Abstract
Many studies have investigated the association between CYP1A1 rs1048943 and rs4646903 polymorphisms and laryngeal cancer risk, but their results have been inconsistent. The PubMed and CNKI were searched for case–control studies published up to 01 July 2015. Data were extracted and pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated. In this meta‐analysis, we assessed 10 published studies involving comprising 748 laryngeal cancer cases and 1558 controls of the association between CYP1A1 rs1048943 and rs4646903 polymorphisms and laryngeal cancer risk. For CYP1A1 rs1048943 of the homozygote G/G and G allele carriers (A/G + G/G) versus A/A, the pooled ORs were 1.77 (95% CI = 1.28–2.81, P = 0.007 for heterogeneity) and 1.86 (95% CI = 1.45–2.40, P = 0.000 for heterogeneity). For CYP1A1 rs4646903 of the homozygote G/G and G allele carriers (A/G + G/G) versus A/A, the pooled ORs were 1.53 (95% CI = 1.31–2.21, P = 0.012 for heterogeneity) and 1.33(95% CI = 1.04–1.71, P = 0.029 for heterogeneity). In the stratified analysis by ethnicity, the significantly risks were found among Asians for both the G allele carriers and homozygote G/G. However, no significant associations were found in Caucasian population all genetic models. These results from the meta‐analysis suggest that CYP1A1 rs1048943 and rs4646903 polymorphisms contribute to risk of laryngeal cancer among Asian populations.
Keywords: CYP1A1, rs1048943, rs4646903, polymorphism, laryngeal cancer, susceptibility, meta‐analysis
Introduction
Laryngeal cancer is one of the most common malignant tumours among head and neck cancers 1. It is considered as a complex disease resulting from the interaction between genetic background and environmental factors 1, 2. A growing body of epidemiological evidence suggests that cigarette smoking and alcohol consumption are probable important aetiological factors increasing the risk of developing laryngeal carcinoma 3. Besides, it is believed that environmental chemical pollutions, widely spread carcinogens, are difficult to be degraded in the environment and thus may have a long‐term effect on human health. Accumulating evidence indicates that genetic polymorphisms have also been extensively investigated to identify inherited genetic risk for laryngeal Squamous cell carcinoma (SCC) 4.
Cytochromes P450 (CYP450s) are haeme‐containing enzymes important to phase I‐dependent metabolism of drugs and other xenobiotics 5. Studies indicate that CYP enzymes participate in cellular functions such as the metabolism of eicosanoids, the biosynthesis of cholesterol and bile acids, synthesis and metabolism of steroids and vitamin D3, synthesis and degradation of biogenic amines, and the hydroxylation of retinoic acid and presumably other morphogens. However, the functions of several CYP enzymes remain unknown 6, 7. Many important single nucleotide polymorphisms (SNPs) have been identified in CYP genes, and such polymorphisms within these genes may play an important role in determining individual susceptibility to many cancers. Two functional SNPs in CYP1A1 [CYP1A1 Ile462Val (CYP1A1*2B and *2C; rs1048943) and MspI (3798 T > C; CYP1A1*2A and *2B; rs4646903)] have been identified. CYP1A1 rs1048943 and rs4646903 polymorphisms have been extensively studied in many cancer sites among different populations, with the inconsistent and inconclusive results.
Several studies have investigated associations between CYP1A1 rs1048943 and rs4646903 polymorphisms and laryngeal cancer risk, but there is the perception that the findings have been inconsistent. A single study may be too underpowered to detect a possible small effect of the polymorphisms on laryngeal cancer, especially when the sample size is relatively small. Different types of study populations may also contribute the disparate findings. Hence, we performed a meta‐analysis of all eligible studies to derive a more precise estimation of the associations of CYP1A1 rs1048943 and rs4646903 polymorphisms with laryngeal cancer.
Materials and methods
Publication search
The electronic databases PubMed and CNKI were searched for studies to include in the present meta‐analysis, using the terms: ‘CYP1A1’ or ‘rs1048943’ or ‘rs4646903’, ‘polymorphism’ and ‘laryngeal cancer’. An upper date limit of 01 July 2015 was applied; no lower date limit was used. The search was performed without any restrictions on language and was focused on studies that had been conducted in humans. Concurrently, the reference lists of reviews and retrieved articles were searched manually. Only full‐text articles were included. Disagreement was resolved by discussion between the two authors. When the same patient population appeared in several publications, only the most recent or complete study was included in this meta‐analysis.
Inclusion criteria
The included studies have to meet the following criteria: (i) evaluating the CYP1A1 rs1048943 and/or rs4646903 polymorphism and laryngeal cancer risk; (ii) case–control studies; and (iii) supply the number of individual genotypes for CYP1A1 rs1048943 and/or rs4646903 genotype in laryngeal cancer cases and controls respectively.
Data extraction
Information was carefully extracted from all eligible publications independently by two authors according to the inclusion criteria listed above. Disagreement was resolved by discussion between the two authors.
The following data were collected from each study: first author's surname, year of publication, ethnicity, total numbers of cases and controls, and numbers of cases and controls with the AA, AG and GG genotypes respectively. Different ethnicity descents were categorized as Asian and Caucasian.
Statistical analysis
Odds ratio (OR) with 95% confidence intervals (CI) was used to assess the strength of association between the CYP1A1 rs1048943 and/or rs4646903 and laryngeal cancer risk. The pooled ORs for the risk associated with the genotypes of homozygote G/G and G allele carriers (A/G + G/G) with the A/A genotype were calculated. Heterogeneity assumption was checked by the chi‐square‐based Q‐test 8. A P‐value greater than 0.10 for the Q‐test indicates a lack of heterogeneity among studies, so the pooled OR estimate of the each study was calculated by the fixed‐effects model (the Mantel–Haenszel method) 9. Otherwise, the random‐effects model (the DerSimonian and Laird method) was used 10. One‐way sensitivity analyses were performed to assess the stability of the results, namely, a single study in the meta‐analysis was deleted each time to reflect the influence of the individual data set to the pooled OR 11. An estimate of potential publication bias was carried out by the funnel plot, in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot suggests a possible publication bias. Funnel plot asymmetry was assessed by the method of Egger's linear regression test, a linear regression approach to measure the funnel plot asymmetry on the natural logarithm scale of the OR. The significance of the intercept was determined by the t‐test suggested by Egger (P < 0.05 was considered representative of statistically significant publication bias) 12. All of the calculations were performed with STATA version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study characteristics
The flow diagram was illustrated graphically in Figure 1. A total of 10 publications involving 748 laryngeal cancer cases and 1558 controls met the inclusion criteria and were ultimately analysed 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. Table 1 presents the main characteristics of these studies. Among the 10 publications, eight were published in English, the remaining two were publish in Chinese. The sample sizes ranged from 46 to 466. Almost all of the cases were histologically confirmed. Controls were mainly healthy populations. Six studies were hospital‐based case–control studies and four were population‐based case–control studies.
Figure 1.
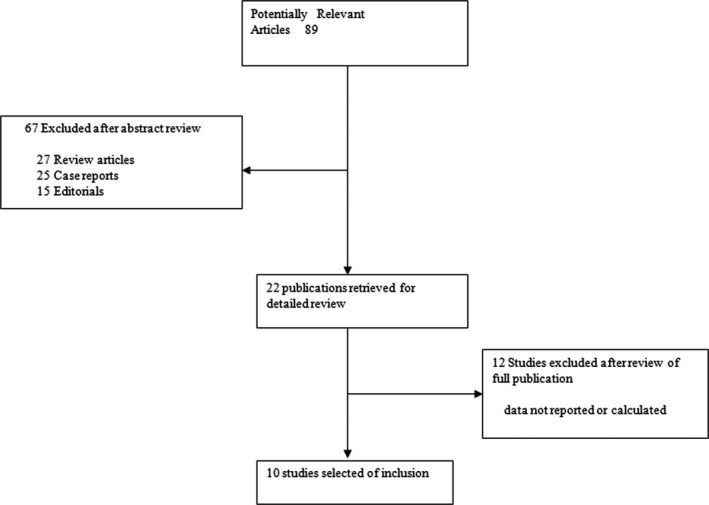
The flow diagram explaining the selection of included publications in the meta‐analysis.
Table 1.
Distribution of CYP1A1 rs1048943 among laryngeal cancer cases and controls included in this meta‐analysis
| First author‐year | Ethnicity (country of origin) | Total sample (case/control) | Source of control | Laryngeal cancer | Controls | ||||
|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | AA | AG | GG | ||||
| Lei‐2002 | Asian (China) | 62/56 | HB | 17 | 36 | 9 | 30 | 22 | 4 |
| Varzim‐2003 | Caucasian (Portugal) | 88/178 | PB | 66 | 19 | 3 | 135 | 43 | 0 |
| Gajecka‐2005 | Caucasian (Poland) | 206/260 | HB | 191 | 15a | 230 | 30a | ||
| Sam‐2008 | Asian (India) | 80/220 | HB | 24 | 44 | 12 | 180 | 36 | 4 |
| Sharma‐2010 | Asian (India) | 46/201 | HB | 35 | 10 | 1 | 156 | 34 | 11 |
| Lourenco‐2011 | Mixed (Brazil) | 37/142 | HB | 25 | 12a | 104 | 38a | ||
The number of the combined AG and GG genotypes.
PB: population based; HB: hospital based. AA indicates wild‐type, AG indicates heterozygote, GG indicates variant homozygote.
Meta‐analysis results
The association between CYP1A1 rs1048943 and laryngeal cancer risk was performed by meta‐analysis. Overall, for the homozygote G/G and G allele carriers (A/G + G/G), the pooled ORs for six studies were 1.77 (95% CI = 1.28–2.81, P = 0.007 for heterogeneity) and 1.86 (95% CI = 1.45–2.40, P = 0.000 for heterogeneity) (Fig. 2), when compared with the homozygous wild‐type genotype (A/A). In the stratified analysis by ethnicity, significant risks were found among Asians for both the G allele carriers (OR = 3.90, 95% CI = 2.70–5.64; P = 0.000 for heterogeneity) and homozygote G/G (OR = 3.29; 95% CI = 1.88–4.49; P = 0.006 for heterogeneity). Among Caucasian populations, no significant association was found in G/G versus A/A (OR = 0.93; 95% CI = 0.49–1.39), and G allele carriers versus A/A (OR = 0.81; 95% CI = 0.52–1.25) (Tables 2 and 3).
Figure 2.
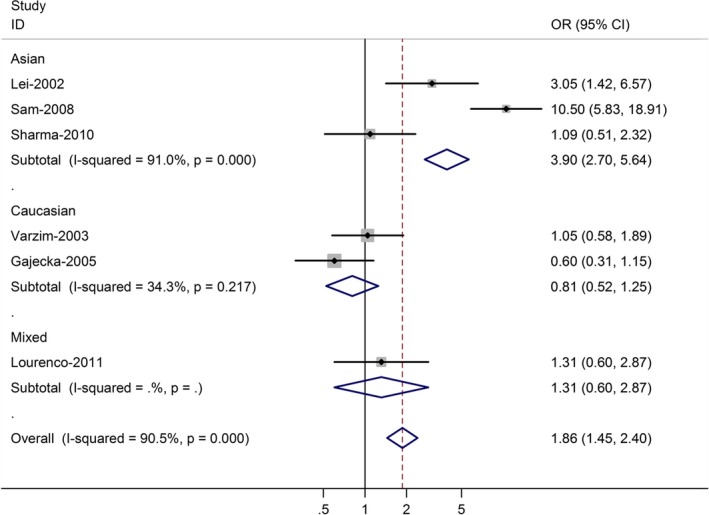
Forest plot (random‐effects model) of laryngeal cancer risk associated with CYP1A1 rs1048943 polymorphism for (AG + GG) versus AA. Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (OR = 1.0).
Table 2.
Distribution of CYP1A1 rs4646903 among laryngeal cancer cases and controls included in this meta‐analysis
| First author‐year | Ethnicity (country of origin) | Total sample (case/control) | Source of control | Laryngeal cancer | Controls | ||||
|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | AA | AG | GG | ||||
| Park‐1997 | Mixed (USA) | 23/23 | HB | 18 | 5a | 21 | 2a | ||
| Morita‐1999 | Asian (China) | 69/164 | PB | 48 | 16 | 5 | 104 | 54 | 6 |
| Varzim‐2003 | Caucasian (Portugal) | 88/178 | PB | 68 | 19 | 1 | 135 | 43 | 0 |
| Li‐2004 | Asian (China) | 89/164 | HB | 33 | 24 | 32 | 77 | 60 | 27 |
| Sam‐2008 | Asian (India) | 80/220 | HB | 64 | 15 | 1 | 180 | 36 | 4 |
| Sharma‐2010 | Asian (India) | 46/201 | HB | 23 | 13 | 10 | 156 | 34 | 11 |
| Szanyi‐2012 | Caucasian (Hungary) | 48/150 | HB | 34 | 14 | 0 | 119 | 31 | 0 |
The number of the combined AG and GG genotypes.
PB: population based; HB: hospital based. AA indicates wild‐type, AG indicates heterozygote, GG indicates variant homozygote.
Table 3.
Summary ORs for various contrasts of CYP1A1 rs1048943 and rs4646903 polymorphisms in this meta‐analysis
| Number of studies | (AG + GG) versus AA | GG versus AA | |
|---|---|---|---|
| OR (95% CI) P (Q‐test) | OR (95% CI) P (Q‐test) | ||
| rs1048943 | |||
| Total | 6 | 1.86 (1.45–2.40) 0.000 | 1.77 (1.28–2.81) 0.007 |
| Caucasian | 2 | 0.81 (0.52–1.25) 0.217 | 0.93 (0.49–1.39) 0.293 |
| Asian | 3 | 3.90 (2.70–5.64) 0.000 | 3.29 (1.88–4.49) 0.006 |
| rs4646903 | |||
| Total | 7 | 1.33 (1.04–1.71) 0.029 | 1.53 (1.31–2.21) 0.012 |
| Caucasian | 2 | 1.14 (0.71–1.81) 0.269 | 1.36 (0.79–2.31) 0.193 |
| Asian | 4 | 1.39 (1.03–1.87) 0.009 | 1.52 (1.25–2.32) 0.002 |
P (Q‐test): P‐value of Q‐test for heterogeneity test; OR: odds ratio; CI: confidence interval.
The association between CYP1A1 rs4646903 and laryngeal cancer risk was performed by meta‐analysis. Overall, for the homozygote G/G and G allele carriers (A/G + G/G), the pooled ORs for seven studies were 1.53 (95% CI = 1.31–2.21, P = 0.012 for heterogeneity) and 1.33 (95% CI = 1.04–1.71, P = 0.029 for heterogeneity) (Fig. 3), when compared with the homozygous wild‐type genotype (A/A). In the stratified analysis by ethnicity, significant risks were found among Asians for both the G allele carriers (OR = 1.39, 95% CI = 1.03–1.87; P = 0.009 for heterogeneity) and homozygote G/G (OR = 1.52; 95% CI = 1.25–2.32; P = 0.002 for heterogeneity). Among Caucasian populations, no significant association was found in G/G versus A/A (OR = 1.14; 95% CI = 0.71–1.81), and G allele carriers versus A/A (OR = 1.36; 95% CI = 0.79–2.31).
Figure 3.
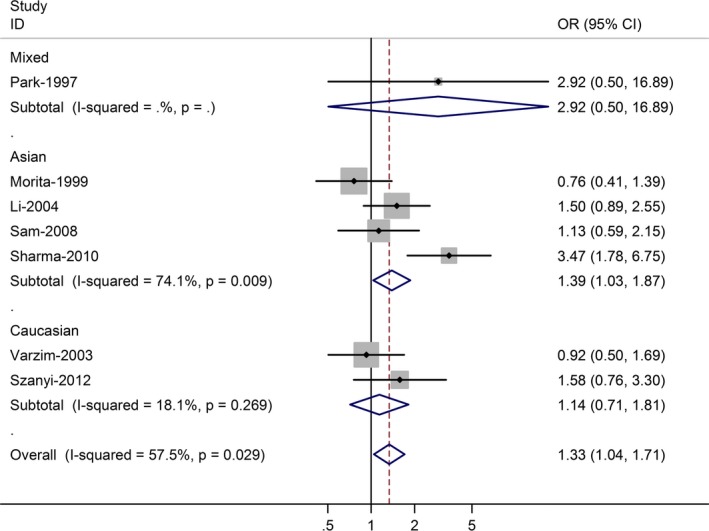
Forest plot (random‐effects model) of laryngeal cancer risk associated with CYP1A1 rs4646903 polymorphism for (AG + GG) versus AA.
Sensitivity analyses
A single study involved in the meta‐analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs, and the corresponding pooled ORs were not materially altered (data not shown).
Publication bias
Begg's funnel plot and Egger's test were performed to access the publication bias of literatures. Evaluation of publication bias for G allele carriers versus AA between CYP1A1 rs1048943 and laryngeal cancer risk showed that the Egger test was not significant (P = 0.615). The funnel plots for publication bias (Fig. 4) also did not show some asymmetry. The similar results were found between CYP1A1 rs4646903 and laryngeal cancer risk for G allele carriers versus AA (Fig. 5). These results did not indicate a potential for publication bias.
Figure 4.
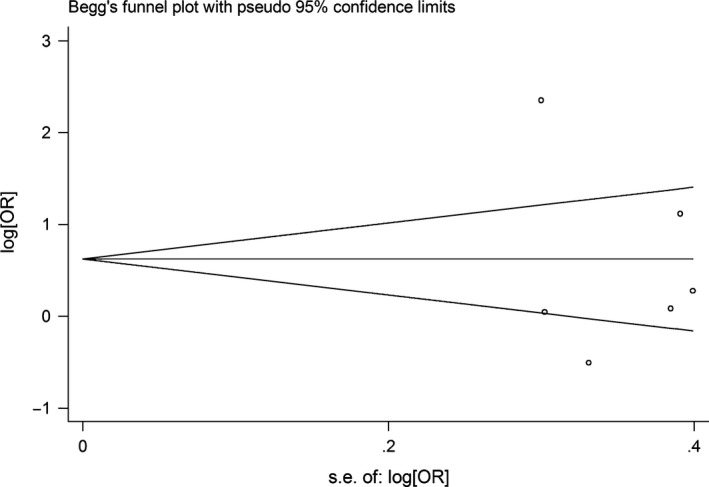
Begg's funnel plot of CYP1A1 rs1048943 polymorphism and laryngeal cancer risk for (AG + GG) versus AA.
Figure 5.
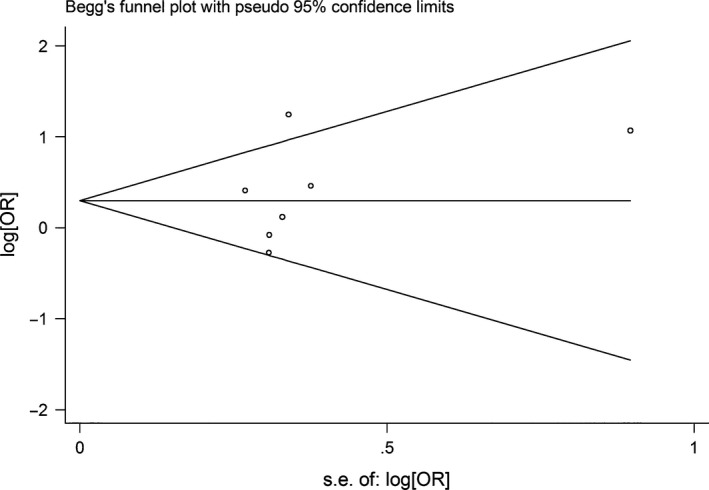
Begg's funnel plot of CYP1A1 rs4646903 polymorphism and laryngeal cancer risk for (AG + GG) versus AA.
Discussion
CYP genes which are comprised of large families of endoplasmic and cytosolic enzymes play a role in drug, steroid hormone and pro‐carcinogen metabolism. In humans, the CYPP450s complex (metalloproteins) contains more than 15 different enzymes 23. Some CYP haeme‐thiolate enzymes participate in the detoxification and formation of reactive intermediates of thousands of chemicals that can damage DNA, lipids and proteins. CYP1A1 is a critical CYPP450 and studies suggest that a CYP1A1 polymorphism may be a risk factor for several malignancies even in the face of its role in detoxification of environmental carcinogens and metabolic activation of dietary compounds that protect against cancer. Two functional non‐synonymous polymorphisms have been recently studied. Specifically, a T‐to‐C mutation in the non‐coding 3′‐flanking region has been reported to cause the creation a new MspI restriction site (MspI polymorphism m1, T6235C, rs4646903). Another CYP1A1 polymorphism is a G‐to‐A transition (A4889G) in exon 7, resulting in the replacement of isoleucine (Ile) by valine (Val), which is a haeme‐binding site (Ile462Val polymorphism m2, A4889G, rs1048943) 24.
It has been accepted that polymorphism of genes involved in the metabolism of tobacco carcinogen is candidate loci for laryngeal cancer susceptibility. Accumulating studies regarding gene variants in the carcinogen metabolism pathway have been performed. It is well recognized that there is a range of individual susceptibility to the same kind of cancer even with identical environmental exposure. Host factors, including polymorphisms of genes involved in carcinogenesis, may have accounted for this difference. Therefore, genetic susceptibility to cancer has been a research focus in scientific community.
Previous meta‐analyses suggest that genetic variation of CYP1A1 rs1048943 and rs4646903 might be associated with increased risks of various cancers, such as lung cancer 25 and oesophageal cancer 26. However, no significant association of CYP1A1 rs1048943 and rs4646903 polymorphisms with increased susceptibility to breast cancer has been reported by a recent meta‐analysis 27. The present meta‐analysis investigating the relationship between CYP1A1 rs1048943 and rs4646903 polymorphisms and laryngeal cancer risk provided the most comprehensive evidence. Significant association of CYP1A1 rs1048943 and rs4646903 polymorphisms and laryngeal cancer risk was demonstrated. Meta‐analyses of total studies showed that CYP1A1 rs1048943 and rs4646903 polymorphisms were associated with laryngeal cancer risk for the homozygote G/G and G allele carriers (A/G + G/G) when compared with the homozygous wild‐type genotype (A/A). In the stratified analysis by ethnicity, the significantly risks were found among Asians for both the G allele carriers and homozygote G/G. However, no significant associations were found in Caucasian population all genetic models. Sensitivity analyses by sequential omission of any individual studies and subgroup analyses by case sample size and ethnicity suggested the findings were highly unlikely because of chance. In the stratified analysis by ethnicity, the significantly risks were found among Asians, but no among Caucasians. Publication bias analysis demonstrated that bias favouring publications of positive studies was inexistent. Meanwhile, because the same polymorphism seemed to play different roles in CYP1A1 rs1048943 and rs4646903 susceptibility among different ethnic populations and because the frequencies of SNPs were different among different ethnic groups, subgroup analyses based on ethnicity were conducted.
Our data were consistent with the results of a previous meta‐analysis 28 included only seven studies published in 2009. We have improved upon that previous meta‐analysis by including more recent related studies and by generally using a more comprehensive search strategy. Screening, study selection and quality assessment were performed independently and reproducibly by two reviewers. We also explored heterogeneity and potential publication bias in accordance with published guidelines.
In statistics, meta‐analysis comprises statistical methods for contrasting and combining results from different studies in the hope of identifying patterns among study results, sources of disagreement among those results or other interesting relationships that may come to light in the context of multiple studies. However, some limitations of this meta‐analysis should be acknowledged. Firstly, heterogeneity is a potential problem when interpreting all the results of meta‐analyses. Although we minimized the likelihood by performing a careful search for published studies, using the explicit criteria for study inclusion, performing data extraction and data analysis strictly, the significant between‐study heterogeneity still existed in almost each comparison. The presence of heterogeneity can result from differences in the selection of controls, age distribution, lifestyle factors and so on. Although most of the controls were selected from healthy populations, some studies had selected controls among friends or family of laryngeal cancer patients or patients with other diseases. Secondly, only published studies were included in this meta‐analysis. The presence of publication bias indicates that non‐significant or negative findings may be unpublished. Lastly, our results were based on unadjusted estimates, while a more precise analysis should be conducted if individual data were available, which would allow for the adjustment by other covariates including age, ethnicity, family history, environmental factors and lifestyle.
In conclusion, this meta‐analysis suggests that the CYP1A1 rs1048943 and rs4646903 polymorphisms are associated with laryngeal cancer risk among Asian populations. In addition, it is necessary to conduct large trials using standardized unbiased methods, homogeneous laryngeal cancer patients and well‐matched controls, with the assessors blinded to the data.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- 1. Tahtali A, Hey C, Geissler C, et al HPV status and overall survival of patients with oropharyngeal squamous cell carcinoma—a retrospective study of a German head and neck cancer center. Anticancer Res. 2013; 33: 3481–5. [PubMed] [Google Scholar]
- 2. Hakenewerth AM, Millikan RC, Rusyn I, et al Joint effects of alcohol consumption and polymorphisms in alcohol and oxidative stress metabolism genes on risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2011; 20: 2438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hashibe M, BoVetta P, Zaridze D, et al Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol. 2007; 165: 814–20. [DOI] [PubMed] [Google Scholar]
- 4. Boccia S, Cadoni G, Sayed‐Tabatabaei FA, et al CYP1A1, CYP2E1, GSTM1, GSTT1, EPHX1 exons 3 and 4, and NAT2 polymorphisms, smoking, consumption of alcohol and fruit and vegetables and risk of head and neck cancer. J Cancer Res Clin Oncol. 2008; 134: 93–100. [DOI] [PubMed] [Google Scholar]
- 5. Rodriguez‐Antona C, Ingelman‐Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006; 25: 1679–91. [DOI] [PubMed] [Google Scholar]
- 6. Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006; 6: 947–60. [DOI] [PubMed] [Google Scholar]
- 7. Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002; 360: 1155–62. [DOI] [PubMed] [Google Scholar]
- 8. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954; 10: 101–29. [Google Scholar]
- 9. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 22: 719–48. [PubMed] [Google Scholar]
- 10. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 11. Tobias A. Assessing the influence of a single study in the meta‐analysis estimate. Stata Tech Bull. 1999; 8: 15–7. [Google Scholar]
- 12. Egger M, Davey Smith G, Schneider M, et al Bias in metaanalysis detected by a simple, graphical test. BMJ. 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morita S, Yano M, Tsujinaka T, et al Genetic polymorphisms of drug‐metabolizing enzymes and susceptibility to head‐and‐neck squamous‐cell carcinoma. Int J Cancer. 1999; 80: 685–8. [DOI] [PubMed] [Google Scholar]
- 14. Lei D, Pan X, Guo C, et al Genetic polymorphism of cytochrome P4501A1 and susceptibility to laryngeal carcinoma. Chin J Otorhinolaryngol. 2002; 37: 373–6. [PubMed] [Google Scholar]
- 15. Varzim G, Monteiro E, Silva RA, et al CYP1A1 and XRCC1 gene polymorphisms in SCC of the larynx. Eur J Cancer Prev. 2003; 12: 495–9. [DOI] [PubMed] [Google Scholar]
- 16. Sam SS, Thomas V, Reddy SK, et al CYP1A1 polymorphisms and the risk of upper aerodigestive tract cancers in an Indian population. Head Neck. 2008; 30: 1566–74. [DOI] [PubMed] [Google Scholar]
- 17. Sharma R, Ahuja M, Panda NK, et al Combined effect of smoking and polymorphisms in tobacco carcinogen‐metabolizing enzymes CYP1A1 and GSTM1 on the head and neck cancer risk in North Indians. DNA Cell Biol. 2010; 29: 441–8. [DOI] [PubMed] [Google Scholar]
- 18. Szanyi I, Ráth G, Móricz P, et al Effects of cytochrome P450 1A1 and uridine‐diphosphate‐glucuronosyltransferase 1A1 allelic polymorphisms on the risk of development and the prognosis of head and neck cancers. Eur J Cancer Prev. 2012; 21: 560–8. [DOI] [PubMed] [Google Scholar]
- 19. Lourenco GJ, Silva EF, Rinck‐Junior JA, et al CYP1A1, GSTM1 and GSTT1 polymorphisms, tobacco and alcohol status and risk of head and neck squamous cell carcinoma. Tumour Biol. 2011; 32: 1209–15. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Lin P, Deng YF, et al Relationship between susceptibility and prognosis of laryngeal cancer and genetic polymorphisms in CYP1A1 and GSTM1. Chin J Otorhinolaryngol. 2004; 39: 2–7. [PubMed] [Google Scholar]
- 21. Park JY, Muscat JE, Ren Q, et al CYP1A1 and GSTM1 polymorphisms and oral cancer risk. Cancer Epidemiol Biomarkers Prev. 1997; 6: 791–7. [PubMed] [Google Scholar]
- 22. Gajecka M, Rydzanicz M, Jaskula‐Sztul R, et al CYP1A1, CYP2D6, CYP2E1, NAT2, GSTM1 and GSTT1 polymorphisms or their combinations are associated with the increased risk of the laryngeal squamous cell carcinoma. Mutat Res. 2005; 574: 112–23. [DOI] [PubMed] [Google Scholar]
- 23. Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P‐450 enzymes. Chem Res Toxicol. 1991; 4: 391–407. [DOI] [PubMed] [Google Scholar]
- 24. Sugawara T, Nomura E, Sagawa T, et al CYP1A1 polymorphism and risk of gynecological malignancy in Japan. Int J Gynecol Cancer. 2003; 13: 785–90. [DOI] [PubMed] [Google Scholar]
- 25. Shi X, Zhou S, Wang Z, et al CYP1A1 and GSTM1 polymorphisms and lung cancer risk in Chinese populations: a meta‐analysis. Lung Cancer. 2008; 59: 155–63. [DOI] [PubMed] [Google Scholar]
- 26. Yang CX, Matsuo K, Wang ZM, et al Phase I/II enzyme gene polymorphisms and esophageal cancer risk: a meta‐analysis of the literature. World J Gastroenterol. 2005; 11: 2531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masson LF, Sharp L, Cotton SC, et al Cytochrome P‐450 1A1 gene polymorphisms and risk of breast cancer: a HuGE review. Am J Epidemiol. 2005; 161: 901–15. [DOI] [PubMed] [Google Scholar]
- 28. Zhuo WL, Wang Y, Zhuo XL, et al Polymorphisms of CYP1A1 and GSTM1 and laryngeal cancer risk: evidence‐based meta‐analyses. J Cancer Res Clin Oncol. 2009; 135: 1081–90. [DOI] [PubMed] [Google Scholar]


