Supplemental Digital Content is available in the text.
Summary:
A subcutaneous, prepectoral, muscle-sparing approach has been recently described for implant-based breast reconstruction. This is a preliminary series of 2-stage breast reconstructions by means of tissue expander placed subcutaneously with the support of a titanium-coated polypropylene mesh. A pilot series of cases was started in 2012. Inclusion criteria were informed consent, age less than 80 years, normal body mass index (range, 18.5–24.9), no T4 and metastatic cancers, no comorbidities, and nonsmoking patients. Expander losses, infections, seromas, skin/nipple necrosis, wound dehiscence, and reinterventions were registered in follow-up visits. Furthermore, patients were followed up in second-stage procedures and for at least 1 year from implant positioning to collect any surgical complication, reinterventions, cosmetic outcome, and oncological data. Between June 2012 and March 2014, 25 cases were enrolled in the study. Expander/implant loss rate was 0%. Skin/nipple necrosis rate was 4%. Infections rate was 12% after first-stage and 4% after second-stage procedure. Seromas rate was 0%. Five (20%) fat graft procedures were performed over the expander before second-stage reconstruction, and no reinterventions were required after second stage. Patients mean score was 99 for cosmetic outcome satisfaction, in a 0–100 scale. Subcutaneous 2-stage reconstruction with synthetic mesh proved safe and feasible. Patients satisfaction is very good after 14 months median follow-up form definitive implant placement. Although the present study involved only a small number of cases, a tissue-expander subcutaneous reconstruction seems to have promising results. Whenever pectoralis major muscle can be spared, a conservative reconstruction might be an option.
In the United States, 2-stage implant-based breast reconstruction (IBBR) accounts for approximately 70% of reconstructions after mastectomy,1 and majority of reconstructions are performed by means of acellular dermal matrices (ADMs).2 Biological matrixes are the most used worldwide,3 although synthetic meshes are widespread too, as titanium-coated polypropylene mesh (TiLOOOP Bra, pfm medical, Cologne, Germany) in Europe.4 Such devices, either ADMs or meshes, are traditionally adopted as a muscle extension, creating a “dual plane” coverage of tissue expander (TE)/implant, as described by Spear et al.5 In 2014, a different approach with subcutaneous implant placement by means of a full synthetic mesh or ADMs coverage was described in direct-to-implant reconstructions.6–8 Moreover, a recent article shows a significant difference in capsular thickness between TEs placed subcutaneously and those placed in standard submuscular position.9
Aim of this report is to analyze surgical safety and long-term outcomes of subcutaneous TE 2-stage reconstruction using a synthetic mesh.
PATIENTS AND METHODS
In 2011, a pilot study was approved by the Hospital Drugs and Devices Service Committee, according with Institutional Ethical Committee rules on nonrandomized clinical studies, for evaluation of performances of a titanium-coated polypropylene synthetic mesh (TiLOOOP Bra) in IBBR.
Patients scheduled at our institution for conservative mastectomies, either nipple-sparing or skin-sparing mastectomy, were thoroughly informed of different reconstruction options, either autologous or prosthetic. If TE approach was chosen, patients were informed of the muscle-sparing subcutaneous option with synthetic mesh coverage only (totally subcutaneous, prepectoral TE adjustment, and wrapped in a mesh bag; Fig. 1). Inclusion criteria were age less than 80 years and normal body mass index (range, 18.5–24.9). Exclusion criteria were previous breast surgery, T4 and metastatic cancers, refusal to sign the consent, comorbidities (diabetes, renal failure, heart failure, cardiovascular diseases, hypertension at oral medications, pulmonary diseases, hepatic diseases, and metabolic diseases), smoking, and previous radiotherapy to chest wall. An informed consent, with description of surgical technique and complications, was signed by every woman. A prospective digital database was created to encompass all baseline characteristics, complications, outpatient visits, reinterventions, second-stage reconstructions, final cosmetic outcome, and oncological follow-up.
Fig. 1.
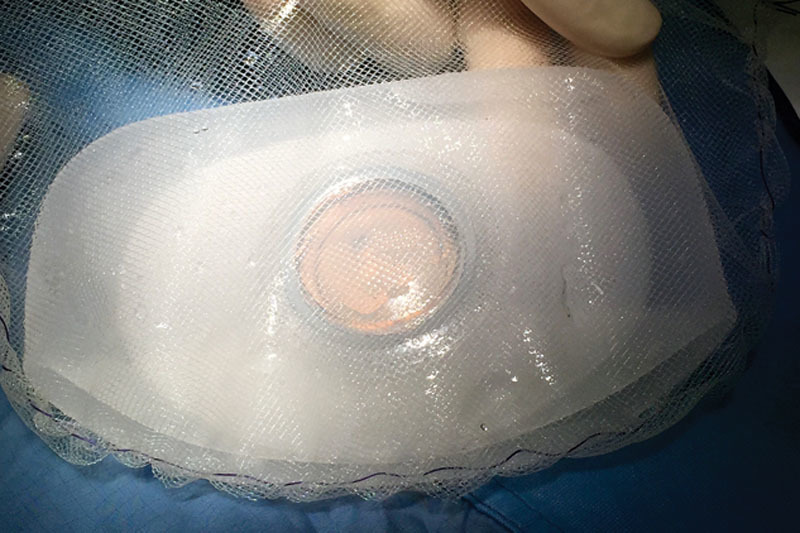
Ex vivo TE preparation with titanium-coated polypropylene mesh. Complete TE wrapping by means of a titanium-coated polypropylene synthetic mesh bag. A mesh pocket is tailored to embrace TE and is left loosely bigger than TE itself considering the maximum TE diameter at final expansion.
Mesh wrapping around TE was loose, considering final expansion diameter of TE. Outpatient expansions were done every week or every 2 weeks for the first 2 months, with 40–50mL of sterile solution each time. Follow-up visits were performed every 2 months thereafter, even after TE exchange with implant.
Subjective cosmetic evaluation was conducted using the postoperative BREAST-Q module (Memorial Sloan-Kettering Cancer Center and The University of British Columbia © 2006, all rights reserved). According to a study on patient-reported quality of life,10 the module was divided into multiple independent scales: satisfaction with breasts (16 items), satisfaction with outcome (7 items), psychosocial well being (10 items), physical well being (16 items), and sexual well being (6 items). For each scale, responses were summed and transformed into a score, in a 0–100 scale. Higher scores indicate greater satisfaction or quality of life. Cosmetic evaluation with BREAST-Q was completed within May 2015 for all patients.
RESULTS
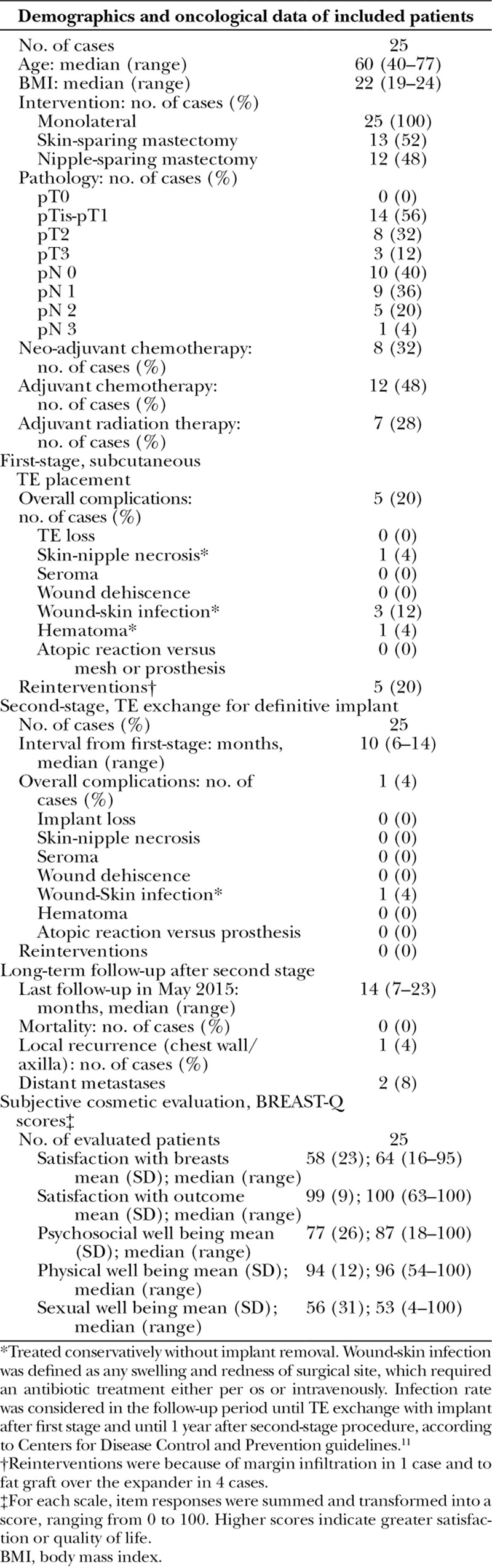
Between June 2012 and March 2014, 25 cases were submitted to a subcutaneous TE/first-stage IBBR. After 10 month median interval, second-stage procedures were performed in all cases. Demographics, oncological data, complications after first stage, reinterventions, further complications after second stage, and long-term cosmetic outcome, with 14-month median follow-up from final reconstruction, are shown in Table 1.
Table 1.
Demographics, Oncological Data, Surgical Complications and Long-Term Cosmetic Outcome of Subcutaneous Implant-Based 2-Stage Breast Reconstruction
DISCUSSION
On the basics of the rationale that both ADMs and synthetic meshes are safe under the mastectomy flaps in the inferolateral pole in a combined submuscular “dual plane” coverage, we assume that it could be safe to cover breast prostheses entirely with such devices. Results in subcutaneous direct-to-implant reconstructions are published in three 2014 studies, with either titanium-coated mesh or ADMs.6–8 Same approach can be used in 2-stage TE reconstruction as well, particularly because of lesser tension on skin flaps, whenever a 2-stage procedure is chosen either for oncological or for surgical reasons. Subcutaneous reconstructions recreate the anatomical position and ptosis of breast gland. Besides, pectoralis muscle can be spared, avoiding drawbacks such as prosthesis “animation” (See video, Supplemental Digital Content 1, which displays a subcutaneous TE. Monolateral subcutaneous TE (full height, full projection) 3 months after intervention during chemotherapy. This video is available in the “Related Videos” section of the full-text article on http://links.lww.com/PRSGO/A159).
Video 1.
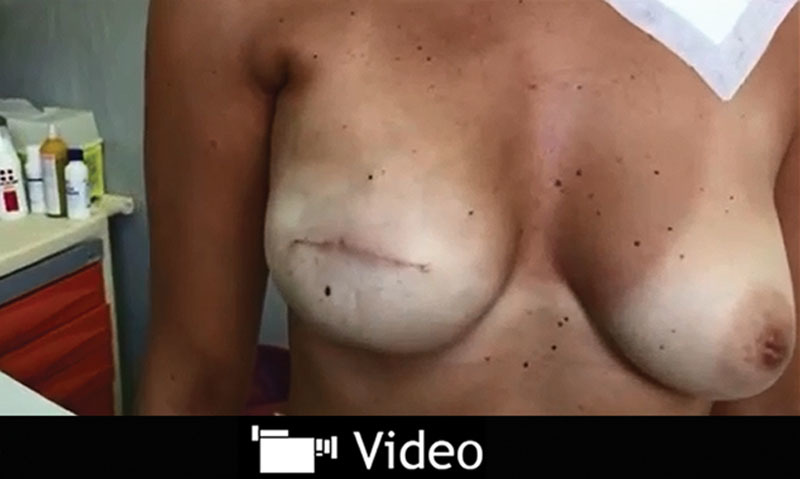
See video, Supplemental Digital Content 1, which displays a subcutaneous TE. Monolateral subcutaneous TE (full height, full projection) 3 months after intervention during chemotherapy. This video is available in the “Related Videos” section of the full-text article on http://links.lww.com/PRSGO/A159.
In present series, no TE failure (TE removal), 1 (4%) skin/nipple necrosis, 3 (12%) skin/wound infections, and 1 (4%) hematoma are reported. Complications were all treated conservatively. Five (20%) reinterventions in between first and second stage were all fat graft procedures over the TE, performed in cases submitted to postoperative radiation (always performed before second-stage reconstruction) to ameliorate the subcutaneous softness and thickness.
Only 1 (4%) complication, infection, occurred after second-stage procedures. Final cosmetic outcome, after 14-month median follow-up, was judged with a mean score of 99 in a 0–100 scale by patient-reported evaluations.
Intraoperatively, mesh appears thin, flexible, and completely integrated within TE capsule (Figs. 2,3). The use of ADMs or synthetic meshes provides a scaffold between TE and skin flaps. Such devices are effective to fix TEs or implants in the proper position, giving support and preventing displacements. Direct-to-implant studies6–8 show a seemingly equal safety with either technique. Our results both in a previous study6 and in this series show a 0% seroma rate, probably because of the loose knitwork of mesh that allows an easy fluid drainage without closed spaces. Seroma still remains the most frequent complication with ADMs.2 A possible drawback of a subcutaneous TE placement is the thin medial aspect of reconstructed breast, which can cause rippling and make visible the TE or implant later on. This limit can be addressed, whenever significant, with a fat graft procedure.
Fig. 2.
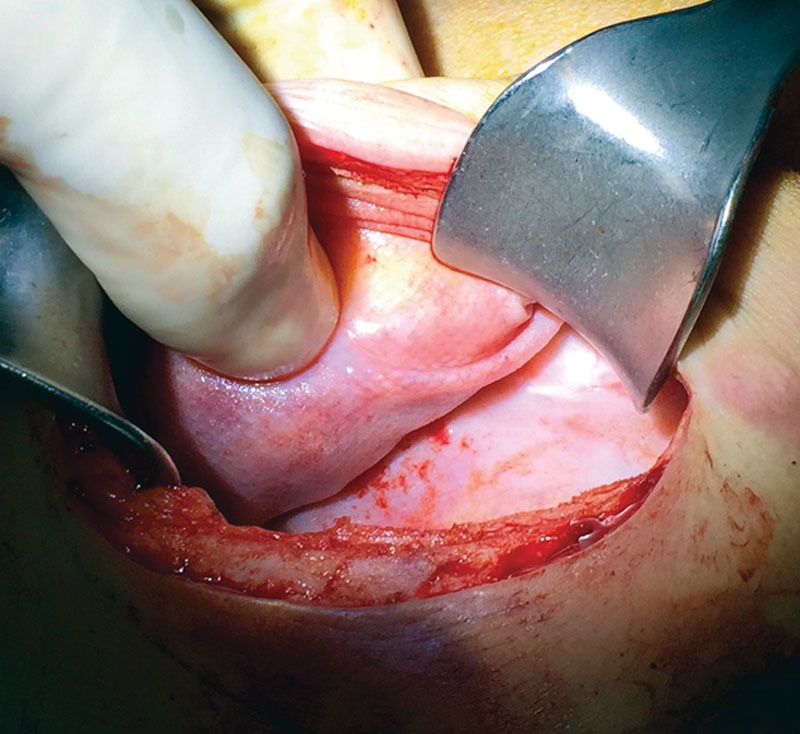
Titanium-coated synthetic mesh integration within capsule. Appearance of capsule during a second-stage procedure, after TE removal of a reconstructed breast by means of a titanium-coated polypropylene mesh.
Fig. 3.
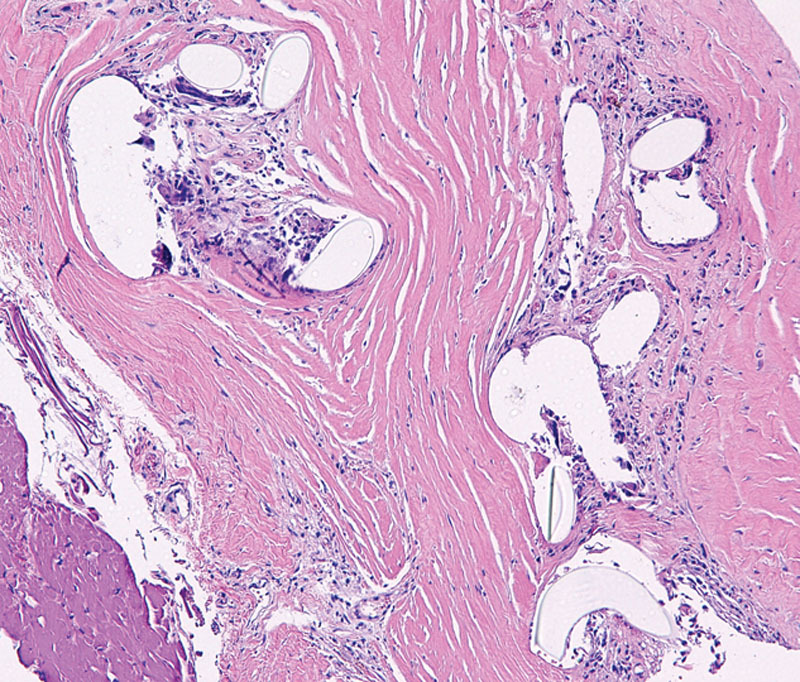
Microscopic appearance of titanium-coated synthetic mesh integration within capsule. Histology shows cystic spaces containing pale material consistent with the titanium-coated polypropylene mesh. Mild chronic inflammatory response with histiocytes and foreign body giant cells surround the mesh. All elements are completely integrated within fibroblastic tissue.
Obvious limits of this report are small number of cases and absence of a randomized control group.
In conclusion, these results might open a way toward the subcutaneous TE reconstruction as an alternative. As conservative mastectomies have changed the breast surgical oncology scenario, it is time to consider also a “conservative reconstruction” approach sparing muscles.
CONCLUSIONS
After the introduction of subcutaneous direct-to-implant breast reconstructions by means of soft tissue replacement devices, such as ADMs and synthetic meshes, a preliminary experience of subcutaneous TEs placement is reported in this series. Twenty-five patients were submitted to subcutaneous 2-stage reconstruction with TE completely wrapped in a titanium-coated polypropylene mesh and followed up even after second-stage procedure with a 14-month median long-term evaluation using BREAST-Q self reported module.
No TE/implant failures with removal were registered, and complications were minor and treated conservatively. Long-term subjective cosmetic results are excellent. This pilot experience with subcutaneous TE 2-stage reconstruction shows that, granted some selection criteria, this type of “anatomical” reconstruction is feasible, avoiding the drawbacks of muscle “animation” over the implant.
ACKNOWLEDGMENT
We thank Monica Macguire for English proofreading.
Ethical Approval: Pilot study approved by Hospital Drugs and Devices Service Committee (CAD 20110517), within Institutional Ethical Committee rules on nonrandomized studies. Written informed consent required, according to ethical standards laid down in 1964 Helsinki Declaration and later amendments.
Supplementary Material
Footnotes
Disclosure: Authors themselves and Institutional University Hospital funds covered all the expenses for the present study. No third party funding was received. Authors are all members of a public-system University hospital and have no consults, royalties or any type of research funds issued with any private company as for the present study purposes. The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by pfm medical, Cologne, Germany.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.American Society of Plastic Surgeons. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics. Accessed August 1, 2015.
- 2.Ibrahim AM, Koolen PG, Ashraf AA, et al. Acellular dermal matrix in reconstructive breast surgery: survey of current practice among plastic surgeons. Plast Reconstr Surg Glob Open. 2015;3:e381. doi: 10.1097/GOX.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocak E, Nagel TW, Hulsen JH, et al. Biologic matrices in oncologic breast reconstruction after mastectomy. Expert Rev Med Devices. 2014;11:65–75. doi: 10.1586/17434440.2014.864087. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich M, Paepke S, Zwiefel K, et al. Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases. Plast Reconstr Surg. 2013;132:8e–19e. doi: 10.1097/PRS.0b013e318290f8a0. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Parikh PM, Peisin E, et al. Acellulardermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 6.Casella D, Bernini M, Bencini L, et al. TiLoop Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg. 2014;37:599–604. doi: 10.1007/s00238-014-1001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg. 2014 doi: 10.1111/ans.12849. September 29. doi: 10.1111/ans.12849. [DOI] [PubMed] [Google Scholar]
- 8.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. doi: 10.1016/j.bjps.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Tomita K, Yano K, Nishibayashi A, et al. Effects of subcutaneous versus submuscular tissue expander placement on breast capsule formation. Plast Reconstr Surg Glob Open. 2015;3:e432. doi: 10.1097/GOX.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Ann Surg Oncol. 2013;20:3422–3429. doi: 10.1245/s10434-013-3026-2. [DOI] [PubMed] [Google Scholar]
- 11.Degnim AC, Throckmorton AD, Boostrom SY, et al. Surgical site infection after breast surgery: impact of 2010 Centers for Disease Control and Prevention reporting guidelines. Ann Surg Oncol. 2012;19:4099–4103. doi: 10.1245/s10434-012-2448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


