Summary:
Primary squamous cell carcinoma (SCC) of the breast comprises less than 0.1% of all breast cancers. Literature review reveals only 1 reported case of an SCC arising from the capsule of a breast implant. The authors describe, herein, a primary SCC arising from the capsule of a long-standing silicone breast implant.
Primary squamous cell carcinoma (SCC) of the breast is rare, characterized by large tumor size, rapid progression, frequent relapse, and poor prognosis.1–5 The diagnosis requires 3 conditions to be met: (1) more than 90% of the malignant cells must have squamous differentiation, (2) there are no other primary sites of SCC, and (3) the lesion must be separate from the skin and nipple.6
Implant-associated SCC of the breast has only been described once in 1992, although there are several reports of primary breast SCC arising from injection of free silicone.7–10 In 1999, the Institute of Medicine concluded that there was no evidence to suggest a causal relationship between silicone implants and either autoimmune disease or cancer.11 This is the first case report of a primary SCC arising from a breast implant capsule since the silicone implant moratorium was lifted.
CASE REPORT
The patient is a 58-year-old otherwise healthy woman who underwent primary bilateral augmentation mammoplasty in the 1980s with silicone implants. She required multiple subsequent procedures for a right-sided capsular contracture, including exchange to saline implants and then back to smooth silicone implants, with concomitant bilateral mastopexy and right-sided subtotal capsulectomy in 2000. Details concerning the brand and style of her implants are not available.
The patient presented to her primary care provider in 2015 with a sudden onset of right breast pain, swelling, and erythema. She was diagnosed with mastitis by her primary care physician and was prescribed antibiotics without any symptomatic improvement. She was subsequently evaluated by her community plastic surgeon. Physical examination revealed 2–3× enlargement of the right breast relative to the left with associated erythema and thinning of the overlying skin. She was taken to the operating room where a 500 mL gray fluid collection with keratinous debris was drained. The implant was intact and was removed from its capsule. A 5-cm fungating mass was noted on the posterior aspect of the capsule. A biopsy was taken and sent for pathology. A drain was placed, and the patient was referred to our tertiary care hospital for further care.
Pathology demonstrated a well-differentiated SCC. A positron emission tomography-computed tomographic scan demonstrated markedly increased F-18 fluorodeoxyglucose uptake localized to the right anterior chest wall (Fig. 1). Further extensive workup ruled out another primary site of SCC.
Fig. 1.
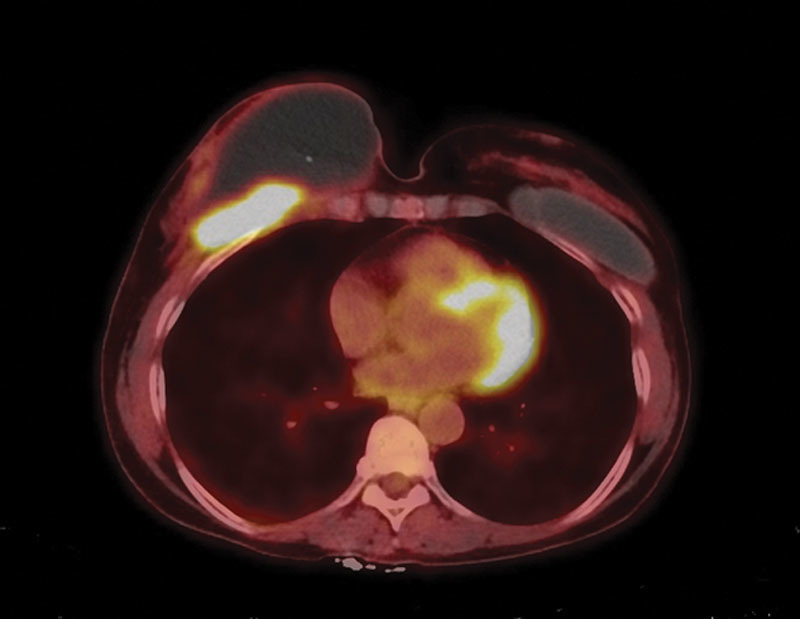
F-18 fluorodeoxyglucose (FDG) positron emission tomography-computed tomographic study demonstrates an ill-defined hypermetabolic soft tissue lesion located deep to the right breast and along the right anterior chest wall, concerning for neoplastic process. There is a large non-FDG avid fluid density collection in the right breast and overlying the hypermetabolic lesion, likely representing a seroma. Left breast implant is noted. No other suspicious FDG avid lesion or lymph node to suggest systemic involvement of malignancy was identified.
She underwent right total mastectomy, sentinel lymph node biopsy, and complete capsulectomy with concurrent left explant and simple mastectomy (per patient request). Intraoperatively, a large recurrent right breast seroma was evident (Fig. 2). The seroma containing keratinous debris was evacuated and sent for cytology. A fungating mass involving the posterior aspect of the subglandular capsule was noted (Fig. 3). To perform en bloc resection, portions of the pectoralis major and minor were removed along with the subglandular capsule (Fig. 4). Sentinel lymph node biopsy was transitioned to a complete right axillary lymph node dissection.
Fig. 2.
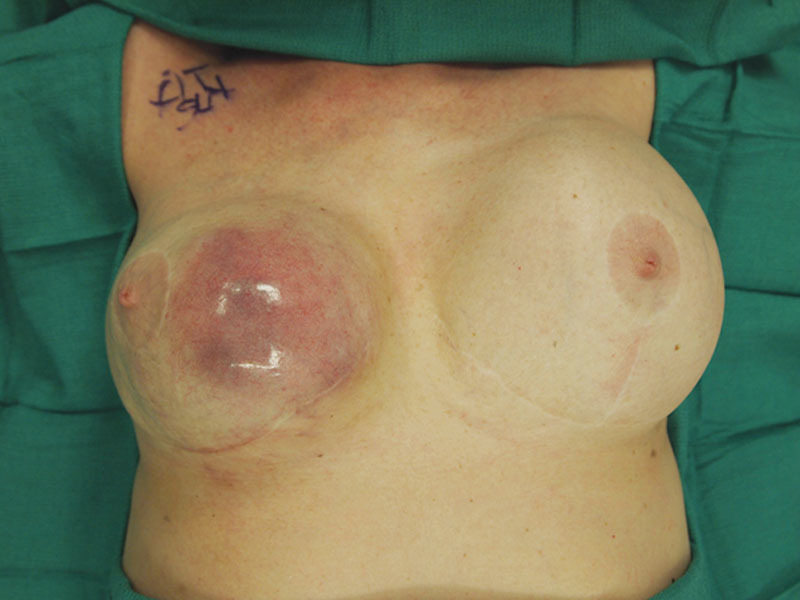
Intraoperative view of right breast (which has previously been explanted) demonstrating dramatic enlargement with obvious thinning and attenuation of the soft tissue envelope.
Fig. 3.
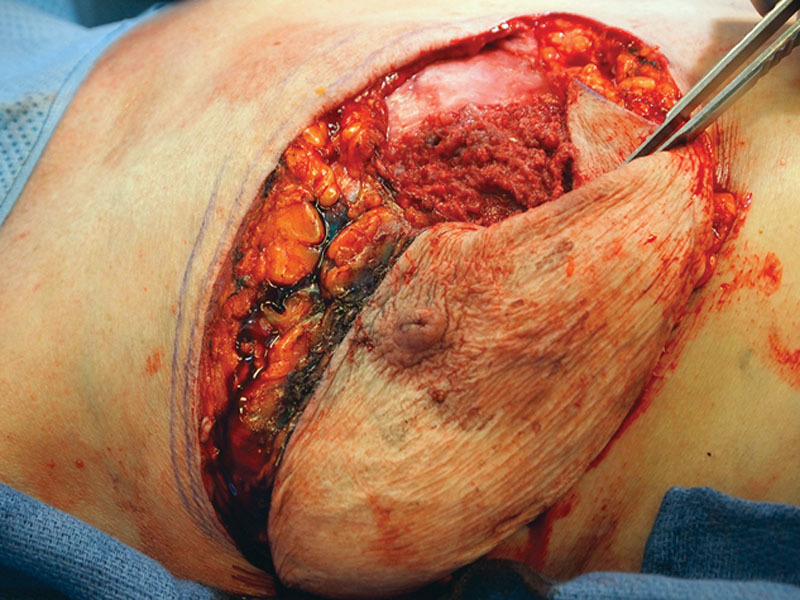
Intraoperative appearance of in situ posterior capsule.
Fig. 4.
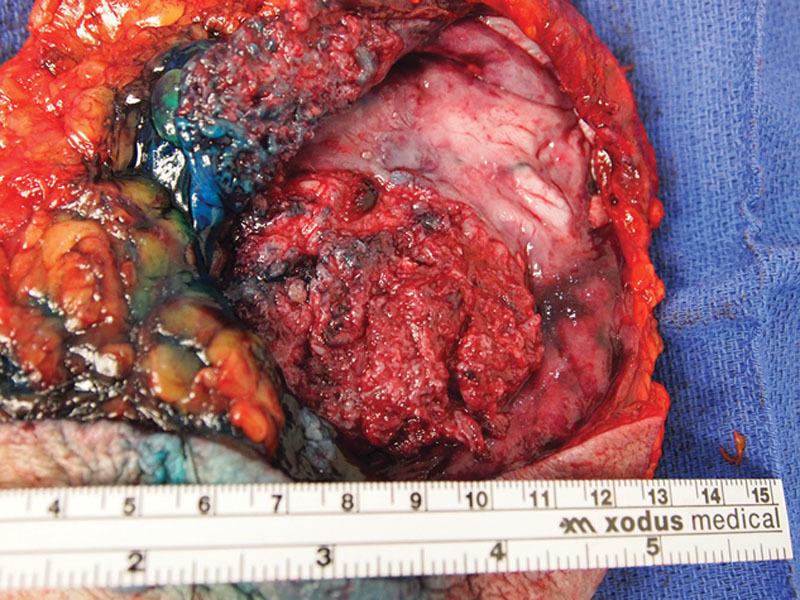
Mastectomy specimen with visible fungating mass on posterior capsule. Isosulfan blue dye present from aborted sentinel lymph node biopsy.
The patient was discharged from the hospital on postoperative day 1. Final pathology revealed 2 foci of invasive, moderately differentiated SCC arising from the implant capsule, measuring 5.5 and 3.2 cm. The capsule showed extensive squamous metaplasia and acute and chronic inflammation. The tumors were negative for estrogen receptor, progesterone receptor, and HER2/neu, and 30 lymph nodes were negative for metastatic disease. Cytology was positive for keratinizing SCC.
DISCUSSION
Primary breast SCC accounts for less than 0.1% of all breast cancers, with only 137 reported cases in the United States between 1975 and 2012.12 Likewise, breast implant-related primary malignancies are also extremely rare, with approximately 112 cases of breast implant-related anaplastic large cell lymphoma and 1 case of primary SCC having been reported in the United States.7,13
In a 2010 retrospective review of 434 capsule pathology specimens obtained from 264 patients, no new neoplasms or occult disease were discovered. However, all patients that were found to have implant-associated cancers presented with breast pain, enlargement, seroma, and painful capsular contracture outside of the typical postoperative window.14 The importance of pathological evaluation of implant-related symptoms is underscored by a 2011 consensus panel that provides guidance on managing patients presenting with delayed periprosthetic fluid collections.15
The histogenesis of SCC of the breast is unclear. Hypotheses include metaplasia from benign disease of the breast parenchyma, malignant growth of previously quiescent intrinsic epidermal elements (such as an epidermal cyst), or chronic abscess. Malignant squamous transformation is known to develop in wounds involved with chronic inflammation such as osteomyelitis and burns. However, it is unclear how epithelial cells come to reside inside the breast tissue.16 One hypothesis suggests this may occur at the time of augmentation or reconstruction. However, there is no experimental or clinical evidence to support expected findings of epithelialization of the implant capsule and subsequent squamous dysplasia in capsulectomy pathologic specimens.17,18 Current study around primary breast implant-associated anaplastic large cell lymphoma, which also presents in similar clinical fashion, may help reveal more information about this issue.13,19
CONCLUSIONS
Primary SCC arising from a breast implant capsule is an exceedingly rare occurrence with only 1 previously reported case in the literature. Although the etiology of cancer arising from an implant capsule remains unclear, many hypotheses exist. Practitioners should maintain a high index of suspicion for implant-related cancers in patients who present with delayed breast pain, enlargement, or late seroma.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Menes T, Schachter J, Morgenstern S, et al. Primary squamous cell carcinoma (SqCC) of the breast. Am J Clin Oncol. 2003;26:571–573. doi: 10.1097/01.coc.0000045809.85995.3B. [DOI] [PubMed] [Google Scholar]
- 2.Macia M, Ces JA, Becerra E, et al. Pure squamous carcinoma of the breast. Report of a case diagnosed by aspiration cytology. Acta Cytol. 1989;33:201–204. [PubMed] [Google Scholar]
- 3.Hennessy BT, Krishnamurthy S, Giordano S, et al. Squamous cell carcinoma of the breast. J Clin Oncol. 2005;23:7827–7835. doi: 10.1200/JCO.2004.00.9589. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski J, Saltzstein SL, Sadler G, et al. Squamous cell carcinoma of the breast: a review of 177 cases. Am Surg. 2009;75:914–917. [PubMed] [Google Scholar]
- 5.Behranwala KA, Nasiri N, Abdullah N, et al. Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29:386–389. doi: 10.1053/ejso.2002.1422. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO Classification of Tumors. Lyon: IARC Press; 2012. [Google Scholar]
- 7.Paletta C, Paletta FX, Jr, Paletta FX., Sr Squamous cell carcinoma following breast augmentation. Ann Plast Surg. 1992;29:425–429; discussion 429. doi: 10.1097/00000637-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Smith LF, Smith TT, Yeary E, et al. Squamous cell carcinoma of the breast following silicone injection of the breasts. J Okla State Med Assoc. 1999;92:126–130. [PubMed] [Google Scholar]
- 9.van Diest PJ, Beekman WH, Hage JJ. Pathology of silicone leakage from breast implants. J Clin Pathol. 1998;51:493–497. doi: 10.1136/jcp.51.7.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmor M, Rothaus KO, Shannahan E, et al. Squamous cell carcinoma of the breast after augmentation with liquid silicone injection. Ann Plast Surg. 1995;34:619–623. doi: 10.1097/00000637-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Bondurant S, Ernster VL, Herdman R Institute of Medicine (US). Committee on the Safety of Silicone Breast Implants . . Safety of Silicone Breast Implants, Vol. xvi. Washington, DC: Institute of Medicine; 2000:. p. 540. [Google Scholar]
- 12.Howlader N NA, Krapcho M, Garshell J, editors. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; Available at: http://seercancergov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 13.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. doi: 10.1097/PRS.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 14.Roth FS, Felder JM, Friedman JD. Breast capsulectomy specimens and their clinical implications. Plast Reconstr Surg. 2010;126:1848–1852. doi: 10.1097/PRS.0b013e3181f4463c. [DOI] [PubMed] [Google Scholar]
- 15.Bengtson B, Brody GS, Brown MH, et al. Managing late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011;128:1–7. doi: 10.1097/PRS.0b013e318217fdb0. [DOI] [PubMed] [Google Scholar]
- 16.Kitchen SB, Paletta CE, Shehadi SI, et al. Epithelialization of the lining of a breast implant capsule. Possible origins of squamous cell carcinoma associated with a breast implant capsule. Cancer. 1994;73:1449–1452. doi: 10.1002/1097-0142(19940301)73:5<1449::aid-cncr2820730520>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Peters W, Fornasier V. Late unilateral breast enlargement after insertion of silicone gel implants: a histopathological study. Can J Plast Surg. 2007;15:19–28. doi: 10.1177/229255030701500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alikhan MB, Nassar A, Mansoor I. Squamous metaplasia on the breast implant capsule. Int J Surg Pathol. 2010;18:570–574. doi: 10.1177/1066896908329587. [DOI] [PubMed] [Google Scholar]
- 19.Jewell M, Spear SL, Largent J, et al. Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plast Reconstr Surg. 2011;128:651–661. doi: 10.1097/PRS.0b013e318221db81. [DOI] [PubMed] [Google Scholar]


