Abstract
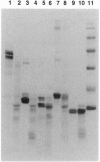
Kedarcidin is a potent antitumor antibiotic chromoprotein, composed of an enediyne-containing chromophore embedded in a highly acidic single chain polypeptide. The chromophore was shown to cleave duplex DNA site-specifically in a single-stranded manner. Herein, we report that in vitro, the kedarcidin apoprotein, which lacks any detectable chromophore, cleaves proteins selectively. Histones that are the most opposite in net charge to the apoprotein are cleaved most readily. Our findings imply that the potency of kedarcidin results from the combination of a DNA damaging-chromophore and a protease-like apoprotein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grunstein M. Histones as regulators of genes. Sci Am. 1992 Oct;267(4):68–74B. doi: 10.1038/scientificamerican1092-68. [DOI] [PubMed] [Google Scholar]
- Hensens O. D., Goldberg I. H. Mechanism of activation of the antitumor antibiotic neocarzinostatin by mercaptan and sodium borohydride. J Antibiot (Tokyo) 1989 May;42(5):761–768. doi: 10.7164/antibiotics.42.761. [DOI] [PubMed] [Google Scholar]
- Hofstead S. J., Matson J. A., Malacko A. R., Marquardt H. Kedarcidin, a new chromoprotein antitumor antibiotic. II. Isolation, purification and physico-chemical properties. J Antibiot (Tokyo) 1992 Aug;45(8):1250–1254. doi: 10.7164/antibiotics.45.1250. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Stabilization of neocarzinostatin nonprotein chromophore activity by interaction with apoprotein and with HeLa cells. Biochemistry. 1980 Oct 14;19(21):4786–4790. doi: 10.1021/bi00562a011. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Napier M. A., Goldberg I. H. Roles of chromophore and apo-protein in neocarzinostatin action. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1970–1974. doi: 10.1073/pnas.77.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Napier M. A., Goldberg I. H., Samy T. S. Requirement for reducing agents in deoxyribonucleic acid strand scisson by the purified chromophore of auromomycin. Biochemistry. 1980 Oct 14;19(21):4780–4785. doi: 10.1021/bi00562a010. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Hesler G. A., Gustavson D. R., Crosswell A. R., Veitch J. M., Forenza S., Tomita K. Kedarcidin, a new chromoprotein antitumor antibiotic. I. Taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo) 1991 May;44(5):472–478. doi: 10.7164/antibiotics.44.472. [DOI] [PubMed] [Google Scholar]
- Long B. H., Golik J., Forenza S., Ward B., Rehfuss R., Dabrowiak J. C., Catino J. J., Musial S. T., Brookshire K. W., Doyle T. W. Esperamicins, a class of potent antitumor antibiotics: mechanism of action. Proc Natl Acad Sci U S A. 1989 Jan;86(1):2–6. doi: 10.1073/pnas.86.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamber S. W., Okasinski W. G., Pinter C. D., Tunac J. B. The Escherichia coli K-12 SOS chromotest agar spot test for simple, rapid detection of genotoxic agents. Mutat Res. 1986 Aug-Sep;171(2-3):83–90. doi: 10.1016/0165-1218(86)90039-x. [DOI] [PubMed] [Google Scholar]
- Pederson D. S., Thoma F., Simpson R. T. Core particle, fiber, and transcriptionally active chromatin structure. Annu Rev Cell Biol. 1986;2:117–147. doi: 10.1146/annurev.cb.02.110186.001001. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Huisman O., D'Ari R., Hofnung M. SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K-12 to measure genotoxicity. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5971–5975. doi: 10.1073/pnas.79.19.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R., Wachtel E. J. The histones. Adv Protein Chem. 1981;34:1–60. doi: 10.1016/s0065-3233(08)60517-3. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Shiraki T., Konishi M., Oki T. DNA intercalation and cleavage of an antitumor antibiotic dynemicin that contains anthracycline and enediyne cores. Proc Natl Acad Sci U S A. 1990 May;87(10):3831–3835. doi: 10.1073/pnas.87.10.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y., Uesawa Y., Takahashi Y., Kuwahara J., Golik J., Doyle T. W. Nucleotide-specific cleavage and minor-groove interaction of DNA with esperamicin antitumor antibiotics. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7672–7676. doi: 10.1073/pnas.86.20.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P. New insights into chromatin function in transcriptional control. FASEB J. 1992 Dec;6(15):3354–3361. doi: 10.1096/fasebj.6.15.1464369. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Naoi N., Hidaka T., Watanabe K., Kumada Y., Takeuchi T., Umezawa H. Studies on auromomycin. J Antibiot (Tokyo) 1979 Apr;32(4):330–339. doi: 10.7164/antibiotics.32.330. [DOI] [PubMed] [Google Scholar]
- Zaheer A., Zaheer S., Montgomery R. Peptidase activity of macromomycin apoprotein. J Biol Chem. 1985 Sep 25;260(21):11787–11792. [PubMed] [Google Scholar]
- Zein N., Colson K. L., Leet J. E., Schroeder D. R., Solomon W., Doyle T. W., Casazza A. M. Kedarcidin chromophore: an enediyne that cleaves DNA in a sequence-specific manner. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2822–2826. doi: 10.1073/pnas.90.7.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein N., Poncin M., Nilakantan R., Ellestad G. A. Calicheamicin gamma 1I and DNA: molecular recognition process responsible for site-specificity. Science. 1989 May 12;244(4905):697–699. doi: 10.1126/science.2717946. [DOI] [PubMed] [Google Scholar]
- Zein N., Sinha A. M., McGahren W. J., Ellestad G. A. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988 May 27;240(4856):1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]