Supplemental Digital Content is available in the text.
Abstract
Background:
Direct-to-implant breast reconstruction can be achieved more easily by means of soft-tissue replacement devices such as dermal matrices and synthetic meshes. The feasibility of a subcutaneous approach has been recently investigated by some studies with different devices functioning as implant support. Aim of this study is to analyze the long-term results, both objective and subjective, of a previous nonrandomized trial comparing prepectoral (subcutaneous) and retropectoral breast reconstructions.
Methods:
Patients enrolled in a nonrandomized prospective trial, comparing the standard retropectoral reconstruction and the prepectoral subcutaneous approach, using a titanium-coated mesh in both techniques, were followed up and evaluated for long-term results. Cases were compared in terms of the causes and rate of reinterventions, of the postoperative BREAST-Q questionnaire results, and of an objective surgical evaluation.
Results:
The subcutaneous group had a rate of implant failure and removal of 5.1% when compared with 0% in the retropectoral group. Aesthetic outcome was significantly better for the subcutaneous group both at a subjective and at an objective evaluation. Capsular contracture rate was 0% in the subcutaneous group.
Conclusions:
A higher rate of implant failure and removal, although not significant, always because of skin flaps and wound problems, should be taken into account for a careful patients selection. The subcutaneous breast reconstruction shows good long-term results. A coherent subjective and objective cosmetic advantage of this approach emerges. Moreover, no capsular contracture is evident, albeit in a relatively limited number of cases.
An implant-based breast reconstruction (IBBR) is by far the preferred way of restoring a female breast after mastectomy and a 2-stage breast reconstruction, by means of a tissue expander, accounts for approximately 70% of all reconstructions according to the American Society of Plastic Surgeons statistics.1 Nonetheless, the opportunity of a direct-to-implant breast reconstruction in breast surgical oncology is very fascinating and tempting for surgeons and for women as well. Whenever surgical conditions, tumor stage and adjuvant treatments allow this option, it is definitely worth considering the avoidance of a temporary tissue expander and its discomfort.2 A 1-stage procedure is quite demanding in technical terms. A full muscular pocket, to cover the prosthesis, allows the use of small to medium size implants and sometimes does not let the surgeon recreate a good lower pole shape and inframammary fold contour. The introduction of soft-tissue replacement devices in IBBR dramatically expands this field in breast surgery. Acellular dermal matrices (ADMs) are by far the most frequently used worldwide.3 A lot of data are present in literature for ADMs use in breast reconstruction.4–18 Synthetic meshes are used as well, as an alternative to ADMs. A titanium-coated polypropylene mesh (TCPM), TiLoop Bra (pfm medical, Cologne, Germany), is approved for use in breast surgery in Europe since 2008. There are studies showing its safety and effectiveness in IBBR,19,20 with promising results in terms of capsular contracture.21
Soft-tissue replacement devices are traditionally used as an inferolateral extension of pectoralis major muscle. ADMs or synthetic meshes function as a hammock to adjust an implant after its placement in a retropectoral position and after muscle detachment from its inferior aspect. Recently, a novel approach, with a prepectoral, subcutaneous, muscle-sparing implant positioning, has been described.22–24 In a previous study, we described a prospective nonrandomized clinical trial designed to compare a muscle-sparing method of using TCPM, as a complete coverage for a prepectoral implant, with the standard retropectoral muscular mesh implant coverage.22 Results are limited to a short-term follow-up, with surgical complications and implant loss showing no difference between the 2 groups.
Aim of this study is to analyze and evaluate long-term results on the same patients enrolled in the aforementioned trial, with 25 months of median follow-up. To ascertain reliability and quality of the subcutaneous reconstructions performed in the previous study, the analysis is focused on long-term surgical complications, requiring reintervention, along with objective parameters such as rippling, capsular contracture, and cosmetic outcome. Another primary endpoint of present evaluation is women’s quality of life (QOL), analyzing functional and aesthetic subjective parameters.
PATIENTS AND METHODS
In 2011, a prospective nonrandomized clinical study was started to compare the use of TCPM in direct-to-implant reconstructions either in the standard muscular mesh implant coverage (group 1, G-1, breast implants in partial retropectoral position, with synthetic TCPM placed as a hammock-like elongation of the muscle over the inferolateral pole) or in a different muscle-sparing technique (group 2, G-2, totally subcutaneous, prepectoral implant adjustment, wrapped in a TCPM bag). Enrollment ended in January 2014. Study design and surgical details were previously described.22 Briefly, inclusion criteria were age less than 80 years, normal body mass index (BMI; range, 18.5–24.9), small-to-medium size breasts, and ptosis grade of the first and second degree according to the 3-tier Regnault ptosis scale.25 Exclusion criteria were previous breast surgery, T4 and metastatic cancers, refusal to sign the consent, comorbidities (diabetes, renal failure, congestive heart failure, pulmonary diseases, hypertension, chronic hepatic diseases, and metabolic diseases), smoking, and previous radiotherapy to the chest wall. Cases baseline characteristics and oncological data of the 2 groups are shown in Table 1. In May 2015, with a minimum follow-up of 16 months from the last enrolled case, we proceeded to a surgical, functional, and aesthetic analysis of all cases. Patients were called and invited to participate in a long-term clinic evaluation. All patients signed a consent to accept the visit/questionnaire within a standard out-patient clinic scheduled activity. No ethical committee approval was required.
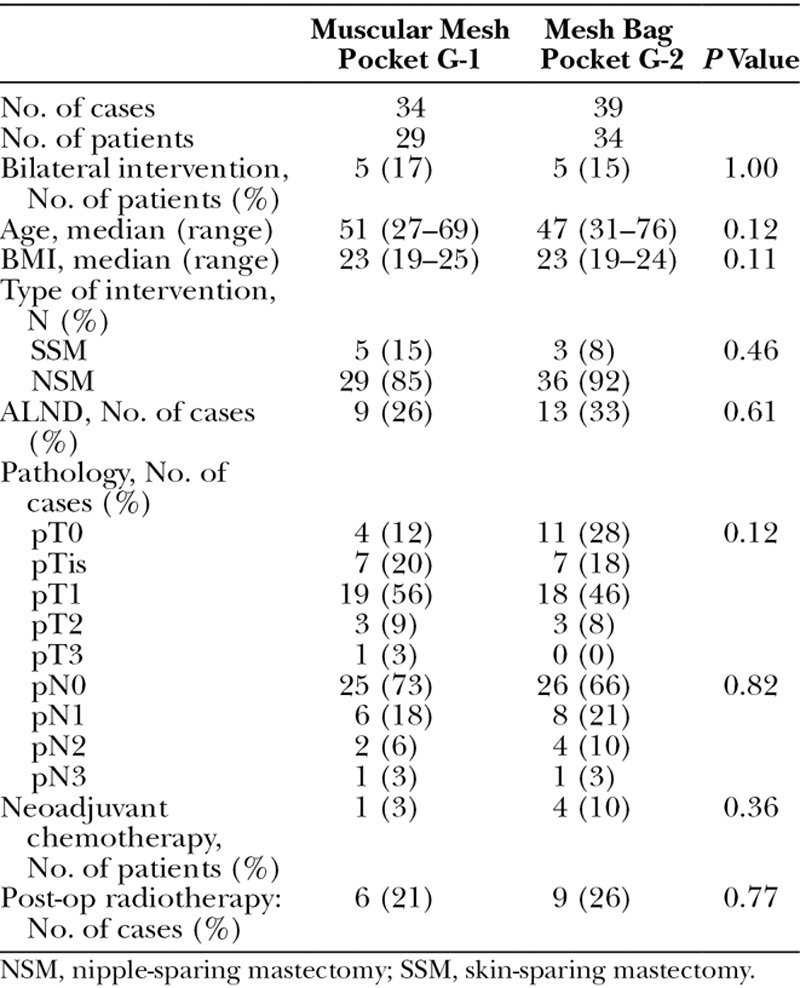
Table 1.
Demographics and Oncological Data of the 2 Groups
All further surgical procedures and postoperative radiation therapies, between the first reconstruction and last follow-up, were investigated and registered. Furthermore, both an objective and a subjective evaluation were conducted. All the evaluations started and were completed within May 2015. The subjective evaluation was conducted using the postoperative section of BREAST-Q (Memorial Sloan-Kettering Cancer Center and The University of British Columbia © 2006, all rights reserved). According to a recent study on long-term patient-reported QOL after breast implant reconstruction,26 the BREAST-Q reconstruction module was divided into multiple independent scales: satisfaction with breasts (16 items), satisfaction with outcome (7 items), psychosocial well being (10 items), physical well being (16 items), and sexual well being (6 items). For each scale, item responses were summed and transformed into a score, ranging from 0 to 100. Higher scores indicate greater satisfaction or QOL. On the other hand, the objective evaluation was performed by 2 surgeons simultaneously. The evaluating surgeons were staff members of the Breast Unit but not those who operated on the patients of the study. A 5-item form was fulfilled for every reconstruction case. The form was structured as follows: a, capsular contracture evaluation, using the 4-grade Baker classification; b, registration of signs of rippling, using a 5-grade Likert scale (score: 1, strongly disagree; 2, disagree; 3, undecided; 4, agree; 5, strongly agree) to judge the statement “this case has no signs at all of rippling”; c, registration of signs of implant’s profile visibility, using a 5-grade Likert scale to judge the statement “this case has no signs at all of a visible implant”; d, registration of signs of implant borders tangibility at smooth touch, using a 5-grade Likert scale to judge the statement “this case has no signs at all of palpable implant borders”; e, aesthetic result, judging the statement “this case has an excellent aesthetic outcome,” still using a 5-grade Likert scale. This evaluation was conducted by 2 different surgeons at the same time, giving a single agreed score for every item.
Subjective evaluation was conducted by every single patient, whereas the objective evaluation was performed on every single operated breast, therefore, for both reconstructions in bilateral cases.
Statistical Analysis
Comparison between muscular mesh pocket and mesh bag pocket patients was made by Wilcoxon’s rank sum test for continuous variables and χ2 test or Fisher’s exact test for categorical variables. BREAST-Q scores were compared as a continuous variable. For each scale, we reported median, range, mean, and standard deviation of the score.
All statistical analyses were performed by STATA 12.1 (StataCorp. 2011, Stata Statistical Software: Release 12, StataCorp LP, College Station, Tex.)
RESULTS
Twenty-nine patients had been initially enrolled in G-1 and 34 patients in G-2; there were 5 bilateral reconstructions in each group, meaning that an overall number of 34 cases in G-1 and 39 cases in G-2 had been performed. Cases from the 2 groups were not significantly different in terms of type of intervention (nipple-sparing mastectomy vs skin-sparing mastectomy), concurrent axillary lymph nodes dissection (ALND), preoperative chemotherapy, and postoperative radiation therapy (Table 1). One patient of G-2 died before this study and was not evaluated. Therefore, 29 patients from G-1 and 33 patients from G-2 were invited and visited as for the purposes of this analysis. One patient in G-2 had her implant removed in the early postoperative course because of a large skin-flap necrosis (as reported in the previous study on short-term complications). Two more patients in G-2 had their implant removed later on because of wound dehiscence during chemotherapy in one case and ipsilateral chest wall local recurrence in the other. Because all these 3 cases had a different type of reconstruction, with autologous flaps, they were not submitted to present study objective and subjective functional and aesthetic evaluation. Eventually, 29 (49%) patients, 34 (49%) cases, were evaluated from G-1 and 30 (51%) patients, 35 (51%) cases, from G-2, with 26 and 25 months of median follow-up, respectively. Early surgical complications from a previous study and long-term results with data analyses from this study are shown in Table 2.
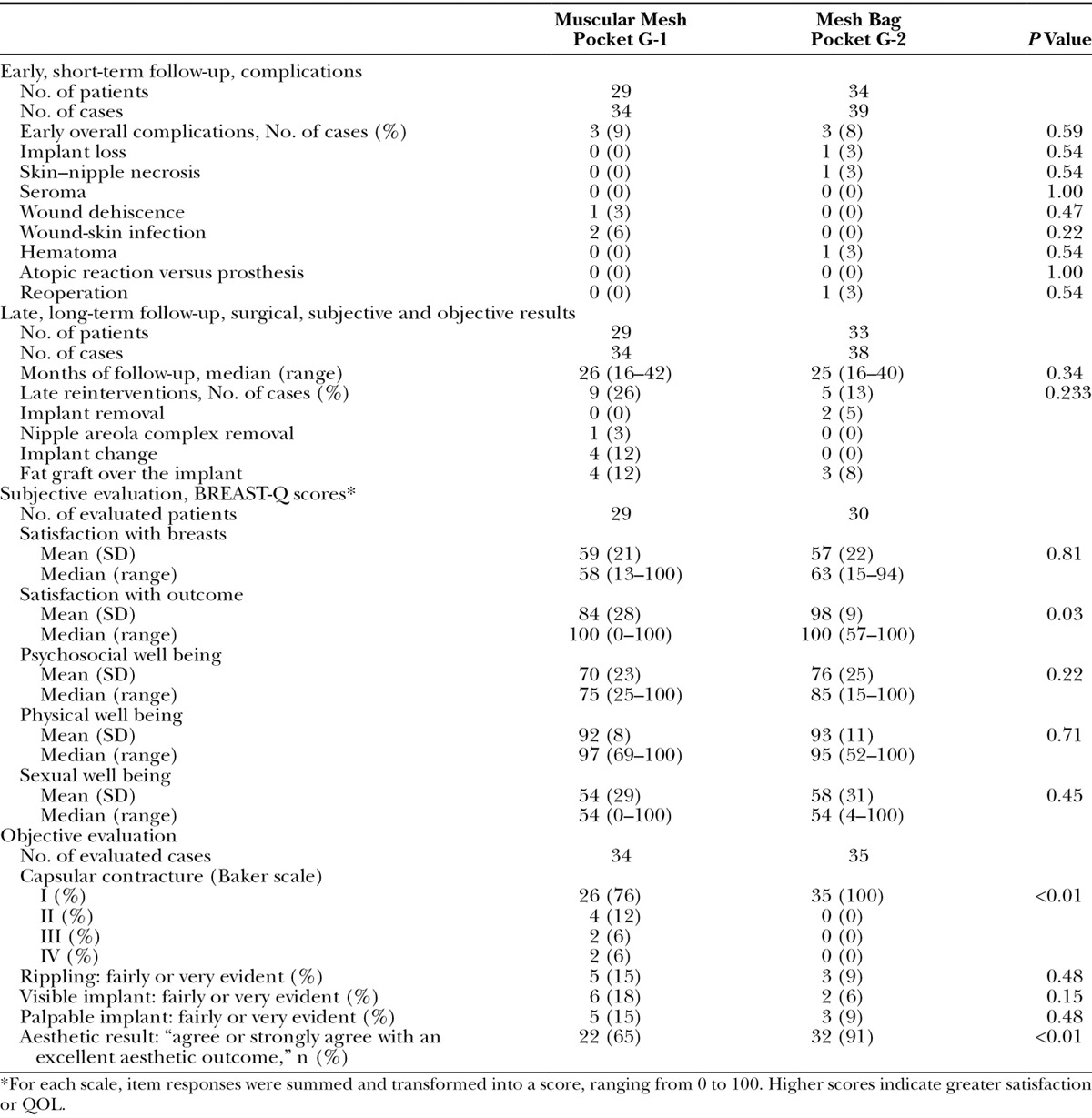
Table 2.
Short-Term Complications and Long-Term Results of Surgical, Subjective, and Objective Comparison of the 2 Groups
DISCUSSION
Breast reconstruction is nowadays a goal that can be achieved more easily and with a much greater cosmetic satisfaction for both women and surgeons. An implant reconstruction is the preferred way of achieving such a result, and a 2-stage procedure is the commonest solution. A single-stage procedure is an interesting option whenever anatomical and oncological characteristics allow it. In a recent study, the single-stage and 2-stage procedures are compared in terms of surgical complications and women’s satisfaction.27 Results show no differences in terms of surgical complications, but the single-stage approach is associated with higher sexual well-being satisfaction, even though more than 80% of patients required reinterventions with additional surgical revisions.
Even in terms of costs, a 2013 study shows a slight advantage for the 1-stage procedure, although only during the first 18 months and without statistical significance.28 A 1-stage technique with a direct-to-implant approach is, therefore, more often adapted particularly after the introduction of soft-tissue replacement devices such as ADMs and synthetic meshes. The standard use of these entails their placement as an elongation of the pectoralis major muscle, previously detached from its inferior aspect. The implant is, hence, covered by the muscle on the medial-upper pole and by such devices on the inferolateral pole to better define the lower profile and inframammary fold contour. Based on the rationale that prosthetic devices are actually subcutaneous under the mastectomy flaps in all the inferolateral reconstructed breast, a complete subcutaneous approach can possibly be used in selected cases. The direct-to-implant subcutaneous reconstruction with a muscle-sparing technique, wrapping the implant entirely within a TCPM bag (Fig. 1) and placing it underneath skin flaps (Figs. 2, 3), is described in a previous study, which constitutes the background of this long-term analysis.22 The subcutaneous procedure means shifting the position of the implant from a retropectoral site to a prepectoral one, as schematically shown in Figs. 4, 5.
Fig.1.
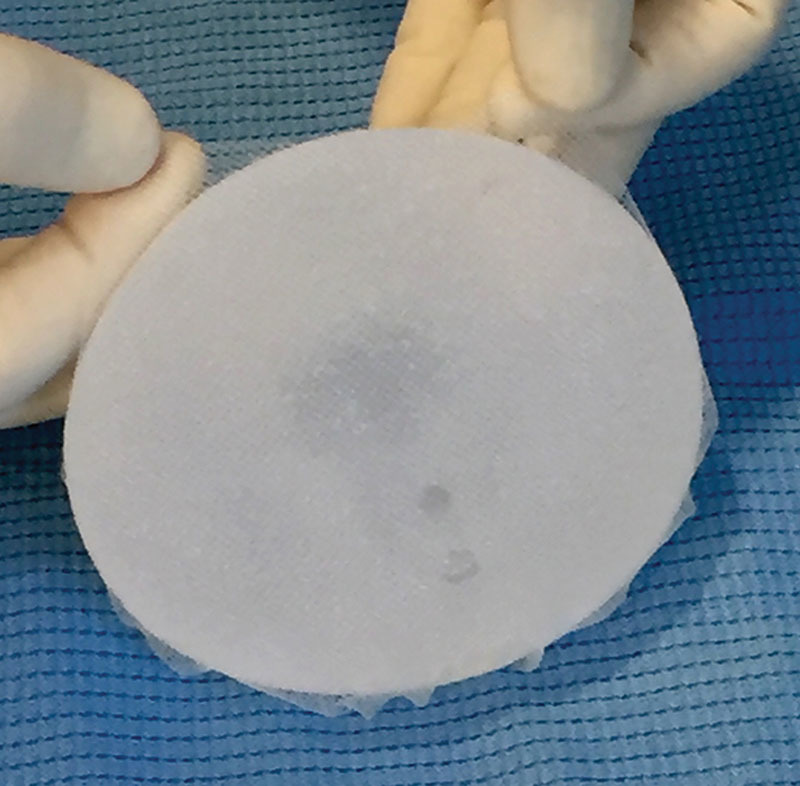
Implant preparation. Complete implant wrapping by means of a TCPM.
Fig. 2.
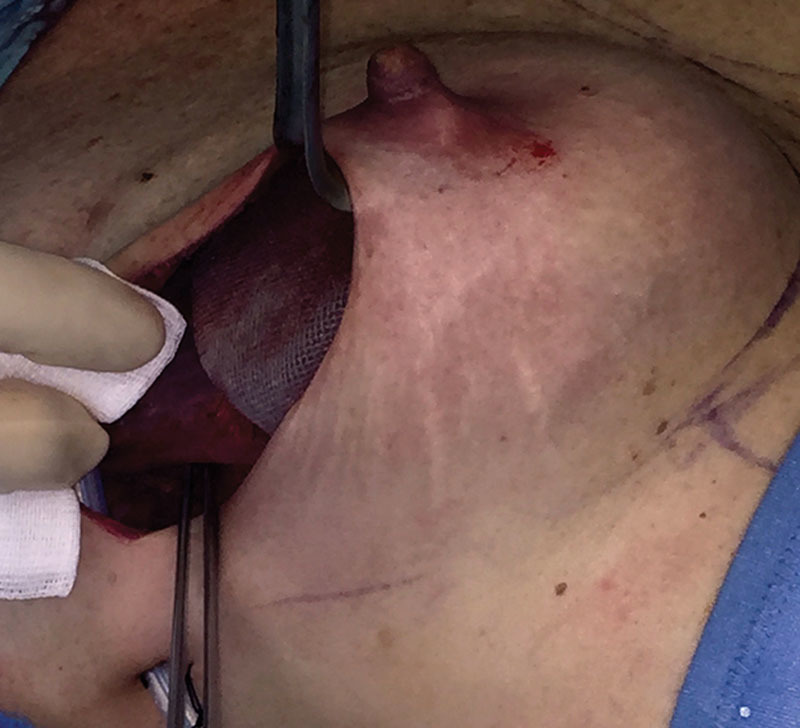
Subcutaneous implant positioning. The implant and the mesh are placed directly underneath the skin flaps in a prepectoral position.
Fig. 3.
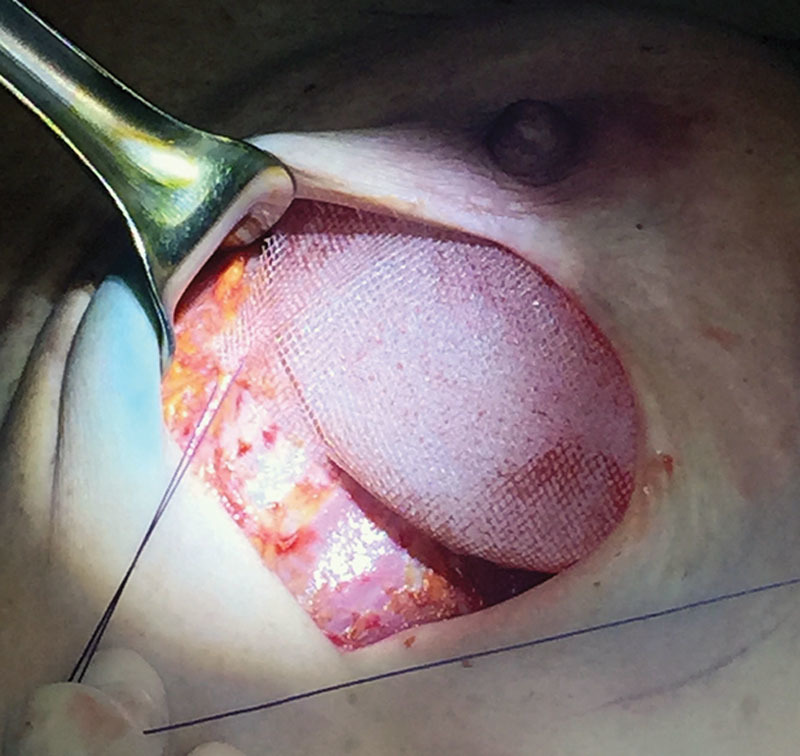
Subcutaneous implant fixation. Few interrupted stitches are placed between the mesh and the muscular fascia to secure the implant in the desired position.
Fig. 4.
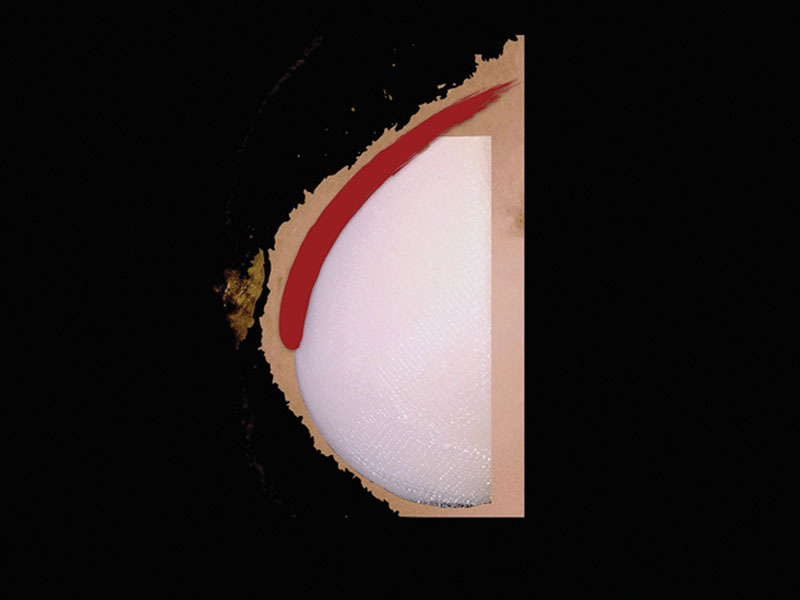
Retropectoral implant position. Implant in the traditional retropectoral pocket created by dissecting the muscle from chest wall.
Fig. 5.

Prepectoral, subcutaneous, implant position. Implant in the subcutaneous space, above the muscle, which is not dissected.
The subcutaneous approach is described, shortly after our study,22 also in 2 more papers, although in a smaller number of cases and using ADMs as coverage for the implant.23,24 Results of our previously published prospective nonrandomized clinical study are limited to short-term complications and show that there are no differences in terms of surgical complications between the 2 groups of patients.22 Studies on the subcutaneous approach conclude that it is safe and feasible. Unfortunately, so far, none of them present long-term results. Moreover, few article deal with the QOL in terms of cosmetic outcomes of breast cancer survivors submitted to any type of reconstruction, as highlighted in a recent study.29 This study faces the issue of subjective cosmetic outcome in the restricted sample of breast cancer survivors enrolled in the previous trial comparing the prepectoral and retropectoral breast reconstructions. In addition to the cosmetic aspect, a subjective functional evaluation (as part of BREAST-Q) and an objective assessment, from surgeons’ perspective, are considered too. Results show that in terms of long-term outcomes the muscle-sparing technique has an encouraging performance.
First, it should be acknowledged that 1 late implant failure and removal, because of poor incision borders healing during chemotherapy, was recorded in G-2. This, summed with 1 early implant loss from the short-term evaluation, makes a surgical failure rate for the subcutaneous technique of 2 out of 39 cases (5.1%) versus 0% in the standard muscular mesh pocket group (G-1). The other registered implant removal in G-2 was because of a locoregional chest wall recurrence.
Notwithstanding this difference in surgical failure, due in both cases to skin flaps and wound problems, the implant change rate for functional and aesthetic reasons definitely favors the subcutaneous technique with 4 cases (12%) and 0 cases (0%) for G-1 and G-2, respectively. A similar number of fat grafts over the implant procedures, to ameliorate the cosmetic outcome, were performed in the 2 groups. In terms of subjective QOL parameters, the BREAST-Q evaluation gives a statistically significant difference in the “satisfaction with outcome” group of items, with the subcutaneous technique favored over the retropectoral one. In our opinion, this excellent result in the subjective satisfaction with reconstructed breast is because of the more natural appearance that a subcutaneous implant entails. This approach, in fact, naturally recreates the innate ptosis of the breast, being the implant in the original anatomical position of the breast gland (Figs. 6–8).
Fig. 6.

Subcutaneous, muscle-sparing, direct-to-implant breast reconstruction. Unilateral breast reconstruction without any surgical procedure on the contralateral side.
Fig. 8.
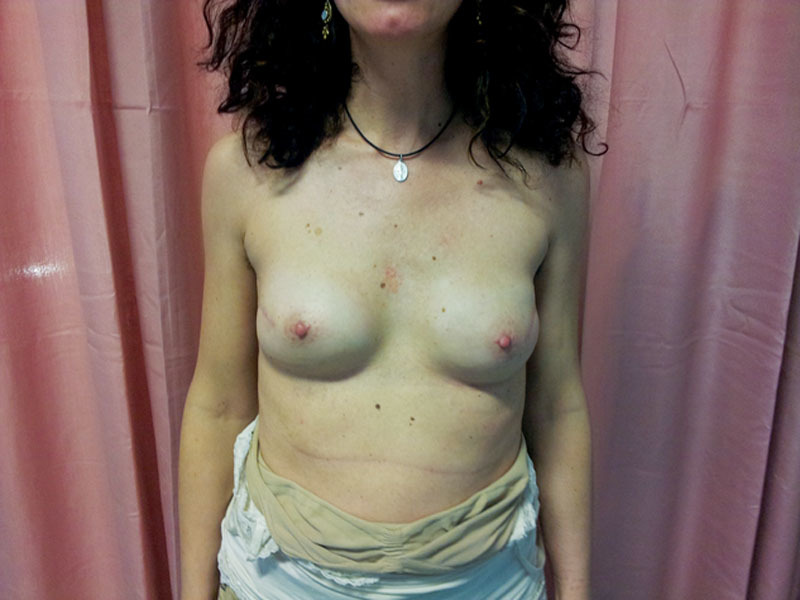
Subcutaneous, muscle-sparing, direct-to-implant breast reconstruction. Bilateral mastectomy with a bilateral direct-to-implant subcutaneous reconstruction using the same implant and technique on both sides.
Fig. 7.

Subcutaneous, muscle-sparing, direct-to-implant breast reconstruction. Unilateral breast reconstruction without any surgical procedure on the contralateral side.
Among the objective evaluation parameters, a significant difference is exhibited for the capsular contracture rate and for the aesthetic outcome. In both cases, the subcutaneous technique results are superior to the retropectoral standard procedure. As for the capsular contracture rate, a III–IV grade contracture is recorded in 4 (12%) G-1 cases, compared with 0% in G-2. A theoretical explanation for such a good result in capsular contracture is that the subcutaneous approach avoids any mechanical stress over the implant and over its capsule as opposed to a retropectoral technique. This hypothesis is corroborated by a recent article showing a significant difference in capsular thickness between tissue expanders placed subcutaneously and those placed in the standard submuscular position.30 Interestingly, no grade III–IV capsular contractures were encountered in G-2 even considering that 9 (26%) cases in this group received a postoperative radiation therapy. Furthermore, the chemically and biologically inert nature of the titanium-coated mesh might play a role in this as hypothesized in a recent study.21 Synthetic mesh constitutes an interface between implant and skin flaps, recreating a new fascia and supporting the implant itself to prevent rotations in the early postoperative course. The same goal can be achieved by ADMs as described in the aforementioned studies.23,24 A TCPM has a good flexibility, which helps in placement. Its loose knitwork helps in fluids drainage, avoiding closed space. Moreover, the new fascia created by mesh integration within tissues appears very thin and soft when exposed (Figs. 9, 10). A formal histologic analysis of TCPM integration within tissues was performed in 1 study,21 with good results in terms of fibrosis and subsequent capsular contracture. Besides, costs are definitely lower for TCPM when compared with ADMs overall.
Fig. 9.
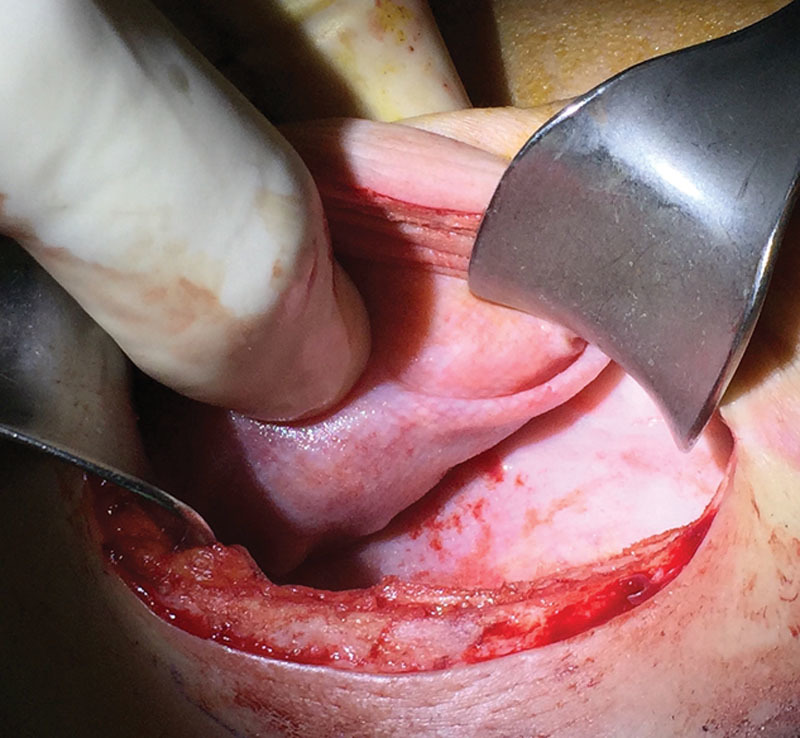
Titanium-coated synthetic mesh integration within capsule. Appearance of capsule during a second-stage procedure, after tissue expander removal of a reconstructed breast by means of a TCPM. The synthetic mesh results completely integrated within the capsule, which results in thin and soft tissues.
Fig. 10.
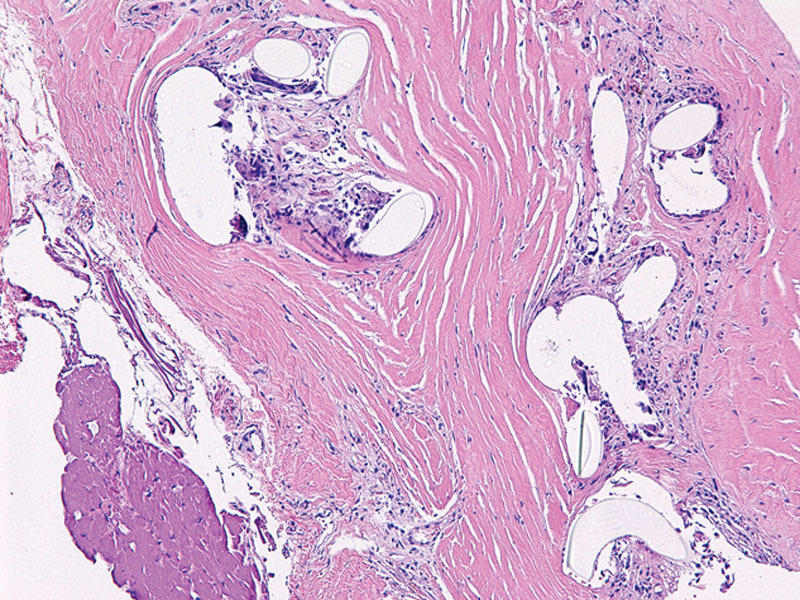
Microscopic appearance of titanium-coated synthetic mesh integration within capsule. Fibrous capsule shows regular cystic spaces containing pale material consistent with the TCPM, surrounded by a mild chronic inflammatory response with histiocytes and foreign body giant cells. These elements are completely integrated within fibroblastic tissue.
Nonetheless, a subcutaneous reconstruction can be done, as reported in the cited articles from different authors,23,24 with TCPM or ADMs alternatively and seemingly with good results in both cases.
The surgeons’ aesthetic outcome judgment is deemed excellent in 91% of G-2 cases versus 65% of G-1, and this is coherent with the women subjective evaluation, with a significant advantage of the subcutaneous approach. A retropectoral implant has the disadvantage of the muscle presence, which can retract or somehow flatten the implant itself, with high ridden breast appearance and unpleasant “animated” implants. A subcutaneous reconstruction, instead, allows a more natural ptosis and appearance of the breast (See video, See Supplemental Digital Content 1, which displays cosmetic long-term result of a bilateral subcutaneous breast reconstruction, bilateral mastectomy with direct-to-implant subcutaneous breast reconstruction in a skinny woman, and appearance at 24 month follow-up, in a patient submitted to systemic adjuvant chemotherapy and postoperative radiation therapy on the right side. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A152).
Video 1.

See Video, Supplemental Digital Content 1, which displays cosmetic long-term result of a bilateral subcutaneous breast reconstruction, bilateral mastectomy with direct-to-implant subcutaneous breast reconstruction in a skinny woman and appearance at 24 month follow-up, in a patient submitted to systemic adjuvant chemotherapy and postoperative radiation therapy on the right side. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A152.
On the other hand, it is important to highlight that a careful patients selection is mandatory for a subcutaneous approach. The absence of muscle coverage over the implant can expose the medial aspect of the reconstructed breast to more visible implant borders and signs of rippling. These drawbacks can be corrected with a fat graft procedure over the implant capsule, as occurred in 3 cases (9%) in G-2. The surgeon choice of the subcutaneous technique over the submuscular one can be made intraoperatively on the basis of skin flaps viability and thickness. Also the reconstructed breast medial-upper aspect and sternal border contour is deemed important in the decision of using or sparing the muscle. A BMI lower than 18.5 was an exclusion criterion in patients enrollment. Nonetheless, even with normal BMI patients, in case of skinny flaps on the upper/medial pole of the reconstructed breast, a submuscular approach might be chosen if implant borders visibility and some ripples are predictable.
Some oncological aspects are of utmost importance as well, in the surgical planning beforehand. In case, if a postoperative radiation, and a chemotherapy as well, is anticipated, which could jeopardize a correct wound and skin flaps healing, then a subcutaneous approach and maybe the entire direct-to-implant reconstruction strategy would be better changed.
All results are limited by the number of cases and by the nonrandomization nature of the previous study upon which this long-term analysis was conducted. Further analysis and larger numbers should be used to confirm present results. Nonetheless, this is, to our knowledge, the first study evaluating the subcutaneous approach with a median follow-up of 25 months.
In conclusion, subcutaneous breast reconstruction exhibits encouraging results in terms of aesthetic outcome and capsular contracture over a long-term period of evaluation. Skin flaps viability and wound healing are of utmost importance for its successful performance. Present results might lead to a consideration of the muscle-sparing subcutaneous approach as a valid alternative to the standard retropectoral technique. As much as conservative, mastectomies31 have changed the breast surgical oncology scenario, a “conservative reconstruction” paradigm is worth considering.
ACKNOWLEDGMENT
We thank Monica Macguire for English proofreading.
Ethical approval: No institutional ethical committee approval required. Follow-up observational study of a previously approved nonrandomized clinical study. Study protocol was performed after obtaining informed consent and in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Supplementary Material
Footnotes
Authors themselves and Institutional University Hospital funds covered all the expenses for the present study. No third party funding was received. Authors are all members of a public-system University hospital and have no consults, royalties or any type of research funds issued with any private company as for the present study purposes.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge has been paid for by PFM Medical, Cologne, Germany.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.American Society of Plastic Surgeons. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2014-statistics/plastic-surgery-statsitics-full-report.pdf. Accessed June 3rd, 2015.
- 2.Salzberg CA. Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg. 2012;130(5 Suppl 2):S95–S103. doi: 10.1097/PRS.0b013e318262e1a1. [DOI] [PubMed] [Google Scholar]
- 3.Kocak E, Nagel TW, Hulsen JH, 3rd, et al. Biologic matrices in oncologic breast reconstruction after mastectomy. Expert Rev Med Devices. 2014;11:65–75. doi: 10.1586/17434440.2014.864087. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 5.Rawlani V, Buck DW, 2nd, Johnson SA, et al. Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg. 2011;66:593–597. doi: 10.1097/SAP.0b013e3181f3ed0a. [DOI] [PubMed] [Google Scholar]
- 6.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. doi: 10.1097/PRS.0b013e31822b6637. [DOI] [PubMed] [Google Scholar]
- 7.Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg. 2011;128:1162–1169. doi: 10.1097/PRS.0b013e318230c29e. [DOI] [PubMed] [Google Scholar]
- 8.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 10.Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol. 2012;19:3402–3409. doi: 10.1245/s10434-012-2362-y. [DOI] [PubMed] [Google Scholar]
- 11.Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg. 2012;129:901e–908e. doi: 10.1097/PRS.0b013e31824ec447. [DOI] [PubMed] [Google Scholar]
- 12.Hill JL, Wong L, Kemper P, et al. Infectious complications associated with the use of acellular dermal matrix in implant-based bilateral breast reconstruction. Ann Plast Surg. 2012;68:432–434. doi: 10.1097/SAP.0b013e31823b6ac6. [DOI] [PubMed] [Google Scholar]
- 13.Newman MI, Swartz KA, Samson MC, et al. The true incidence of near-term postoperative complications in prosthetic breast reconstruction utilizing human acellular dermal matrices: a meta-analysis. Aesthetic Plast Surg. 2011;35:100–106. doi: 10.1007/s00266-010-9631-6. [DOI] [PubMed] [Google Scholar]
- 14.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 15.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 16.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- 17.Breuing KH, Colwell AS. Immediate breast tissue expander-implant reconstruction with inferolateral AlloDerm hammock and postoperative radiation: a preliminary report. Eplasty. 2009;9:e16. [PMC free article] [PubMed] [Google Scholar]
- 18.Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg. 2009;123:1–6; discussion 107. doi: 10.1097/PRS.0b013e3181904bff. [DOI] [PubMed] [Google Scholar]
- 19.Dieterich M, Reimer T, Dieterich H, et al. A short-term follow-up of implant based breast reconstruction using a titanium-coated polypropylene mesh (TiLoop(®) Bra). Eur J Surg Oncol. 2012;38:1225–1230. doi: 10.1016/j.ejso.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Dieterich M, Paepke S, Zwiefel K, et al. Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases. Plast Reconstr Surg. 2013;132:8e–19e. doi: 10.1097/PRS.0b013e318290f8a0. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann PA, Becker B, Mauss KL, et al. Titanium-coated polypropylene mesh (TiLoop Bra®)—an effective prevention for capsular contracture? Eur J Plast Surg. 2014;37:339–346. [Google Scholar]
- 22.Casella D, Bernini M, Bencini L, et al. TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg. 2014;37:599–604. doi: 10.1007/s00238-014-1001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berna G, Cawthorn SJ, Papaccio G, Balestrieri N. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg. 2014 doi: 10.1111/ans.12849. September 29. doi: 10.1111/ans.12849. [DOI] [PubMed] [Google Scholar]
- 24.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. doi: 10.1016/j.bjps.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3:193–203. [PubMed] [Google Scholar]
- 26.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Ann Surg Oncol. 2013;20:3422–3429. doi: 10.1245/s10434-013-3026-2. [DOI] [PubMed] [Google Scholar]
- 27.Susarla SM, Ganske I, Helliwell L, et al. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg. 2015;135:1e–8e. doi: 10.1097/PRS.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 28.Singh NK, Reaven NL, Funk SE. Cost comparison of immediate one-stage and tissue-expander breast reconstructions after mastectomy in commercially insured patients. Manag Care. 2013;22:36–43. [PubMed] [Google Scholar]
- 29.Jagsi R, Li Y, Morrow M, et al. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction. Ann Surg. 2015;261:1198–1206. doi: 10.1097/SLA.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomita K, Yano K, Nishibayashi A, et al. Effects of subcutaneous versus submuscular tissue expander placement on breast capsule formation. Plast Reconstr Surg Glob Open. 2015;3:e432. doi: 10.1097/GOX.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veronesi U, Stafyla V, Petit JY, et al. Conservative mastectomy: extending the idea of breast conservation. Lancet Oncol. 2012;13:e311–e317. doi: 10.1016/S1470-2045(12)70133-X. [DOI] [PubMed] [Google Scholar]


