Abstract
Objective:
To investigate the correlations between morphological parameters and rupture status in cerebral aneurysm patients.
Methods:
We conducted a retrospective study of 34 patient records from March 2010 to December 2012. The morphological parameters of 34 ruptured and 42 unruptured cerebral aneurysms in 34 patients (males: female, 15:19; mean age 55.79±10.64 years) leading to subarachnoid hemorrhage were examined using 3D (dimension) digital subtraction angiography (DSA) models, to identify the correlation between 2D morphological parameters and risk factors of rupture status with univariate and multivariate analysis.
Results:
The 2D morphological parameters in ruptured aneurysms were significantly different from those observed in unruptured aneurysms (p<0.05), though only size and height-width ratios independently predicted rupture status. Dmax, Hmax, bottleneck factor, and size ratio significantly correlated with height-width ratio in ruptured but not unruptured aneurysms.
Conclusions:
A specific set of morphological characteristics, most notably size and height-width ratios, may help to understand rupture risk by indicating arterial stretch character in cerebral aneurysms patients.
Cerebral and intracranial aneurysms occur when weak blood vessel walls give way in the brain, producing a bulging or ballooning effect that may or may not be symptomatic or lead to rupture.1,2 The pathophysiological mechanisms associated with rupture occurrence remain poorly understood, and rupture risk is most commonly assessed using aneurysm size, location, and shape.1 With CT technique application on imaging of intracranial aneurysms, more detailed measurements on aneurysm morphologies have become available to better assess rupture risk in diverse cerebral aneurysms. In one of the largest international studies of cerebral aneurysms to date, the International Study of Unruptured Intracranial Aneurysms (ISUIA) reported in 1998-1999 that 0.1-0.2% of intracranial aneurysm patients experienced rupture, with the risk of morbidity and mortality related to surgery greatly exceeding the 7.5-year risk of rupture in patients with relatively small diameters (<10 mm).3,4 Thus, preventative surgical treatment may not be appropriate for many patients, despite a mortality rate of 40-50%, and a morbidity rate of 10-20%5-7 in patients that develop rupture leading to aneurysmal subarachnoid hemorrhage. Over the last decade, numerous strategies have been proposed for assessing rupture risk;7-9 however, these broad guidelines may not consistently predict outcomes and rupture risk in many patient subpopulations. Thus, detailed stratification of intracranial aneurysm patients with aneurysm morphology and patient status such as gender, age, and the clinical condition (namely, hypertension, body mass index, smoking habits) help to understand the prevention of potentially life-threatening aneurysmal subarachnoid hemorrhage occurrence.
The geometrical morphological characteristics of aneurysm rupture are mostly location, shape, and size.1 More recently, aspect ratios greater than 1.6 have been shown to be significantly associated with rupture, with 80% of patients that experienced rupture exhibiting greater aspect ratios.10 Similarly, increased bottleneck factor and height-width ratio were consistently associated with rupture.11 The increasingly wide availability of 3 dimensional (3D) angiography imaging has also led to the employment of advanced computations and hemodynamics models for rupture assessment, that carefully consider individual physiological and morphological parameters related to aneurysm rupture.12,13 In these models, trends toward simple stable patterns, large impingement regions, and jet sizes have been associated with unruptured aneurysms, while disturbed flow patterns, small impingement regions, and narrow jets were indicative of rupture.
Due to the diversity in morphological and clinical characteristics and methodologies in these reports; however, accurate meta-analysis is all but impossible.14 Though comprehensive reviews have been conducted on strategies for assessing rupture in aneurysm patients,14 few clinical studies have examined aneurysm morphology and clinical status in a single patient cohort. Thus, most reports on aneurysm rupture risk fail to account for confounding variables between diverse patient cohorts, potentially overlooking the impact of key clinical parameters. Therefore, improved cerebral aneurysm patient stratification requires comprehensive assessment of key clinical and morphological characteristics in a single group of patients, which may yield valuable information on the interrelationships between these parameters of rupture risk. The current study investigated the relationship between morphological and clinical parameters and rupture status in cerebral aneurysm patients using 3D models produced by digital subtraction angiography (DSA). Notably, clinical performance was minimized in order to most effectively assess morphological parameters.
Methods
Patients
This study was approved by the Ethics Review Committee of the Shaoxing City People’s Hospital, Shaoxing, China. Informed consent was obtained from all participants through follow-up phone calls and special clinic reexamination, and all subjects were willing to participate in this study. A retrospective study of 34 multiple cerebral aneurysm patients that experienced aneurysm with rupture leading to acute subarachnoid hemorrhage was conducted using the clinical database of the Shaoxing City People’s Hospital for patients treated between March 2010 and December 2012.
Patients were included that (1) were diagnosed with intracranial cerebral aneurysm based on 3-dimensional DSA data confirming multiple aneurysms; (2) exhibited saccular aneurysms with a discrete dome and neck; (3) exhibited at least one ruptured and one unruptured aneurysm within 3 days of admission; (4) exhibited acute subarachnoid hemorrhage on CT; and (5) had complete clinical records, including age, gender, height, weight, and other clinical parameters assessed by the current study. Patients were excluded that exhibited (1) aneurysms that had mycotic, peripheral, fusiform, or extradural lesion characteristics, (2) aneurysms associated with an arteriovenous malformation based on variant natural history or pathological type; and (3) complicating conditions, such as cancer, infection, or other significant comorbidities.
Assessment of aneurysm rupture
The 3D DSA was conducted with a flat panel Allura Xper FD10 DSA unit (Philips Medical Systems, Eindhoven, Netherlands). Rupture was determined based on CT data, exhibiting one or more thick areas of hemorrhage on CT scan. Subarachnoid hemorrhage was determined when these areas were centralized in the basal and ambiens cistern, indicating rupture of the vertebrobasilar artery. Additionally, daughter blebs and aspheric surfaces on CT scans and DSA were used to indicate ruptured aneurysms, as previously described.15
Two-dimensional morphological assessments of aneurysms
The geometrical indices for cerebral aneurysms applied in the current study were adapted from those previously described. Two-dimensional (2D) measurements included: maximal diameter (Dmax), minimum neck diameter (Dneck), aneurysm height (Hmax), maximum width (Wortho, maximal longitudinal diameter parallel with the neck plane), bulge height (Bh, height from the neck plane to the Wortho plane), average parent artery diameter (Dparent), inflow angle (parent artery versus aneurysm apex), and dome width (Wmax, dome width perpendicular to height) (Figures 1 & 2). All data can be measured in 3D DSA. Measurements may not be in the same plane, but we selected the 2 aneurysms in which all 2D measurements were in the same plane to explain the measuring method more intuitively. Based on these measurements, 2D indices were calculated, as follows: aspect ratio (Hmax/Dneck), bottleneck factor (Wortho/Dneck), bulge location (bulge height/Hmax), size ratio (Hmax/Dparent), and height-width ratio (Hmax/Wmax). As minimum neck diameter is preferable to maximum neck diameter or average neck diameter in 2D indices,16 the final measurements selected for use in statistical analyses were Dmax, Hmax, aspect ratio, bottleneck factor, bulge location, size ratio, inflow angle, and height-width ratio.
Figure 1.
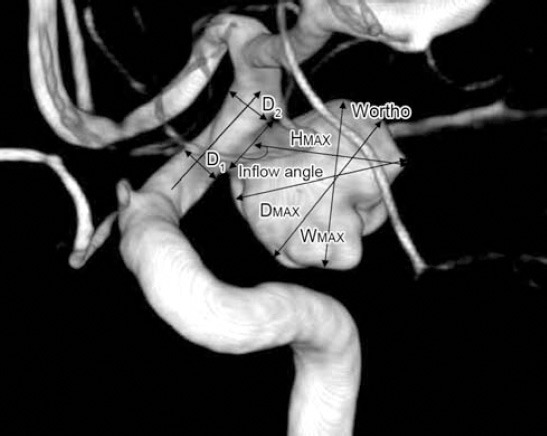
Sidewall aneurysm measurements. Patient (male, 46 years) was hospitalized because of acute headache for one day. Head CT showed subarachnoid hemorrhage. Two days later 2 aneurysms in each carotid were found on digital subtraction angiography. The left carotid aneurysm is shown: maximal diameter (Dmax), longest dimension from the center of the neck to the dome tip (Hmax or height), maximal longitudinal diameter parallel with the neck plane (Wortho), dome width perpendicular to height (Wmax), the angle between axis of flow in the parent vessel at the level of the aneurysm neck and the aneurysm’s main axis from the center of the neck to the tip of the dome (inflow angle). The average value of both sides of the aneurysm neck=D1+D2/2 (average parent artery diameter).
Figure 2.
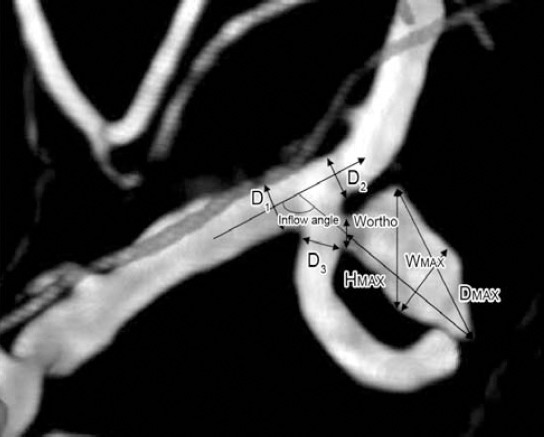
Bifurcation aneurysm assessments. Patient (male, 66 years) was hospitalized because of acute headache for one day. Head CT showed subarachnoid hemorrhage. Two days later 2 aneurysms in the left middle cerebral artery and right posterior communicating artery respectively were found on digital subtraction angiography. The left middle cerebral aneurysm is shown: Maximal diameter (Dmax), longest dimension from the center of the neck to the dome tip (Hmax or Height), maximal longitudinal diameter parallel with the neck plane (Wortho), dome width perpendicular to height (Wmax), the angle between axis of flow in the parent vessel and the aneurysm’s main axis from the center of the neck to the tip of the dome (inflow angle). The average value of artery diameter=D1+D2+D3/3.
Classification of variables
The Dmax and Hmax were classified into 3 groups, as <5, ≥5, and >10 mm. To evaluate the risk factors of size ratio, aspect ratio, bottleneck factor, and height-width ratio with aneurysm rupture, size ratio, aspect ratio, and bottleneck factor were divided into 3 groups: <1 (as control), ≤ 1.6 ≥1, and >1.6;10,12 height-width ratio was divided into 2 groups: ≤1.0 (as control) and > 1.0. Inflow angles were classified into 3 groups: ≤ 90°, ≤120°, and >120°.
Statistical analysis
Data were analyzed with the Statistical Package for Social Sciences version 18 for Windows (SPSS Inc., Chicago, IL, USA). All data were tested with Kolmogorov-Smirnov test; most did not fit for normal distribution. Univariate analysis was conducted for individual risk factors, and Mann-Whitney test was used to assess differences between ruptured and unruptured groups as nonparametric statistics. Binary logistic regression analyses of morphological risk factors with systematic removal of least significant variables were performed. The groups of Dmax, Hmax, aspect ratio, bottleneck factor, size ratio, inflow angle, and height-width ratio were also subjected to forward stepwise regression with the independent variable of rupture status based on variable entry and removal by Wald score (0.05 = keep; 0.1 = removal). Spearman correlation coefficients of morphological risk factors were performed. P-values of less than 0.05 were considered statistically significant.
Results
Base-line data of patients with aneurysms
Thirty-four patients (mean age 55.79±10.64 years; male:female 15:19) met the inclusion criteria, including 76 aneurysms (34 ruptured and 42 unruptured). Each patient had one ruptured aneurysm. Of these 34 patients, 27 (79.4%) had one, 6 (17.7%) had 2, and one patient (2.9%) had 3 unruptured aneurysms. All patients were hospitalized for more than 3 days and exhibited acute subarachnoid hemorrhage on CT scans. Hypertension was reported in 13 (38.2%) patients (Table 1).
Table 1.
Basic data of 34 multiple cerebral aneurysm patients with 76 aneurysms.*
Two-dimensional morphological assessments of Dmax, Hmax, and inflow angle significantly indicates rupture
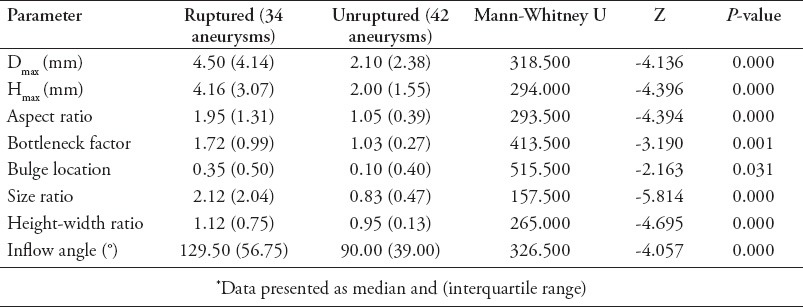
All of the 2D parameters were statistically significantly different between ruptured and unruptured aneurysms. Median and interquartile range (Q) of inflow angles was 129.5° and 56.75° in ruptured aneurysms; while they were 90° and 39° in unruptured aneurysms (p<0.0001). Median and interquartile range (Q) of Dmax in ruptured aneurysms were 4.50 and 4.14 mm, by contrast, these of Dmax in unruptured aneurysms were 2.10 and 2.38 mm, (p<0.0001). The median and interquartile range (Q) of Hmax in ruptured aneurysms were 4.16 and 3.07 mm, while these of Hmax in unruptured aneurysms were 2.00 and 1.55 mm (p<0.0001) (Table 2). The 2D assessments between patients with different types of aneurysms were highly variable (data not shown).
Table 2.
Comparisons of morphological aneurysm parameters between ruptured and unruptured aneurysms.*
Calculated two-dimensional morphological parameters indicate rupture
The 2D morphological parameters in ruptured aneurysms were significantly different from those observed in unruptured aneurysms (all p<0.05) (Table 2). The median and interquartile range (Q) of size ratio, height-width ratio, and aspect ratio were all significantly different in ruptured and unruptured aneurysms, (all p<0.0001). The median and interquartile range (Q) of bottleneck factor was found to be significantly different between ruptured and unruptured aneurysms (p=0.001). The median and interquartile range (Q) of bulge location was also significantly different between ruptured and unruptured aneurysms (p=0.031) (Table 2).
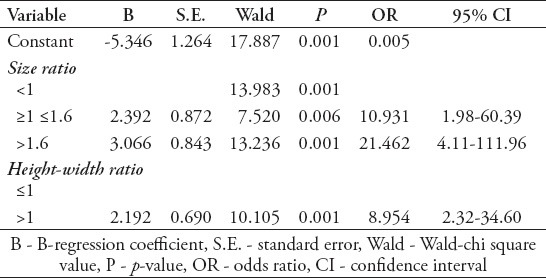
Size ratio and height-width ratio predict rupture in binary logistic regression
Forward stepwise binary logistic regression indicated that size ratio and height-width ratio were the only 2D morphological assessments significantly predictive of rupture (both p=0.001), unlike Dmax (p=0.08), Hmax (p=0.135), aspect ratio (p=0.576), bulge location (p=0.688), bottleneck factor (p=0.283), and inflow angle (p=0.349) (Table 3). The model fit analysis showed results with Cox & Snell R2=0.465, Nagelkerke R =0.622, likelihood X2=47.468, p<0.001. Hosmer and Lemeshow test also showed a good fitting of the predictive model (X2=2.086, p=0.720).
Table 3.
Evaluation of the risk factors of size ratio and height-width ratio with aneurysm rupture by binary logistic regression analyses.
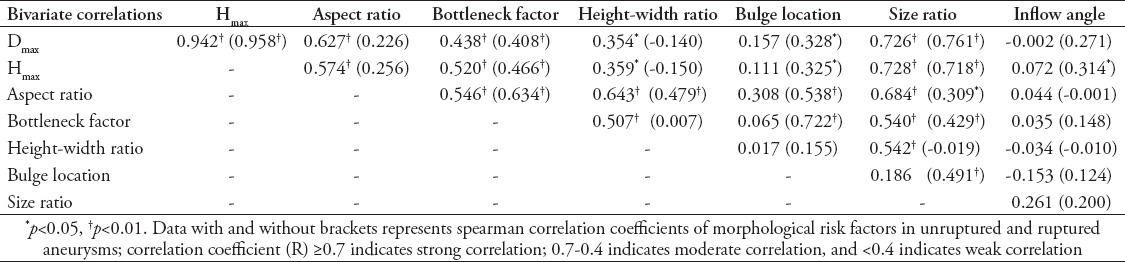
Correlations between two-dimensional morphological parameters for rupture
Inflow angle did not significantly correlate to any other parameter except for Hmax. The Dmax and Hmax were closely related in both ruptured and unruptured aneurysms. They correlated to bottleneck factor (p<0.01) and size ratio (p<0.05) in ruptured aneurysms and unruptured aneurysms, and correlated to aspect ratio (p<0.01) and height-width ratio (p<0.05) in ruptured aneurysms, but did not correlated (p>0.05) in unruptured aneurysms. On the contrary Dmax and Hmax correlated to bulge location (p<0.05) in unruptured aneurysms, but did not correlated to bulge location (p>0.05) in ruptured aneurysms. Size ratio correlated to all other measures (p<0.05) with the notable exception of inflow angle and bulge location (p>0.05) in ruptured aneurysms, or height-width ratio (p>0.05) in unruptured aneurysms. Height-width ratio correlated to Dmax (p<0.05), Hmax (p<0.05), aspect ratio (p<0.01), bottleneck factor (p<0.01), size ratio (p<0.01), except for bulge location (p>0.05), and inflow angles (p>0.05) in ruptured aneurysms. In unruptured aneurysms, comparatively, height-width ratio only correlated to aspect ratio (p<0.01). The bottleneck factor correlated to aspect ratio (p<0.01), Dmax (p<0.01), Hmax (p<0.01), and size ratio (p<0.01) highly in ruptured aneurysms and unruptured aneurysms, and correlated to height-width ratio (p<0.01) in ruptured aneurysms, by contrast correlated to bulge location (p<0.01) in unruptured aneurysms. Aspect ratio correlated with bottleneck factor (p<0.01), height-width ratio (p<0.01), size ratio (p<0.01 and p<0.05) in both ruptured and unruptured aneurysms, Dmax (p<0.01) and Hmax (p<0.01) in ruptured aneurysms and bulge location (p<0.01) in unruptured aneurysms. Interestingly, bulge location correlated to Dmax (p<0.05), Hmax (p<0.05), aspect ratio (p<0.01), bottleneck factor (p<0.01), size ratio (p<0.01) except for height-width ratio (p>0.05) and inflow angle (p>0.05) in unruptured aneurysms, but had no correlation to any other variables in ruptured aneurysms (Table 4).
Table 4.
Spearman correlation coefficients of morphological risk factors by bivariate correlation analysis among multiple cerebral aneurysm patients.
Discussion
The present retrospective study demonstrated that rupture occurrence in cerebral aneurysm patients correlated with complex and interrelated morphological factors that could be best expressed by utilizing calculated 2D morphological parameters as well as maximum diameter (Dmax), maximum height (Hmax), and inflow angle. The current study indicates that specific morphological characteristics of ruptured aneurysms and unruptured aneurysms in a single patient have distinct characteristics. Though further research will be required to determine potential relationships between clinical variables, such as smoking, hypertension, arteriosclerosis, or even genetic predisposition, and aneurysm rupture, this study indicates that in a single patient, aneurysm rupture risk can be distinguished. These findings potentially indicate that morphological variation, with consideration for clinical variables, is a potentially effective approach for clinical stratification of these patients.
Conventionally, the primary indicator of rupture risk was a large maximum diameter (Dmax), defined by Wiebers et al17 as larger than 10 mm; however, a recent proliferation of inconsistent reports of methods to indicate aneurysm rupture have appeared in the literature.14 For aneurysms with Dmax larger than 10 mm, it has been reported that there is only a 0.05% per year risk of rupture.3,4 Conversely, Baharoglu et al18 reported that though aneurysms larger than 7.76 mm were likely to rupture, Dmax was of no significant use in predicting rupture due to the wide variation in aneurysm type. These findings are consistent with the current results, which indicate that aneurysms of ~6 mm and ~3.5 mm were sometimes subject to rupture, though Dmax was not the most significant predictor of these events. Because the size of aneurysms is closely associated with the geometry of the parent artery diameter,19 it is unsurprising that assessment of maximum aneurysm diameter yields inconsistent results. Sato et al19 demonstrated that the variant aneurysm shapes and configurations, including ratio of aneurysm height to neck diameter and torsion angle of the aneurysm to the upstream parent artery, played a role in determining the shear wall stress. In fact, artery failure was more closely related to these parameters, in terms of materials failure theory, than artery curvature, or aneurysm size or shape.19 Thus, aneurysms must be more carefully considered on an individual basis in order to determine artery wall failure leading to rupture, and more appropriate clinical guidelines should be developed.
Based on the need for better clinical guidelines for aneurysm rupture, researchers have recently proposed aspect ratio, bottleneck factor, size ratio, height-width ratio, and inflow angle as critical measurements of rupture risk.14 Rahman et al20 reported that size ratio was the most significant discriminant of rupture status, while Hoh et al11 reported that bottleneck factor and height-width ratio were most consistently associated with rupture.21 In yet another report, Baharoglu et al18 published that inflow angle was the most significant discriminant of rupture status in sidewall-type aneurysms, potentially due to high-energy transmission of the dome. The current study, however, suggests that size ratio and height-width ratio were the most significant discriminant for rupture, though aspect ratio, bottleneck factor, and inflow angle were also related to rupture risk. The discrepancies between these studies may indicate a need to more carefully consider the geometry of not just the aneurysm, but also the surrounding artery.19 Furthermore, there is an urgent need for better clinical typing of aneurysms.18
In the current study, bifurcation and sidewall aneurysms were mixed despite previous indications that these 2 aneurysm types have very different failure properties.18 Particularly for inflow angle, aspect ratio, and bottleneck factor,18 this may interfere with accurate assessment of the relationship between these parameters and rupture. For the same reason, these parameters may not be broadly applicable to all aneurysm types, necessitating better aneurysm typing in order for more appropriate assessments of rupture risk to be widely implemented. Fortunately, modern angiography has allowed for reliable and consistent centerline techniques for examining artery and aneurysm characteristics.21 Notably, aspect ratio can describe satisfactorily lengthways stretch aneurysm but cannot describe satisfactorily transverse stretch aneurysm; comparatively, bottleneck factor can satisfactorily describe transverse stretch aneurysm, but cannot satisfactorily describe lengthways stretch aneurysm.21 Using the directions suggested in centerline angiography techniques coupled with the current findings and this brief review of the literature, we propose that aneurysms can be classified broadly into 2 types based on stretch direction: lengthwise and transverse. By applying modern CT angiography, rather than DSA,22 it may be possible to distinguish characteristics of these aneurysms more effectively, thereby allowing improved rupture risk assessment and prevention in clinical patients.
More specifically, neck width, dome width, aneurysm shape, aspect ratio (height/neck width), and bottleneck factor (dome width/neck width) have been examined. Among these, aspect ratio has shown the greatest promise as a parameter to associate with rupture risk.14,23,24 Carter et al25 demonstrated that ruptured intracranial aneurysms originating from distal blood vessels were smaller than ruptured intracranial aneurysms originating from proximal, larger blood vessels. Thus, size ratio is useful because it is representative of aneurysm stretch character relative to parent artery. Similarly, Rahman et al20 reported that patients that experienced rupture exhibited significantly larger size ratios compared with patients that did not experience rupture, suggesting that size ratios may be a practical tool for gauging stretch and, thereby, assessing rupture in clinical aneurysm patients. Furthermore, height-width ratio increases with increasing Dmax and Hmax, increasing bottleneck factor and increasing the size ration in ruptured aneurysms but not in unruptured aneurysms, thereby indicating stretch. Thus, aneurysm stretch character more significantly correlates with expansion of the aneurysm in irregular aneurysms that will undergo rupture, unlike other parameters. It is, thus, important to consider than height-width ratio, size ratio, aspect ratio are all interrelated, describing aneurysm characteristics and stretch in different but important ways.
Anther variable, bulge location increases with increasing aspect ratio, increasing bottleneck factor, increasing size ration, and increasing Dmax and Hmax in unruptured aneurysms, but does not correlate to any variables in ruptured aneurysms, which indicates that with larger aneurysm volume the largest transverse may be closer to the tip the aneurysm in unruptured aneurysms. But, it does not happen in ruptured aneurysm, owing to the more irregular sphere.
The relatively small patient group and lack of differentiation between bifurcation and sidewall aneurysm hemodynamics may limit the broad applicability of these results. Further study is required to relate clinical variables to these diverse morphological variables before clinical recommendations can be made. Additionally, further prospective studies will be required to confirm these results, particularly to allow proper blinding and avoid study bias. Thus, these findings can only provide a basis for further study, instead of providing a quantitative clinical standard. More extensive and comprehensive multivariate analysis of both clinical and morphological parameters will be required to generate useful clinical guidelines for aneurysm patient stratification by rupture risk.
In conclusion, this retrospective case-control analysis of multiple aneurysm patients with and without rupture indicates that morphological parameters are useful in predicting rupture leading to acute subarachnoid hemorrhage. In particular, maximum diameter (Dmax), aneurysm height (Hmax), inflow angle, aspect ratio, bottleneck factor, bulge location, size ratio, and height-width ratio was associated with rupture by univariate analyses. Notably, only size ratio and height-width ratio was significantly associated with ruptured aneurysms in binary logistic analyses. Thus, the stretch character of aneurysms is more significantly correlated with expansion in ruptured aneurysms. These findings, however, require confirmation in larger future larger studies that also consider diverse clinical variables as well as morphological characteristics.
Acknowledgments
The authors would like to thank Haiyan Xing for her assistance in statistical analysis.
Footnotes
Disclosure.
Related articles.
Farag A, Al-Yamany M. Surgical clipping of ruptured anterior circulation cerebral aneurysms: KFMC experience. Neurosciences 2008; 13 (Suppl 1): 32.
Jabbour RMD, Khalifeh RMD, El-Kutoubi AMD, Atweh S. Dissecting aneurysm of the basilar trunk in a young man with CNS brucellosis. Neurosciences 2003; 8 (Suppl 1): 59.
Sami AS. Aneurysm coiling in sub-arachnoid hemorrhage. Two years Egyptian experience and follow-up. Neurosciences 2003; 8 (Suppl 1): 45.
References
- 1.UCAS Japan Investigators. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 2.A.D.A.M. Inc. Medical Encyclopedia. Aneurysm in the brain: Aneurysm - cerebral; Cerebral aneurysm. [Updated 2014 May 16] Bethesda (MD): U.S. National Library of Medicine; Available from URL: http://www.nlm.nih.gov/medlineplus/ency/article/001414.htm . [Google Scholar]
- 3.Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 4.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 6.Fogelholm R, Hernesniemi J, Vapalahti M. Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage. A population-based study. Stroke. 1993;24:1649–1654. doi: 10.1161/01.str.24.11.1649. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz M. Guidelines for the surgical treatment of unruptured intracranial aneurysms: the first annual J. Lawrence Pool Memorial Research Symposium--Controversies in the management of cerebral aneurysms. Neurosurgery. 2009;64:E577. doi: 10.1227/01.NEU.0000342785.23826.42. [DOI] [PubMed] [Google Scholar]
- 8.Komotar RJ, Mocco J, Solomon RA. Guidelines for the surgical treatment of unruptured intracranial aneurysms: the first annual J. Lawrence pool memorial research symposium--controversies in the management of cerebral aneurysms. Neurosurgery. 2008;62:183–194. doi: 10.1227/01.NEU.0000311076.64109.2E. [DOI] [PubMed] [Google Scholar]
- 9.Lee T, Baytion M, Sciacca R, Mohr JP, Pile-Spellman J. Aggregate analysis of the literature for unruptured intracranial aneurysm treatment. AJNR Am J Neuroradiol. 2005;26:1902–1908. [PMC free article] [PubMed] [Google Scholar]
- 10.Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. 2001;48:495–503. doi: 10.1097/00006123-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007;61:716–723. doi: 10.1227/01.NEU.0000298899.77097.BF. [DOI] [PubMed] [Google Scholar]
- 12.Baharoglu MI, Schirmer CM, Hoit DA, Gao BL, Malek AM. Aneurysm inflow-angle as a discriminant for rupture in sidewall cerebral aneurysms: morphometric and computational fluid dynamic analysis. Stroke. 2010;41:1423–1430. doi: 10.1161/STROKEAHA.109.570770. [DOI] [PubMed] [Google Scholar]
- 13.Cebral JR, Castro MA, Burgess JE, Pergolizzi RS, Sheridan MJ, Putman CM. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol. 2005;26:2550–2559. [PMC free article] [PubMed] [Google Scholar]
- 14.Lall RR, Eddleman CS, Bendok BR, Batjer HH. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26:E2. doi: 10.3171/2009.2.FOCUS0921. [DOI] [PubMed] [Google Scholar]
- 15.Cebral JR, Sheridan M, Putman CM. Hemodynamics and bleb formation in intracranial aneurysms. AJNR Am J Neuroradiol. 2010;31:304–310. doi: 10.3174/ajnr.A1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauric A, Baharoglu MI, Malek AM. Ruptured status discrimination performance of aspect ratio, height/width, and bottleneck factor is highly dependent on aneurysm sizing methodology. Neurosurgery. 2012;71:38–46. doi: 10.1227/NEU.0b013e3182503bf9. [DOI] [PubMed] [Google Scholar]
- 17.Wiebers DO, Whisnant JP, O’Fallon WM. The natural history of unruptured intracranial aneurysms. N Engl J Med. 1981;304:696–698. doi: 10.1056/NEJM198103193041203. [DOI] [PubMed] [Google Scholar]
- 18.Baharoglu MI, Lauric A, Gao BL, Malek AM. Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg. 2012;116:871–881. doi: 10.3171/2011.11.JNS11311. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Imai Y, Ishikawa T, Matsuki N, Yamaguchi T. The importance of parent artery geometry in intra-aneurysmal hemodynamics. Med Eng Phys. 2008;30:774–782. doi: 10.1016/j.medengphy.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A, et al. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke. 2010;41:916–920. doi: 10.1161/STROKEAHA.109.574244. [DOI] [PubMed] [Google Scholar]
- 21.Rengier F, Weber TF, Giesel FL, Bockler D, Kauczor HU, von Tengg-Kobligk H. Centerline analysis of aortic CT angiographic examinations: benefits and limitations. AJR Am J Roentgenol. 2009;192:W255–W63. doi: 10.2214/AJR.08.1488. [DOI] [PubMed] [Google Scholar]
- 22.Diehm N, Herrmann P, Dinkel HP. Multidetector CT angiography versus digital subtraction angiography for aortoiliac length measurements prior to endovascular AAA repair. J Endovasc Ther. 2004;11:527–534. doi: 10.1583/03-1172.1. [DOI] [PubMed] [Google Scholar]
- 23.Amenta PS, Yadla S, Campbell PG, Maltenfort MG, Dey S, Ghosh S, et al. Analysis of nonmodifiable risk factors for intracranial aneurysm rupture in a large, retrospective cohort. Neurosurgery. 2012;70:693–701. doi: 10.1227/NEU.0b013e3182354d68. [DOI] [PubMed] [Google Scholar]
- 24.Ryu CW, Kwon OK, Koh JS, Kim EJ. Analysis of aneurysm rupture in relation to the geometric indices: aspect ratio, volume, and volume-to-neck ratio. Neuroradiology. 2011;53:883–889. doi: 10.1007/s00234-010-0804-4. [DOI] [PubMed] [Google Scholar]
- 25.Carter BS, Sheth S, Chang E, Sethl M, Ogilvy CS. Epidemiology of the size distribution of intracranial bifurcation aneurysms: smaller size of distal aneurysms and increasing size of unruptured aneurysms with age. Neurosurgery. 2006;58:217–223. doi: 10.1227/01.NEU.0000194639.37803.F8. [DOI] [PubMed] [Google Scholar]


