Abstract
Supplemental Digital Content is available in the text.
Sir:
Brachial plexus nerve root avulsion injuries result in paralysis of the affected arm that will not recover without treatment.1 Cell transplantation therapies at the site of nerve root reimplantation could improve axon regeneration after these injuries. In the most economical animal model, the rat, accurate intradural avulsion and reimplantation of cervical ventral nerve roots to test new cell therapies are difficult.2–5 To address the lack of detailed reports describing this technically challenging procedure, we describe improvements to a unilateral dorsal surgical approach to avulse the C8 and/or T1 nerve roots intradurally. These improvements substantially enhanced the success rate of the procedure in a series of 40 Sprague Dawley rats.
All procedures were approved by the ethical review process of the University College London Institute of Neurology and were in accordance with United Kingdom legislation [Animals (Scientific Procedures) Act 1986]. A custom made animal support molded from plasticine flexes the rat cervical spine to increase the interlaminar space. Using the spinous process of T2 as an anatomical landmark the levator auris longus, platysma and trapezius muscles are sectioned at the midline and retracted (See Figure, Supplemental Digital Content 1, which displays the anatomy of the Sprague Dawley rat brachial plexus. Left shows the brachial plexus nerve roots and ganglia attached to the cervical spine. The lamina of T1 and C7 vertebra have been removed to show the T1 and C8 nerve roots. Right shows the cervical dorsal root ganglia, ventral roots, and brachial plexus. Dorsal roots have been removed. The T1 ventral nerve root consists of 2 main rootlets indicated by white stars. http://links.lww.com/PRSGO/A155; See Video 1, Supplemental Digital Content 2, which demonstrates the location and exposure of the T1/C7 vertebra via a dorsal approach using the spinous process of T2 as an anatomical landmark. The lamina, transverse process and pedicle are drilled and a T1/C7 hemilaminectomy performed to create a sufficient surgical window. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A156). Meticulous hemostasis throughout the procedure using biopolar cauterization, Floseal (Baxalter, Staines-upon-Thames, UK), gauze packing, and blunt dissection techniques are vital to minimize blood pooling in the visual field. Underlying paraspinal muscles are pushed laterally with forceps and retractors. The hemilamina of T1 and C7 are removed with bone rongeurs, along with the associated transverse process and facet joint. Thinning of the lamina and transverse process by drilling with a 0.7-mm burr aids gentle removal (See Video 1, Supplemental Digital Content 2, which demonstrates the location and exposure of the T1/C7 vertebra via a dorsal approach using the spinous process of T2 as an anatomical landmark. The lamina, transverse process and pedicle are drilled and a T1/C7 hemilaminectomy performed to create a sufficient surgical window. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A156). The pedicle of C7 is also drilled until almost level with the posterior aspect of the vertebral body and the anterior aspect of the canal. A dural/arachnoid mater flap is then created by opening the dura close to the midline using a 30G needle and custom-made tungsten nerve hook (Fig. 1 and see Video 2, Supplemental Digital Content 3, which shows the opening of the dura and transection and retraction of the T1 dorsal root using the custom made nerve hook. The dura is retracted with sutures, and 1 of the 2 T1 ventral rootlets is avulsed using the nerve hook. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A157). This is retracted and held in place with two 10/0 sutures. Absorption spears are useful to stem bleeding from epidural vessels. The dorsal root is carefully lifted, transected, and retracted using the nerve hook and needle. The ventral root can now be visualized, and all rootlets accurately avulsed close to the cord surface using the nerve hook and forceps (Fig. 2 and see Video 2, Supplemental Digital Content 3, which shows the opening of the dura and transection and retraction of the T1 dorsal root using the custom made nerve hook. The dura is retracted with sutures, and 1 of the 2 T1 ventral rootlets is avulsed using the nerve hook. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A157; see Video 3, Supplemental Digital Content 4, which demonstrates using the hook and forceps, the second ventral rootlet is passed underneath the obstructing blood vessel and avulsed. The pia is opened and the ventral root reimplanted in the ventrolateral cord. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A158) Before this, denticulate ligaments must be severed to avoid tension on the cord or surrounding blood vessels when the ventral root is hooked (see Video 2, Supplemental Digital Content 3, which shows the opening of the dura and transection and retraction of the T1 dorsal root using the custom made nerve hook. The dura is retracted with sutures, and 1 of the 2 T1 ventral rootlets is avulsed using the nerve hook. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A157). Often large blood vessels are associated with the ventral root, but with careful manipulation, these can be avoided (see Video 3, Supplemental Digital Content 4, which demonstrates using the hook and forceps, the second ventral rootlet is passed underneath the obstructing blood vessel and avulsed. The pia is opened and the ventral root reimplanted in the ventrolateral cord. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A158). The ventral root can now be reimplanted into the ventrolateral cord with experimental cellular therapies and secured with Tisseel (Baxter).
Video 1.
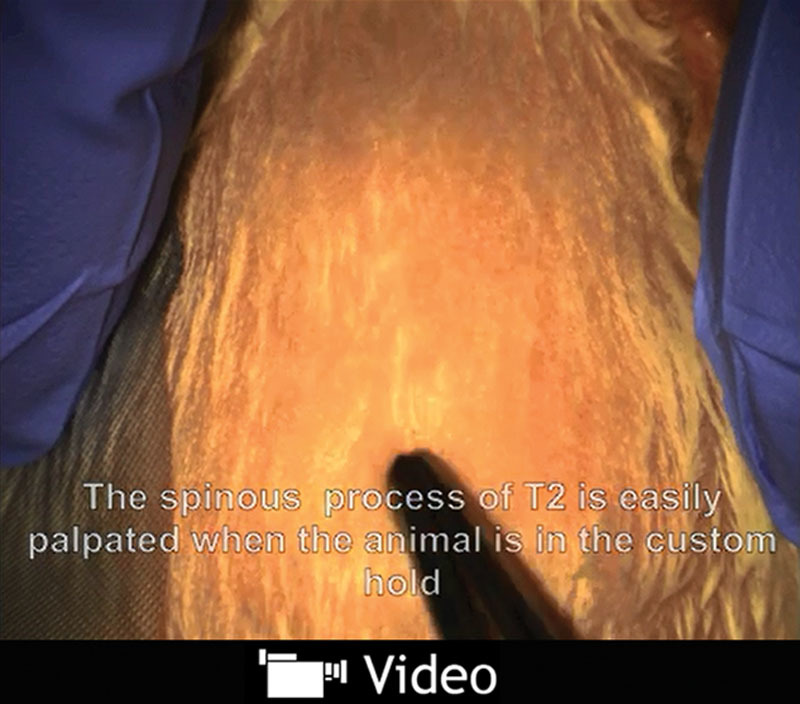
See video, Supplemental Digital Content 2, which demonstrates the location and exposure of the T1/C7 vertebra via a dorsal approach using the spinous process of T2 as an anatomical landmark. The lamina, transverse process and pedicle are drilled and a T1/C7 hemilaminectomy performed to create a sufficient surgical window. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A156.
Fig.1.

Custom-made nerve hook for manipulating the dura and cervical nerve roots. This is made by filing a tungsten needle (bottom) to form a small flat surface with smooth edges that can be curved using forceps to the optimal length and angle for hooking the rat cervical nerve roots. Small blue interval markers indicate 1 mm.
Video 2.

See video, Supplemental Digital Content 3, which shows the opening of the dura and transection and retraction of the T1 dorsal root using the custom made nerve hook. The dura is retracted with sutures, and 1 of the 2 T1 ventral rootlets is avulsed using the nerve hook. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A157.
Fig. 2.

The surgical view of the T1 ventral rootlets created using the described approach. Often blood vessels may run alongside the ventral rootlets. To avulse the nerve root without excessive bleeding, the rootlets must be passed underneath the blood vessel and hooked from the opposite side (see Video 3, Supplemental Digital Content 4 which demonstrates using the hook and forceps, the second ventral rootlet is passed underneath the obstructing blood vessel and avulsed. The pia is opened and the ventral root reimplanted in the ventrolateral cord. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A158).
Video 3.

See video, Supplemental Digital Content 4, which demonstrates using the hook and forceps, the second ventral rootlet is passed underneath the obstructing blood vessel and avulsed. The pia is opened and the ventral root reimplanted in the ventrolateral cord. This video is available in the “Related Videos” section of the full-text article on http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A158.
In summary, the techniques outlined; meticulous hemostasis, lamina, transverse process, and pedicle drilling, creating a dural/arachnoid flap, use of dural sutures, a custom-made animal support and nerve hook, substantially improve the access and visibility of the ventral root from a dorsal surgical approach. Accurate avulsion close to the spinal cord surface is easier to achieve, and success rates were improved from 9 out of 20 to 19 out of 20 procedures.
Supplementary Material
Footnotes
Disclosure: This work was funded by the European Research Council and was carried out at UCL/UCLH Biomedical Research Centre, which receives funding from the National Institute for Health Research. The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by University College London.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Carlstedt T, Anand P, Htut M, et al. Restoration of hand function and so called “breathing arm” after intraspinal repair of C5-T1 brachial plexus avulsion injury. Case report. Neurosurg Focus. 2004;16:E7. doi: 10.3171/foc.2004.16.5.8. [DOI] [PubMed] [Google Scholar]
- 2.Wu W. Potential roles of gene expression change in adult rat spinal motoneurons following axonal injury: a comparison among c-jun, off-affinity nerve growth factor receptor (LNGFR), and nitric oxide synthase (NOS). Exp Neurol. 1996;141:190–200. doi: 10.1006/exnr.1996.0153. [DOI] [PubMed] [Google Scholar]
- 3.Bertelli JA, Mira JC. Brachial plexus repair by peripheral nerve grafts directly into the spinal cord in rats. Behavioral and anatomical evidence of functional recovery. J Neurosurg. 1994;81:107–114. doi: 10.3171/jns.1994.81.1.0107. [DOI] [PubMed] [Google Scholar]
- 4.Zhao S, Pang Y, Beuerman RW, et al. Expression of c-Fos protein in the spinal cord after brachial plexus injury: comparison of root avulsion and distal nerve transection. Neurosurgery. 1998;42:1357–1362; discussion 1362. doi: 10.1097/00006123-199806000-00099. [DOI] [PubMed] [Google Scholar]
- 5.Cao XC, Ling LJ. Anatomic basis and technical aspects of a new brachial plexus avulsion injury model in the rat. Plast Reconstr Surg. 2003;111:2488–2472. doi: 10.1097/01.PRS.0000063136.86358.12. [DOI] [PubMed] [Google Scholar]


