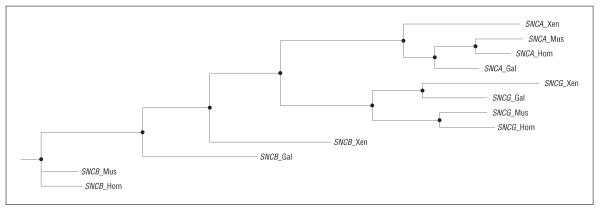
Comparison of the genomic structure of the 3 synuclein genes in human, mouse, chicken, and frog indicates that the SNCA and SNCG genes, which appear to be associated with DLBD, are evolutionarily more closely related than SNCB (Figure 2). This high level of conservation between SNCA and SNCG suggests a similar functional role for these 2 genes; therefore, the well-proven association28-31 and pathogenicity37-39 of SNCA variants and mutations could potentially be mirrored in SNCG. Additionally, interactions between α-synuclein and β-synuclein support a role of the synuclein gene family in LBD. Transgenic α-synuclein A53T mice develop a parkinsonian movement disorder with α-synuclein inclusions and loss of dopaminergic terminals, whereas double transgenic progenies of these mice, also expressing β-synuclein, were shown to present a significantly milder disease phenotype.40 Evaluation of a genetic interaction between the 3 synuclein genes did not yield significant associations; however, the marginal significance of the single SNP association and the relatively low number of DLBD samples indicate that our study is underpowered for this analysis.
Figure 2.
Phylogenic and evolutionary analysis of the DNA sequences for SNCA, SNCB, and SNCG from Homo sapiens (Hom), Mus musculus (Mus), Gallus gallus (Gal), and Xenopus laevis (Xen) were performed using phylemon.36
Our pilot study suggests that genetic variability in the SNCA and SNCG loci influences risk of DLBD. The findings are interesting given the conservation of synuclein homologues, especially SNCA and SNCG, and the encoded proteins. Nevertheless, the results need to be validated in larger clinical series of DLB and further postmortem studies of DLBD. Whether LBD represents a continuum rather than discrete diseases, they clearly share some common etiology, pathological features, and now genetic features. How genetic variability contributes to the disease process such as the quantitative burden or regional distribution of Lewy pathology remains to be determined. However, given that aging increases the incidence of dementia in patients with PD,41 the development of molecular therapeutics targeted to lower synuclein expression may be all the more worthwhile. Further studies addressing the role of the synuclein gene family in LBD are now warranted.
Acknowledgments
Funding/Support: This study was supported by an Eli-Lilly scholarship and a Herb Geist gift for Lewy body research (Dr Nishioka); Swiss National Science Foundation grant PASMP3-123268/1 (Dr Wider); and in part by grants P01 AG017216, R01 NS057567, and R01 AG015866 from the National Institutes of Health and 121849 from the Canadian Institute of Health Research (Dr Wszolek). Mayo Clinic, Jacksonville, is a Morris K. Udall Parkinson’s Disease Research Center of Excellence (grant NINDS P50 #NS40256) and is supported by a Pacific Alzheimer Research Foundation grant C06-01 (Drs Wszolek, Dickson, and Farrer).
Footnotes
Author Contributions: Dr Vilariño-Güell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Vilariño-Güell, Lincoln, Ross, Wszolek, and Farrer. Acquisition of data: Soto-Ortolaza, Rajput, Robinson, Ferman, Wszolek, Dickson, and Farrer. Analysis and interpretation of data: Nishioka, Wider, Soto-Ortolaza, Kachergus, Jasinska-Myga, Ross, and Wszolek. Drafting of the manuscript: Nishioka, Wider, Vilariño-Güell, Ross, and Wszolek. Critical revision of the manuscript for important intellectual content: Soto-Ortolaza, Lincoln, Kachergus, Jasinska-Myga, Ross, Rajput, Robinson, Ferman, Dickson, and Farrer. Statistical analysis: Vilariño-Güell and Wszolek. Obtained funding: Ferman, Wszolek, and Farrer. Administrative, technical, and material support: Nishioka, Vilariño-Güell, Soto-Ortolaza, Lincoln, Kachergus, Jasinska-Myga, Rajput, Robinson, and Farrer. Study supervision: Wszolek.
Online-Only Material: The eTable is available at http://www.archneurol.com.
Financial Disclosure: Dr Rajput reports receiving honoraria from GlaxoSmithKline, Novartis, Prestwick, Teva, and UCB Pharma for speaking or advisory boards; Allergan, Novartis, Teva, and Kyowa for clinical trials; and within the past 12 months, being a paid consultant and receiving honoraria from Serono and University of Calgary for participating in educational events for residents. Dr Farrer reports a US provisional patent application for a device that treats neurodegenerative diseases and has been licensed to Alnylam Pharmaceuticals Inc, and receiving an honorarium for a seminar from H. Lund-beck A/S, GlaxoSmithKline, Elan Pharmaceuticals, and Genzyme.
REFERENCES
- 1.Kosaka K. Diffuse Lewy body disease. Intern Med. 1998;37(1):6–10. doi: 10.2169/internalmedicine.37.6. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 3.Fujishiro H, Ferman TJ, Boeve BF, et al. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol. 2008;67(7):649–656. doi: 10.1097/NEN.0b013e31817d7a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57(8):1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 5.Dickson DW, Liu W, Hardy J, et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol. 1999;155(4):1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 7.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7(4):306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 8.Scholz SW, Houlden H, Schulte C, et al. SNCA variants are associated with increased risk for multiple system atrophy [published correction appears in Ann Neurol. 2010;67(2):277] Ann Neurol. 2009;65(5):610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross OA, Vilariño-Güell C, Zbigniew WK, Farrer MJ, Dickson DW. Reply to: SNCA variants are associated with increased risk of multiple system atrophy. Ann Neurol. 2009;67(3):414–415. doi: 10.1002/ana.21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George JM. The synucleins [published online ahead of print December 20, 2001] Genome Biol. 2002;3(1) REVIEWS3002. [Google Scholar]
- 11.Masliah E, Rockenstein E, Veinbergs I, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32(2):213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- 13.Fan Y, Limprasert P, Murray IV, et al. Beta-synuclein modulates alpha-synuclein neurotoxicity by reducing alpha-synuclein protein expression. Hum Mol Genet. 2006;15(20):3002–3011. doi: 10.1093/hmg/ddl242. [DOI] [PubMed] [Google Scholar]
- 14.Ohtake H, Limprasert P, Fan Y, et al. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63(5):805–811. doi: 10.1212/01.wnl.0000139870.14385.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96(23):13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ninkina N, Peters O, Millership S, Salem H, van der Putten H, Buchman VL. Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein [published online February 26, 2009] Hum Mol Genet. 2009;18(10):1779–1794. doi: 10.1093/hmg/ddp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosaka K. Diffuse Lewy body disease in Japan. J Neurol. 1990;237(3):197–204. doi: 10.1007/BF00314594. [DOI] [PubMed] [Google Scholar]
- 18.Kosaka K, Tsuchiya K, Yoshimura M. Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol. 1988;7(6):299–305. [PubMed] [Google Scholar]
- 19.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree: a new disease? Clin Neuropathol. 1984;3(5):185–192. [PubMed] [Google Scholar]
- 20.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65(7):685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata IF, Kachergus JM, Taylor JP, et al. Lrrk2 pathogenic substitutions in Parkinson’s disease. Neurogenetics. 2005;6(4):171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 23.Siepel A, Bejerano G, Pedersen JS, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15(8):1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flowers JM, Leigh PN, Davies AM, et al. Mutations in the gene encoding human persyn are not associated with amyotrophic lateral sclerosis or familial Parkinson’s disease. Neurosci Lett. 1999;274(1):21–24. doi: 10.1016/s0304-3940(99)00673-4. [DOI] [PubMed] [Google Scholar]
- 26.Krüger R, Schöls L, Müller T, et al. Evaluation of the gamma-synuclein gene in German Parkinson’s disease patients. Neurosci Lett. 2001;310(2-3):191–193. doi: 10.1016/s0304-3940(01)02127-9. [DOI] [PubMed] [Google Scholar]
- 27.Leimeister C, Steidl C, Schumacher N, Erhard S, Gessler M. Developmental expression and biochemical characterization of Emu family members. Dev Biol. 2002;249(2):204–218. doi: 10.1006/dbio.2002.0764. [DOI] [PubMed] [Google Scholar]
- 28.Mizuta I, Satake W, Nakabayashi Y, et al. Multiple candidate gene analysis identifies alpha-synuclein as a susceptibility gene for sporadic Parkinson’s disease. Hum Mol Genet. 2006;15(7):1151–1158. doi: 10.1093/hmg/ddl030. [DOI] [PubMed] [Google Scholar]
- 29.Mueller JC, Fuchs J, Hofer A, et al. Multiple regions of alpha-synuclein are associated with Parkinson’s disease. Ann Neurol. 2005;57(4):535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- 30.Winkler S, Hagenah J, Lincoln S, et al. alpha-Synuclein and Parkinson disease susceptibility. Neurology. 2007;69(18):1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4. [DOI] [PubMed] [Google Scholar]
- 31.Pals P, Lincoln S, Manning J, et al. alpha-Synuclein promoter confers susceptibility to Parkinson’s disease. Ann Neurol. 2004;56(4):591–595. doi: 10.1002/ana.20268. [DOI] [PubMed] [Google Scholar]
- 32.Fung HC, Scholz S, Matarin M, et al. Genome-wide genotyping in Parkinson’s disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 2006;5(11):911–916. doi: 10.1016/S1474-4422(06)70578-6. [DOI] [PubMed] [Google Scholar]
- 33.Brighina L, Okubadejo NU, Schneider NK, et al. Beta-synuclein gene variants and Parkinson’s disease: a preliminary case-control study. Neurosci Lett. 2007;420(3):229–234. doi: 10.1016/j.neulet.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavedan C, Buchholtz S, Auburger G, et al. Absence of mutation in the beta- and gamma-synuclein genes in familial autosomal dominant Parkinson’s disease. DNA Res. 1998;5(6):401–402. doi: 10.1093/dnares/5.6.401. [DOI] [PubMed] [Google Scholar]
- 35.Lincoln S, Crook R, Chartier-Harlin MC, et al. No pathogenic mutations in the beta-synuclein gene in Parkinson’s disease. Neurosci Lett. 1999;269(2):107–109. doi: 10.1016/s0304-3940(99)00420-6. [DOI] [PubMed] [Google Scholar]
- 36.Tárraga J, Medina I, Arbiza L, et al. Phylemon: a suite of web tools for molecular evolution, phylogenetics and phylogenomics [published online ahead of print April 22, 2007] Nucleic Acids Res. 2007;35(Web server issue):W38–W42. doi: 10.1093/nar/gkm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 38.Krüger R, Kuhn W, Müller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 39.Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 40.Park JY, Lansbury PT., Jr Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson’s disease. Biochemistry. 2003;42(13):3696–3700. doi: 10.1021/bi020604a. [DOI] [PubMed] [Google Scholar]
- 41.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]