Abstract
Background:
There is considerable variation in the planning and implementation process for breast augmentation. Although general guidelines are available, the distinctive characteristics of the Natrelle 410 breast implant warrant surgical guidelines specific to this device. This study aimed to develop consensus recommendations for patient selection and preoperative planning for Natrelle 410 in primary breast augmentation.
Methods:
Surgeons were invited to participate in this study, which used a modified Delphi method. Participants completed 2 rounds of online surveys, with the second round (Recommendations Survey) based on responses from the first round. Respondents also listed their top priorities for using Natrelle 410 implants.
Results:
Participants (n = 22) reached consensus on 15 of 18 criteria for patient selection; tuberous breasts, patient preference regarding upper pole shape, and asymmetry of the breasts were the top 3 patient characteristics considered appropriate for the use of Natrelle 410. Consensus was reached on 38 of 51 items related to preoperative planning, with 8 measurements and 6 markings recommended by the participants. Patient-desired outcome was considered the most essential element for Natrelle 410 implant selection; quality of skin envelope and height and width dimension of the breast were selected as the most essential elements for Natrelle 410 implant volume selection.
Conclusions:
The modified Delphi method resulted in consensus recommendations for patient selection and preoperative planning in primary breast augmentation with the Natrelle 410 breast implant. These recommendations and priorities, used in concert with a surgeon’s clinical experience, are designed to optimize surgical outcomes.
Breast augmentation is a common cosmetic surgical procedure, with more than 1.2 million procedures performed annually worldwide.1,2 Well-described approaches to planning and performing breast augmentation represent a progressive understanding of the impact that defined approaches can have on successful outcomes.3–7 However, considerable variations in approach still exist in clinical practice, partly because of aesthetic differences, surgical preferences, and anatomic variations. The unique characteristics of different implant types may warrant refinement of current best practices in breast augmentation.
The Natrelle 410 breast implant (Allergan, Irvine, Calif.) is a teardrop-shaped (anatomic), form-stable, textured silicone implant designed to mimic the natural slope of the breast.7,8 Natrelle 410 is manufactured in 12 styles based on a matrix of 3 implant height options and 4 implant projection options.8 A 10-year, multicenter study8,9 supported the long-term safety and effectiveness of Natrelle 410. As these implants were approved in Europe in 1993, Brazil in 1998, and Canada in 2006, but not until 2013 in the United States,9 US surgeons may benefit from recommendations of those who are more experienced with Natrelle 410. General guidelines for breast augmentation are available in the literature,3–6 but distinctive characteristics of the Natrelle 410 implant—including the textured Biocell shell surface facilitating tissue integration and minimizing the risk of rotation7,10 and the multiple anatomic shapes of the different Natrelle 410 implants with regard to the point of maximum projection, angle of inclination, and slope of the upper pole—warrant surgical guidelines specific to this device. In addition, Natrelle 410 implants are available with 2 types of silicone gel fillers (cohesive and highly cohesive) that differ from other anatomic implants. The present study solicited perspectives from an international group of surgeons using a modified Delphi method to establish consensus recommendations for optimizing outcomes with Natrelle 410 breast implants for primary augmentation. The Delphi method utilizes multiple iterative rounds of questionnaires to gather data and test hypotheses, allowing for objective assessment and statistical analyses of judgment decisions.11 This analysis focuses on consensus recommendations and top planning priorities for patient selection and preoperative planning; recommendations for intraoperative technique and postoperative management are reported separately.
METHODS
The author group comprised surgeons from Brazil, Canada, China, Sweden, and the United States. Surgeons were selected and invited by Allergan to participate in this Delphi study. Invited surgeons were known to Allergan and to the surgical community as having extensive experience with Natrelle 410 implants in clinical practice and/or clinical trials. Survey participants were informed that this Allergan-sponsored study would lead to 2 articles written by 5 preidentified surgeons and 1 Allergan author. The goal was to provide surgeons with a standardized procedural and technical framework for optimizing outcomes in breast augmentation with Natrelle 410 implants. The participants’ identities remained blinded to the authors and to each other; all survey responses remained anonymous.
The authors collaborated to develop 2 rounds of surveys using the Delphi method (Fig. 1). The first (Personal Practice Survey) was based on the authors’ own expertise. The second (Recommendations Survey) was developed based on responses to the first survey. Links to the online surveys were sent to participants via E-mail. The surveys were administered via Survey Monkey (SurveyMonkey; www.surveymonkey.com, Palo Alto, Calif.). Responses to all items were required.
Fig. 1.
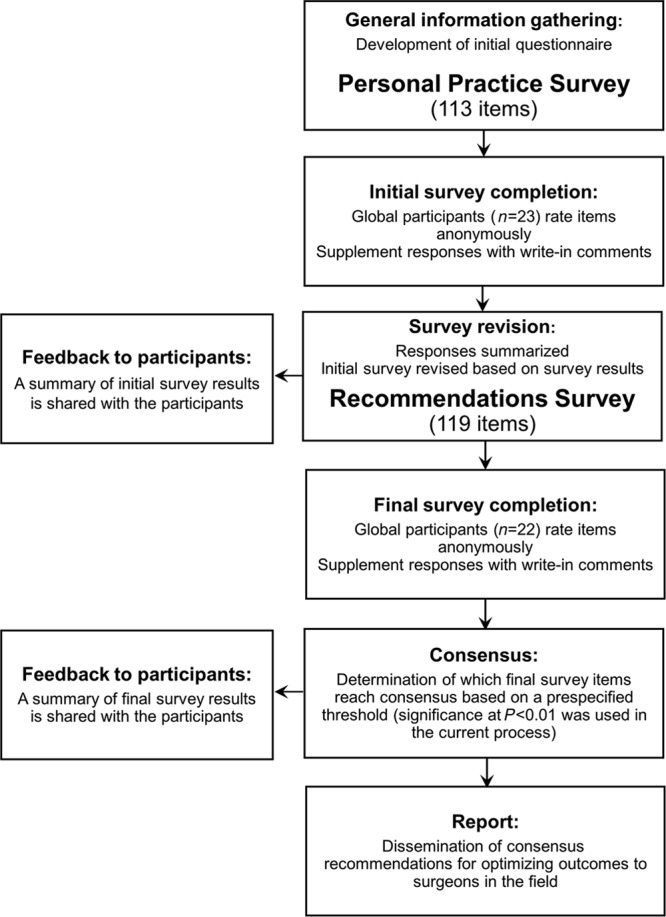
Delphi method, study design.
Personal Practice Survey
The initial phase focused on gathering general information regarding the participants’ practices when using Natrelle 410 implants in primary breast augmentation. Participants provided responses regarding their own approaches to 71 items related to patient selection and preoperative planning. Participants could supplement responses with write-in comments. Results of the Personal Practice Survey were shared with participants and used as a guide for the authors to develop the Recommendations Survey.
Recommendations Survey
The authors restructured the Personal Practice Survey based on participants’ responses and write-in comments to develop the Recommendations Survey. The Recommendations Survey collected the participants’ feedback in 2 ways: it first elicited their agreement (or disagreement) with a list of practices in the use of Natrelle 410, then elicited their choice of the top planning priorities among those practices, specifically regarding the Natrelle 410 implant.
The survey contained a series of Topic Statements worded to elicit participants’ recommendations for use of Natrelle 410 in patient selection and preoperative planning. Each Topic Statement was followed by a corresponding list of items, each requiring a response. The patient selection section comprised 1 Topic Statement with 18 related items (Table 1). The preoperative planning section comprised 7 Topic Statements, each followed by 3–18 items for a total of 51 preoperative planning items (Table 2). Response options for each item were “Agree,” “Neutral,” and “Disagree.” Some items may not have pertained to certain participants. For example, if an earlier response indicated that a participant did not recommend a particular type of preoperative measurement, a response concerning how that measurement should be performed was not expected. Therefore, some items included a fourth response option (ie, “I do not measure…” or “I do not determine…”). After participants provided responses for the items listed under each Topic Statement, they were asked to indicate their top 3 choices from the items associated with that Topic Statement.
Table 1.
Patient Selection with Natrelle 410 Implants for Primary Breast Augmentation: Participant Responses and Percent Agreement.
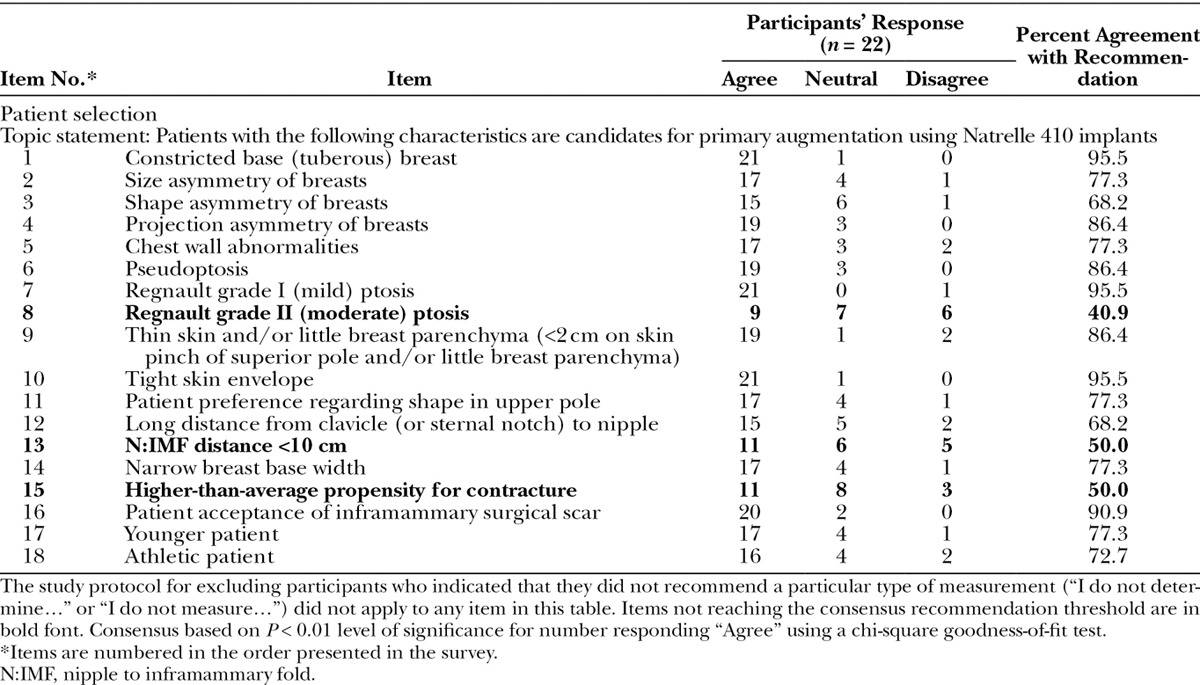
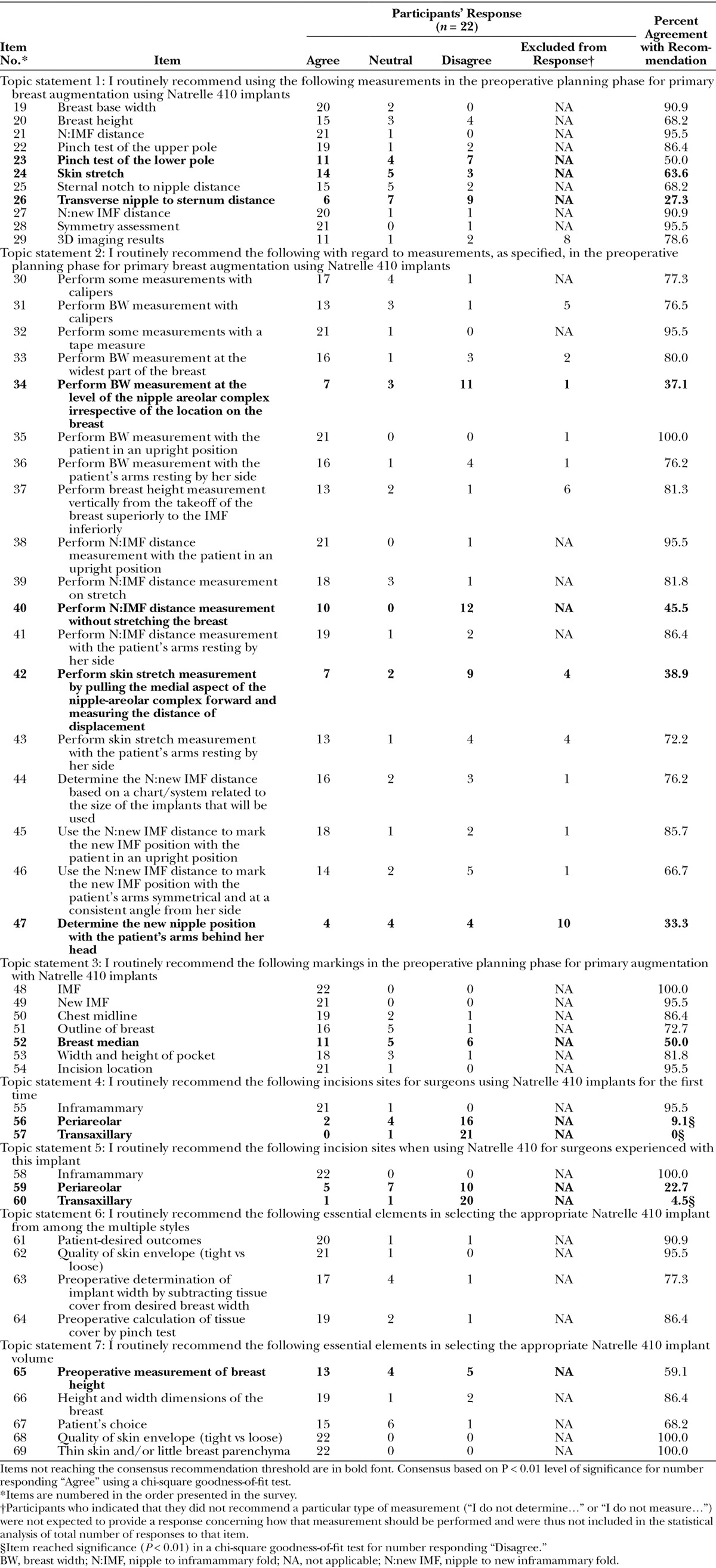
Table 2.
Preoperative Planning with Natrelle 410 Implants for Primary Breast Augmentation: Participant Responses and Percent Agreement.
Statistical Analysis
The threshold criterion for reaching consensus level agreement on items in the Recommendations Survey was based on a chi-square goodness-of-fit test. Response to an item was considered to have reached consensus level of agreement if the number of participants who responded “Agree” reached statistical significance at the P < 0.01 level. A P < 0.01 level of significance for items with 3 possible responses (ie, “Agree,” “Neutral,” and “Disagree”) correlated with at least 63.65% of participants giving the same response for that particular item. Participants choosing the responses “I do not measure…” or “I do not determine…” were excluded from the statistical analysis of that particular item; therefore, threshold percentages for items to which 1 or more participants responded “I do not measure…” or “I do not determine…” varied according to the number of participants included in the analysis.
RESULTS
Of 30 surgeons invited to take part in the consensus process, 23 agreed to participate. All participants completed the Personal Practice Survey. Of these, 22 (96%) completed the Recommendations Survey and are included in this analysis. Survey participants were practicing in Europe/Middle East (n = 7), the United States (n = 6), Canada (n = 4), Latin America (n = 3), Asia (n = 1), and Australia (n = 1).
Patient Selection Recommendations
Participants responding to the Recommendations Survey items regarding candidates for primary breast augmentation with Natrelle 410 implants reached consensus on a range of patient characteristics, including 15 of the 18 items. Responses to the patient selection items are shown in Table 1; items that did not reach consensus for recommendation in boldface.
Preoperative Planning Recommendations
Consensus was reached on 38 of 51 items related to preoperative planning for breast augmentation with Natrelle 410 implants (Table 2; items in boldface did not reach consensus for recommendation). Participants reached consensus on 8 of 11 preoperative measurement selections (Topic Statement 1), 14 of 18 recommendations for how to perform those measurements (Topic Statement 2), and 6 of 7 preoperative marking selections (Topic Statement 3). They reached consensus on recommending an inframammary incision site for surgeons using Natrelle 410 implants for the first time and for those experienced with the implants (Topic Statements 4 and 5, respectively). The number of “Disagree” responses reached statistical significance (P < 0.01) for 3 of the incision site items: Among the 22 participants, 16 disagreed with using the periareolar incision and 21 would not recommend the transaxillary approach for first-time use; 20 participants would not recommend the transaxillary approach for surgeons experienced with the implant. Consensus was reached regarding the determination of the best Natrelle 410 implant type for each patient, with significant agreement on 4 of 5 items for selecting style (Topic Statement 6) and 4 of 4 items for selecting volume (Topic Statement 7).
Top Recommendations for Natrelle 410
Participants most often listed constricted base (tuberous) breast (n = 13), patient preference regarding shape in the upper pole (n = 7), and shape asymmetry of the breasts (n = 6) as the top 3 characteristics of candidates for the use of the Natrelle 410 implant in the patient selection section of the survey (representative examples of appropriate candidates for Natrelle 410 implants are shown in Figures 2–5). Top priorities identified by participants for preoperative measurements, markings, and implant selection with Natrelle 410 implants are listed in Table 3. Priorities endorsed by the greatest number of respondents included breast base width as the top measurement for Natrelle 410 (16/20 participants), new inframammary fold (IMF) as the top marking for Natrelle 410 (19/22), patient-desired outcomes as the most essential element for implant selection (16/22), and quality of skin envelope (21/22) and height and width dimension of breast (20/22) as the most essential elements for implant volume selection.
Fig. 2.
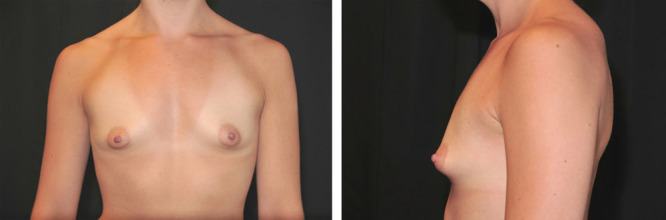
A 31-year-old woman with tuberous breasts, empty lower pole, and convexity in the upper chest wall is shown before implantation.
Fig. 5.

The 39-year-old woman who had breast asymmetry before implantation is shown at 6 months after implantation. Natrelle 410 implants with full height and moderate projection (235 g) and with moderate height and low projection (170 g) were used on the right and left sides, respectively.
Table 3.
Survey Participants’ Priorities for Preoperative Planning With Natrelle 410 Implants

Fig. 3.
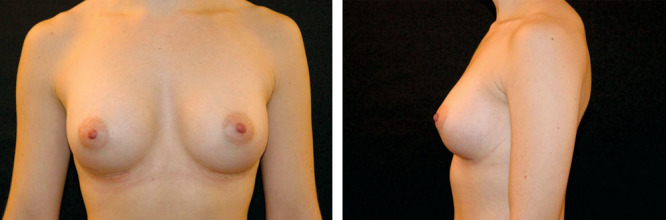
The 31-year-old woman who had tuberous breasts, empty lower pole, and convexity in the upper chest before implantation is shown at 6 months after implantation. Natrelle 410 implants with moderate height and full projection (335 g) were used in each breast. Note the natural slope, despite the preoperatively projecting upper chest wall.
Fig. 4.

A 39-year-old woman with breast asymmetry—her left breast was 60 cc larger and projected 8 mm more than her right breast—is shown before implantation.
DISCUSSION
Consensus recommendations for surgical practice indicate preferred approaches to clinical problems as established by experts in the field. They are based on existing data or on a consensus of expert opinion when few or no data are available. The present recommendations apply to surgeons who use Natrelle 410 for primary breast augmentation. Users of other devices may not find these recommendations useful or suitable to their practice, as the surgeons’ feedback in this study was solicited specifically regarding Natrelle 410 and reflect their experience with this device and its specific characteristics. These recommendations indicate experienced surgeons’ preferred approaches with this device, although they are not necessarily the only approaches, owing to the complexity of aesthetic surgery.
Patient Selection
Most of the surgeons surveyed seemed to have a well-defined perspective on appropriate patient selection and use of biodimensional planning with regard to Natrelle 410. Recommendations for patient selection indicated that Natrelle 410 may be used in a wide variety of patient types, although participants considered some characteristics more valuable than others in identifying the best candidates.
Several consensus items are worth noting. Participants agreed that the form-stable structure of Natrelle 410 is particularly beneficial for cases of tuberous breast, which was among the characteristics reaching the highest level of agreement (95.5%) and was most frequently mentioned in the top 3 characteristics for patients appropriate for augmentation with Natrelle 410 implants. Patient preference regarding shape in the upper pole (eg, more natural appearance) and breast shape asymmetry rounded out the top 3 characteristics. The latter 2 items were not among those receiving the most “agree” responses, but they were most often rated by the respondents as top characteristics in patient selection, specifically regarding the use of the Natrelle 410 implant.
Based on rates of participant agreement, the anatomic shape selections were considered well suited for patients with Regnault grade I ptosis, tight skin envelope, and/or acceptance of inframammary surgical scar (each reaching >90% agreement). Consensus was reached for treatment of pseudoptosis, where height options for the implant may be an advantage over round devices. In the authors’ opinion, patients with asymmetry related to size, shape, and/or projection of breasts or with chest wall abnormalities may be good candidates for Natrelle 410 implants, as the variety of available shapes makes it possible to compensate for these asymmetries.
Some characteristics did not seem to be key decision factors for the use of Natrelle 410. Lack of consensus regarding patients with Regnault grade II ptosis may represent a concern with rotation risk, double-bubble deformity, or the need for mastopexy in addition to augmentation. Prediction of postoperative nipple position and calculation of the amount of skin needed in the lower pole using the lower ventral curvature of the implant7 could be used to evaluate whether a ptotic breast may be corrected with an implant alone. In the authors’ opinion, lower height, higher projection implants are more suitable than others for correcting ptosis. Interestingly, consensus was not reached regarding patients with a higher-than-average propensity for capsular contracture, despite evidence in the literature indicating that Natrelle 410 implants are associated with a low rate of capsular contracture versus smooth surface and other anatomic implants.6,9,12 Participants commented that assessing whether a patient had a high propensity of contracture would be difficult in the setting of primary augmentation.
Preoperative Planning
Previously published approaches to breast augmentation provide details on measurements and markings for anatomic breast implants that are generally in agreement with the consensus recommendations for Natrelle 410 reached in this analysis.6,7 Certain key measurements seem to be generally appropriate for any breast augmentation,4,13 but some are particularly important with shaped implants wherein the device must fit more precisely. Greater than 90% agreement was reached by participants for recommending breast base width, nipple-to-IMF distance, and nipple-to–new IMF distance measurements, and assessment for symmetry when using Natrelle 410 implants. The rates of agreement suggest that participants place more importance on analyzing implant width versus height. Indeed, breast base width was considered the most important measurement for Natrelle 410 by the greatest number of respondents. In the authors’ view, however, implant height is at least equally important to implant width, especially when assessing a breast with well-defined dense glandular tissue and a short lower pole. More than one-third of all participants indicated that they do not have access to 3-dimensional (3D) imaging, but among those who do, a recommendation for using results of this technology in preoperative planning with Natrelle 410 implants reached consensus. Computational modeling based on 3D surface scans has shown promising results for predicted outcomes14; however, commercially available systems do not take biomechanical soft-tissue behavior into account. Patients should be informed of the limitations of this imaging technique before consenting to undergo the assessment.
It is notable that participants did not reach consensus regarding recommendations for a pinch test in the lower pole and a skin stretch assessment, published by some experts to be high-priority measurements.4,7,13 Lack of consensus on assessment of skin stretch may be attributable to difficulty in obtaining an accurate objective assessment. There was also little agreement on the 3 most important measurement-related items specific to Natrelle 410. A recommendation for performing nipple-to-IMF distance measurement without stretching the breast did not reach consensus even though it was the item selected by the greatest percentage of respondents as top priority. Measuring nipple-to-IMF distance without stretch, in the authors’ opinion, is not reproducible and does not reflect accurate assessment. Determining the new nipple position with the patient’s arms behind her head also did not reach consensus; however, this measurement technique may be useful for determining preoperative markings and setting the new IMF. It has been demonstrated that arm elevation 45 degrees above the horizontal plane accurately predicts the new postaugmentation nipple position.7 It is possible that there are sufficient variations in performing new nipple measurements (eg, position of arms) that precluded the participants from reaching consensus. Participants were more united in their selection of the 3 most important markings for Natrelle 410: new IMF, width and height of pocket, and the incision location.
Nearly all participants recommended inframammary incisions for experienced and first-time users of Natrelle 410. A significant percentage of participants disagreed with recommending the transaxillary approach for either first-time or experienced Natrelle 410 users (P < 0.01). Although the transaxillary approach is generally favored in certain geographic regions and can be effective in an experienced surgeon’s hands, its use, particularly with anatomically shaped implants, has some limitations.7,15–17 First, the remote incision may increase the occurrence of malposition, and an overdissection of the pocket could result in an implant-envelope disproportion that may allow the implant to rotate. Additionally, revision surgery often requires an inframammary incision, resulting in a second scar.
Participants significantly disagreed with recommending the periareolar approach for first-time users of Natrelle 410, although results for this approach by surgeons experienced with this implant were mixed. In the authors’ experience, the inframammary approach is well suited for the use of Natrelle 410 because of the ease of dissection and placement, particularly for surgeons without extensive experience with this implant. Additionally, the inframammary approach has been associated with a lower risk for capsular contraction versus periareolar and transaxillary approaches.18,19 Nonetheless, the authors believe that the periareolar approach is a reasonable option, especially in tuberous breast cases, and particularly in the hands of surgeons experienced with Natrelle 410. If it is not necessary to move the IMF, the periareolar incision may be as effective as inframammary incision.
Survey participants recommended consideration of multiple factors when choosing Natrelle 410 style and volume. Consensus was reached on using tissue-based implant selection considering height and width dimensions of the breast and quality of the skin envelope and breast parenchyma, both described as top priorities for Natrelle 410. However, the importance of patient preference was also considered a top priority for the selection of Natrelle 410. Thus, although the ideal implant based on tissue conditions may be determined, some divergence based on patient preference may be acceptable. It is important to emphasize that it is the surgeon’s responsibility to inform patients of what is and is not possible, and that the foundation for selecting proper implant volume and dimension relates to both tissue conditions and patient preferences.
Study Limitations
Several limitations of this study should be noted. There are no universally accepted consensus thresholds for the Delphi method,20,21 which may make interpretation of results challenging. In this study, we chose a P < 0.01 level of significance to define the consensus threshold and provide statistical rigor to the analysis. Also, lack of consensus does not necessarily mean that the item is inappropriate for Natrelle 410; rather, it was not supported by the majority. Differences in the respondents’ geography, practice patterns, and patient distribution may substantially affect their experience with, and perceptions of, the Natrelle 410 implant, and therefore result in important differences in their recommendations that could appear as lack of consensus. Finally, participants did not supply rationales for their responses, as this level of information gathering is not generally part of the Delphi method. Nonetheless, this is the first time the Delphi method has been used to provide consensus recommendations for plastic surgery.
Conclusions and Implications for Clinical Practice
Experts in the field of breast augmentation surgery shared their approaches to optimizing surgical results achieved with the Natrelle 410 implant. Through the Delphi method, they provided consensus recommendations regarding patient characteristics and preoperative planning. Notably, the participants also provided feedback on what they considered the most important recommendations, specifically for the Natrelle 410 implant. These recommendations may serve as a basis for modification or refinement of a surgeon’s current procedures. They may also lend assurance to surgeons having less experience with the Natrelle 410 implant. Evolving information regarding the use of Natrelle 410 may have produced some important factors for patient selection and preoperative planning that are not included in the current recommendations. Such items may be worthy of future reassessment. Additional topics for evaluation could include ptosis management, revision and reconstruction procedures, and the use of Natrelle 410 implants in association with internal support matrices and fat, as well as detailed methods for carrying out the consensus recommendations reached in the current study.
The surgeon must choose the course best suited to individual patients based on a range of variables present at the moment of decision. However, the current consensus recommendations may play an important role in the optimization of aesthetic breast augmentation with Natrelle 410 implants.22
ACKNOWLEDGMENTS
We thank Svetlana Pidasheva, PhD, and Damien Bates, MD, PhD, FRACS, who were employees of Allergan at the time of this study, for their contributions to the study design. We also thank Dr. Bates for data interpretation. Writing and editorial assistance was provided to us by Kathleen Dorries, PhD, of Peloton Advantage, Parsippany, N.J. All authors meet the ICMJE authorship criteria.
Footnotes
Disclosure: This study was sponsored by Allergan, Irvine, Calif. Dr. Hedén serves as a consultant for Allergan and has received honoraria for lectures and travel from that company. Dr. Brown serves as a consultant for Allergan and as a speaker for LifeCell. Dr. Luan has no conflicts of interest to disclose. Dr. Maxwell serves as a consultant for and has received royalties from Allergan and is a consultant for LifeCell. Dr. Mendonça Munhoz serves as a consultant for Allergan. Dr. Carter was an employee of Allergan at the time of this study and during manuscript preparation. Neither honoraria nor other form of payments were made for authorship. The Article Processing Fee was paid for by Allergan.
REFERENCES
- 1.American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank Statistics 2013. Available at: http://www.surgery.org/sites/default/files/Stats2013_4.pdf. Accessed September 24, 2015. [DOI] [PubMed] [Google Scholar]
- 2.International Society of Aesthetic Plastic Surgeons. ISAPS International Survey on Aesthetic/Cosmetic Procedures Performed in 2011. Available at: http://www.isaps.org/Media/Default/global-statistics/ISAPS-Results-Procedures-2011.pdf. Accessed September 24, 2015. [Google Scholar]
- 3.Tebbetts JB. A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics. Plast Reconstr Surg. 2002;109:1396–1409. doi: 10.1097/00006534-200204010-00030. [DOI] [PubMed] [Google Scholar]
- 4.Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. 2005;116:2005–2016. [PubMed] [Google Scholar]
- 5.Berry MG, Cucchiara V, Davies DM. Breast augmentation: Part III–preoperative considerations and planning. J Plast Reconstr Aesthet Surg. 2011;64:1401–1409. doi: 10.1016/j.bjps.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Hedén P, Jernbeck J, Hober M. Breast augmentation with anatomical cohesive gel implants: the world’s largest current experience. Clin Plast Surg. 2001;28:531–552. [PubMed] [Google Scholar]
- 7.Heden P. Breast augmentation with anatomic, high-cohesiveness silicone gel implants (European experience). In: Spear SL, Willey SC, Robb GL, editors. In: Surgery of the Breast: Principles and Art. 3rd ed. Philadelphia, Pa.: Lippincott, Williams and Wilkins; 2011. pp. 1322–1345. [Google Scholar]
- 8.Bengtson BP, Van Natta BW, Murphy DK, et al. Style 410 U.S. Core Clinical Study Group. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):40S–48S. doi: 10.1097/01.prs.0000286666.29101.11. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32:709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 10.Barone FE, Perry L, Keller T, et al. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;90:77–86. doi: 10.1097/00006534-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Dalkey N, Helmer O. An experimental application of the Delphi Method to the use of experts. Manag Sci. 1963;9:458–467. [Google Scholar]
- 12.Namnoum JD, Largent J, Kaplan HM, et al. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66:1165–1172. doi: 10.1016/j.bjps.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Adams WP., Jr . Tissue-based planning. In: Adams WP Jr, editor. In: Breast Augmentation: McGraw-Hill Plastic Surgery Atlas. New York, N.Y.: The McGraw-Hill Companies, Inc.; 2011. pp. 12–19. [Google Scholar]
- 14.Donfrancesco A, Montemurro P, Hedén P. Three-dimensional simulated images in breast augmentation surgery: an investigation of patients’ satisfaction and the correlation between prediction and actual outcome. Plast Reconstr Surg. 2013;132:810–822. doi: 10.1097/PRS.0b013e3182a014cb. [DOI] [PubMed] [Google Scholar]
- 15.Munhoz AM, Fells K, Arruda E, et al. Subfascial transaxillary breast augmentation without endoscopic assistance: technical aspects and outcome. Aesthetic Plast Surg. 2006;30:503–512. doi: 10.1007/s00266-006-0017-8. [DOI] [PubMed] [Google Scholar]
- 16.Adams WP, Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg. 2012;130:597e–611e. doi: 10.1097/PRS.0b013e318262f607. [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;133:567e–583e. doi: 10.1097/PRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 18.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132:1115–1123. doi: 10.1097/01.prs.0000435317.76381.68. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson JM, Gatti ME, Schaffner AD, et al. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32:456–462. doi: 10.1177/1090820X12444267. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CC, Sandford BA. The delphi technique: making sense of consensus. Practical Assess Res Eval. 2007;12:1–8. [Google Scholar]
- 21.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Natrelle Silicone-Filled Breast Implants [Directions for Use] Santa Barbara, Calif.: Allergan, Inc.; 2009. [Google Scholar]


