Abstract
Background:
Anatomically shaped, form-stable Natrelle 410 breast implants were approved in Europe in 1993 and in the United States in 2013. Although general guidelines for breast augmentation are available, the distinctive characteristics of Natrelle 410 warrant specific guidelines for this device. The goal of this study was to generate consensus recommendations for intraoperative technique and postoperative management with Natrelle 410 in primary breast augmentation.
Methods:
Surgeons were invited to participate in the study, which used a modified Delphi method. Participants completed 2 rounds of online surveys; the second survey (Recommendations Survey) was generated based on first survey results. Respondents also listed top priorities for use of Natrelle 410.
Results:
Participants (n = 22) reached consensus on 15 of 18 perioperative and surgical techniques; dual-plane placement, tight pockets, and limiting the boundaries of dissection were among intraoperative techniques considered most important for Natrelle 410. Consensus was reached for 18 of 32 items regarding postoperative management and 6 of 9 open-ended postoperative activity restrictions. Consensus on activity restrictions with specified time limits were similar to consensus recommendations on general restrictions. Top participant-identified intraoperative and postoperative management practices for Natrelle 410 were dual-plane placement of the implant and wearing a bra postoperatively, respectively.
Conclusions:
The Delphi method identified consensus recommendations on a broad range of intraoperative techniques and postoperative management practices for primary breast augmentation with Natrelle 410. These recommendations and priorities provide surgeons with a framework that, together with the surgeon’s experience, will contribute to optimal clinical outcomes with Natrelle 410.
A successful outcome in breast augmentation surgery requires careful consideration of 4 elements: patient selection, preoperative planning, intraoperative technique, and postoperative management. There are numerous published approaches to breast augmentation, but each varies in areas of emphasis and level of detail, particularly regarding intraoperative technique1–4 and postoperative management.3,5,6 In addition, surgeons have developed their own individual processes and procedures based on clinical training and day-to-day experience. Although a variety of approaches to breast augmentation surgery have been used successfully, some aspects of the surgeon’s approach might best be customized to the specific type of implant used in each case.
The Natrelle 410 breast implant (Allergan, Inc., Irvine, Calif.) is an anatomically shaped, form-stable, textured silicone implant designed to resemble the natural slope of the breast.2,7 It was first approved for use in Europe in 1993,7 in South America in 1998, and in Canada in 2006 and became available in the United States in 2013. Consequently, although the long-term safety and effectiveness of Natrelle 410 have been demonstrated in a prospective, 10-year, multicenter study conducted in the United States,7–9 US surgeons are generally less experienced with Natrelle 410 implants compared with their colleagues in other parts of the world. Although more general surgical guidelines are available,1,10–12 recommendations focused specifically on the use of Natrelle 410 will be valuable to surgeons because of the implant’s particular characteristics. These include the textured Biocell (Allergan, Inc., Irvine, Calif.) shell surface facilitating tissue integration13 and the specific anatomic shapes of the different Natrelle 410 implants with regard to point of maximum projection, angle of inclination, and slope of the upper pole. The objective of the current study was to arrive at consensus recommendations among experienced surgeons regarding the optimal use of Natrelle 410 implants for primary breast augmentation.
This study is the first to employ a modification of the Delphi method to generate recommendations for primary breast augmentation surgery. The Delphi method is a consensus-building process using a series of questionnaires to arrive at a convergence of opinion from selected individuals on a specific issue.14,15 No Delphi consensus articles on aesthetic surgery are found in the literature. However, the Delphi method has been used to arrive at consensus evaluations in an analysis of factors contributing to aesthetic outcomes in conservative treatment of breast cancer.16 In the current study, recommendations were generated for optimizing patient selection, preoperative planning, intraoperative techniques, and postoperative management in breast augmentation surgery using Natrelle 410 implants. Consensus recommendations for patient selection and preoperative planning are presented in a separate article.17 We report here on consensus recommendations and top priorities for intraoperative techniques and postoperative management of patients receiving Natrelle 410 implants.
METHODS
A modified Delphi method was used to reach consensus among international surgeons with expertise in breast augmentation surgery. Invited surgeons were known to Allergan and to the surgical community as having extensive experience with Natrelle 410 implants in clinical practice and/or clinical trials. Detailed methods are described by Hedén et al 201517 and are summarized here briefly. The authors collaborated to develop 2 Web-based surveys focused on the topics of intraoperative technique and postoperative management with Natrelle 410 implants. Both surveys were administered via Survey Monkey. The survey participants’ identities remained blinded to the authors and to each other, and their responses remained anonymous. Participants were informed that the study was sponsored by Allergan and that the consensus results would be reported by the authors.
Personal Practice Survey
The initial survey (Personal Practice Survey) queried participants on their specific practices for using Natrelle 410 implants in primary breast augmentation. The Personal Practice Survey included 113 items, 42 related to intraoperative technique and postoperative management. Participants were able to supplement their responses with write-in comments. The results of the Personal Practice Survey were shared with the participants and used as a guide for the authors to develop the subsequent Recommendations Survey.
Recommendations Survey
In the final phase of the modified Delphi process, the Personal Practice Survey was restructured and modified based on the participants’ responses and write-in comments. The resulting Recommendations Survey consisted of several Topic Statements, worded to elicit participants’ recommendations for use of Natrelle 410 implants in intraoperative technique and postoperative management (eg, “I routinely recommend the following intraoperative techniques with Natrelle 410 implants”). Each Topic Statement was followed by a list of corresponding items that required a response from the participants. The Topic Statements and their corresponding items are listed in Tables 1 and 2. The “Intraoperative Technique” section comprised 1 Topic Statement, with 18 related items. Two additional items in this section asked participants to identify antimicrobials/antiseptics that they use to irrigate implants and implant pockets from a list of 5 options. The “Postoperative Management” section comprised 4 Topic Statements, with a total of 32 related items.
Table 1.
Intraoperative Technique with Natrelle 410 Implants for Primary Breast Augmentation

Table 2.
Postoperative Planning with Natrelle 410 Implants for Primary Breast Augmentation
In most cases, there were 3 response options for each item: “Agree,” “Neutral,” and “Disagree.” Response to some items was not applicable to certain participants. For example, if a participant provided an earlier response indicating that they do not recommend a particular type of postoperative restriction, they would not be expected to provide a response concerning the duration of that restriction. For this reason, some items included a fourth response option (eg, “I do not use…” or “I do not restrict…”). Participants were also asked to identify their top 3 priorities for the Natrelle 410 implant from the list of items associated with each Topic Statement. For example, participants were asked to indicate the top 3 intraoperative techniques that are most important for Natrelle 410.
Statistical Analysis
The threshold criterion for responses to an item reaching consensus level in the Recommendations Survey was set at the P < 0.01 level of significance when measuring agreement of answers using a chi-square goodness-of-fit test. Participants were excluded from the statistical analysis of any item for which they chose the “I do not use…” or “I do not restrict…” response. The P < 0.01 threshold correlated with 63.65% “Agree” responses for all items with 3 possible responses (ie, “Agree,” “Neutral,” or “Disagree”); threshold percentages for items that included responses of “I do not use...” or “I do not restrict…” varied according to the number of participants included in the chi-square test. Items for which a statistically significant percentage of participants responded “Disagree” were also noted.
The 2 questions on specific antimicrobials/antiseptics (items 12 and 14) were not statistically analyzed; rather, they were evaluated for trends in usage.
RESULTS
A total of 30 surgeons practicing worldwide were invited to participate: 7 declined and 23 completed the Personal Practice Survey. In all, 22 of 23 surgeons (96%)17 completed the Recommendations Survey.
Intraoperative Technique
Analysis of the responses to items regarding intraoperative technique for breast augmentation with Natrelle 410 revealed consensus on a broad range of perioperative and surgical techniques, including 15 of the 18 items (Table 1). Consensus was reached on recommendations to irrigate implants and implant pockets with antimicrobials or antiseptics. Antimicrobials effective against Gram-positive bacteria and against Gram-negative bacteria were endorsed by the greatest number of participants (Gram-positive bacteria: 15 and 17 participants for implant and implant pocket irrigation, respectively; Gram-negative bacteria: 13 and 15, respectively). Antiseptics such as Betadine (Purdue Products L.P., Stamford, Conn.) were endorsed by 13 participants (implants and pockets), whereas antimicrobials effective against atypical and anaerobic bacteria were each endorsed by fewer than half of the participants. Intraoperative technique items that did not reach consensus are shaded in Table 1.
Postoperative Management
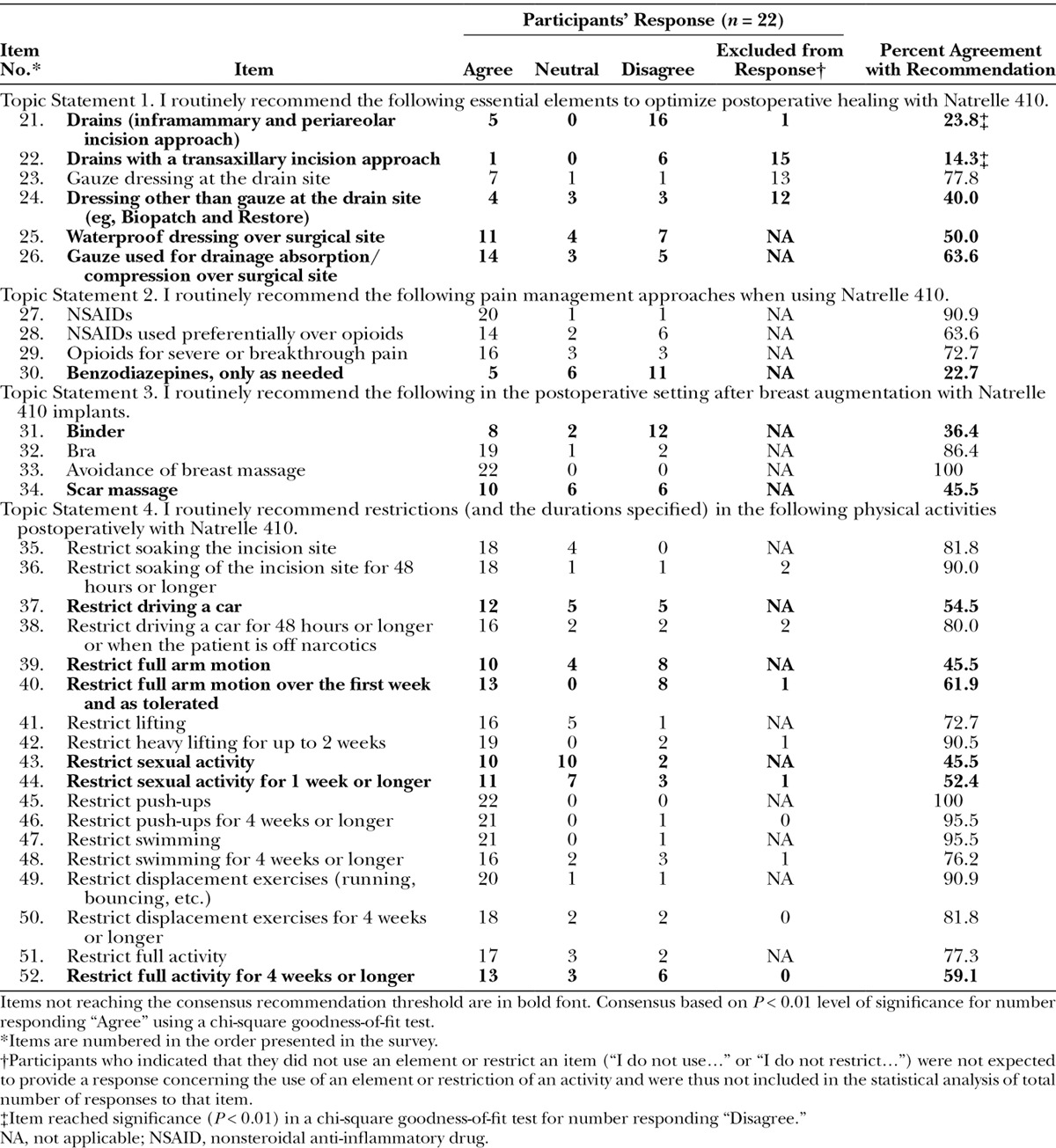
Consensus was reached for 18 of 32 items regarding the postoperative management of patients receiving Natrelle 410 implants (Table 2). Consensus recommendations were similar for postoperative restrictions on activities when the restrictions were described with specific durations and when they were open-ended (6 of 9 items each; Table 2, Statement 4). Exceptions included “Restrict driving a car,” which met consensus only when the time period and narcotic-free timing were specified, and “Restrict full activity,” which met consensus open-ended, but not for a duration of 4 weeks or longer.
Among the items not reaching the consensus threshold for recommendation (shaded items in Table 2), there were 2 items wherein the number of “Disagree” responses reached statistical significance (P < 0.01): 16 of 21 participants (76.2%) disagreed with recommending drains with an inframammary and periareolar incision approach, and 6 of 7 participants (85.7%) disagreed with recommending drains with a transaxillary incision approach (15 of 22 participants were excluded from the analysis of the transaxillary incision item because they responded “I do not use transaxillary incision approach”).
Top Recommendations for the Natrelle 410 Implant
The items selected by the most participants as being among the most important intraoperative techniques specifically for Natrelle 410 were dual-plane placement (selected by 11 of 22 respondents), ensuring tight pockets by limiting the boundaries of dissection (9 of 22) and creating more snug pocket space compared with round implants (8 of 22).
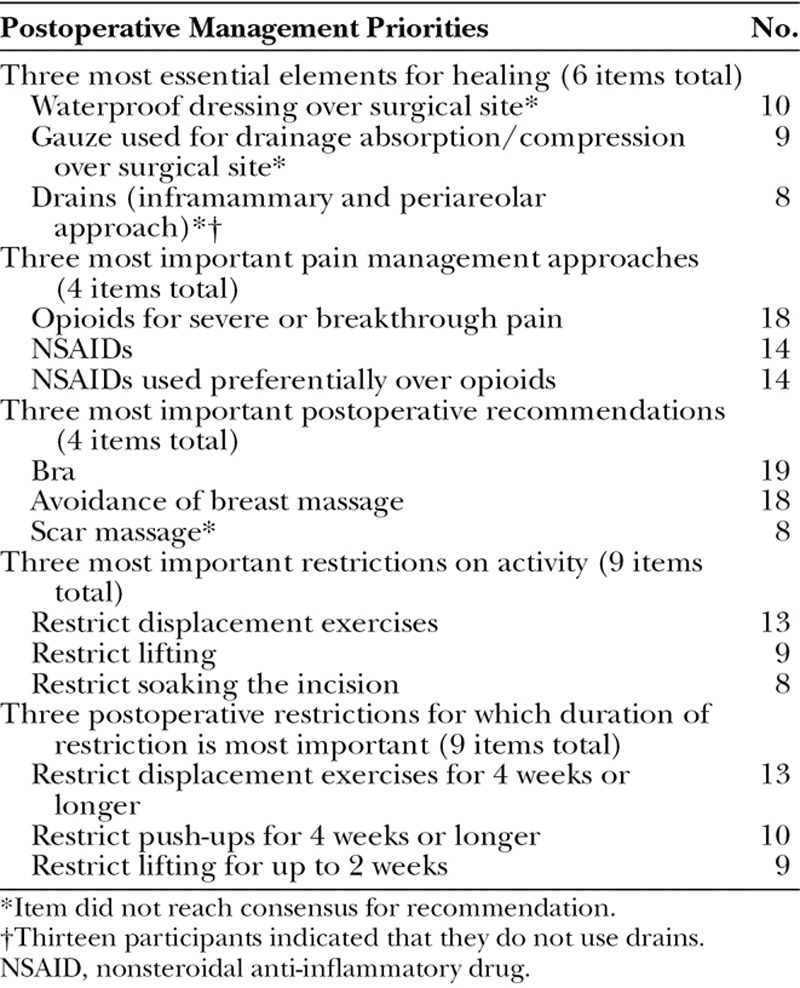
Participant-identified priorities for postoperative management with Natrelle 410 included select wound healing measures, pain management approaches, and activity restrictions (Table 3). Several items were among the top 3 identified by participants as being most important for Natrelle 410, yet did not reach the threshold for consensus recommendation. For example, 8 participants included the use of drains for inframammary and periareolar approach in their top 3 items, but the majority of participants would not recommend the use of drains for any approach. It is possible that participants were providing their top 3 recommendations with only inexperienced surgeons in mind, thus accounting for the apparent discrepancies. Top postoperative management priorities for Natrelle 410 identified by the greatest number of participants included the use of a bra (selected by 19 of 22 respondents), avoidance of breast massage (18 of 22), and opioids for severe or breakthrough pain (18 of 22).
Table 3.
Survey Participant Priorities for Postoperative Management Priorities with Natrelle 410 Implants
DISCUSSION
Using a modification of the Delphi method, a panel of surgeons with extensive experience in breast augmentation surgery using anatomically shaped implants arrived at consensus recommendations for intraoperative technique and postoperative management when using Natrelle 410 breast implants. This report represents the first use of the Delphi method in developing consensus recommendations for breast augmentation surgery in a study designed to collect feedback on surgeons’ experience specifically with the Natrelle 410 implant. Users of other devices may not find these recommendations useful or suitable to their practice, as they reflect the respondents’ experience with this device and its distinctive characteristics, including the textured Biocell shell surface.13 Experienced surgeons have developed preferred approaches to primary breast augmentation that can be tailored to the specific features of each individual case. The recommended approaches presented here are intended to guide clinical decision making for the use of the Natrelle 410 implant.
Intraoperative Technique
There was broad agreement among participants regarding general intraoperative techniques for breast augmentation, such as arm position during surgery, the use of sizers by inexperienced surgeons in complex cases (such as asymmetry cases), electrocautery for dissection, antimicrobial usage, and incision closure techniques. Many of the items that reached consensus for recommendation are long-standing, basic techniques for breast augmentation, in general.18–22
Intraoperative techniques for which consensus was reached that were considered to be of particular importance for Natrelle 410 implants included techniques that contribute to ensuring a hand-in-glove fit for Natrelle 410 in the implant pocket to help maintain stability and minimize the risk of rotation.2,8 These included limiting the boundaries of dissection,4 creating more snug/tight pocket space relative to round implants,22 and the use of electrocautery, which gives better control of the pocket size than blunt dissection.19 Indeed, ensuring tight pockets by limiting the boundaries of dissection and creating more snug pocket space compared with round implants were noted as top intraoperative priorities for Natrelle 410, along with dual-plane placement of the implant. Consensus was reached for the recommendation of an inframammary incision length of approximately 5 cm for Natrelle 410, somewhat longer than generally used for low-cohesive implants. In the authors’ opinion, the length of the incision should be increased for larger implants (up to 6–6.5 cm) and reduced for small implants (down to 4 cm), but an average incision length of 5–5.5 cm allows for proper placement of the highly cohesive implant and reduces the likelihood of gel fracture or deformation during placement.19,22,20,23,24
Dual-plane placement of the Natrelle 410 implant reached consensus for recommendation by the participants, whereas no consensus was reached on subfascial or subglandular placement. General advantages of dual-plane implant placement include a lower likelihood of implant visibility,2 better tissue visualization by mammography,21 and a reduced risk for capsular contraction.6,21 The authors have found that for the Natrelle 410 implant, the dual-plane approach provides good upper pole cover, allows for lower pole subglandular positioning, and still allows for control of rotation when compared with a subglandular pocket on its own.19 In the authors’ opinion, although the risk of rotation is low for the Natrelle 410 implant,7–9 it is increased with nonsubpectoral placement. In patients lacking soft-tissue coverage, the lower likelihood of upper-pole implant visibility with dual-plane placement may be an important consideration.19,20,21 In addition to these observations, the absence of controlled, long-term, prospective clinical studies evaluating the efficacy of the subfascial placement, introduced in the 2000s, may have contributed to the lack of consensus for the subfascial position.25
Postoperative Management
Fewer items reached consensus on postoperative management compared with intraoperative technique, possibly reflecting the diverse training, practices, and experiences of the participants. Specific areas of agreement included the use of a gauze dressing at the drain site, nonsteroidal anti-inflammatory drugs and opioids for pain management, avoidance of breast massage, wearing a bra, and restrictions on selected physical activities such as lifting, displacement exercises, and swimming. These recommendations generally apply to primary breast augmentation with any implant; however, massage is often encouraged for round implants. In the authors’ opinion, massage is contraindicated with the Natrelle 410 implant as it may reduce tissue adhesion to the textured Biocell surface and thereby increase the risk of displacement, rotation of the implant, and capsule complications.25 Immobility of the anatomically shaped Natrelle 410 implant is a necessary outcome that is achieved with placement of the implant in a snug pocket and, perhaps, with tissue adhesion. Immobility combined with low capsule complications leads to immobility with softness or what is described as the “one breast feel.” Similarly, restriction of postoperative physical activity may be particularly important with textured implants; the duration of activity restrictions is generally longer for textured implants compared with round implants. Restriction of displacement exercises for 4 weeks or longer and avoidance of massage were both listed by participants as top postoperative management priorities for Natrelle 410. Subjects’ use of a bra was also a top postoperative priority for Natrelle 410. Areas of disagreement were associated with various surgical dressings and drains, benzodiazepine use, binder use, scar massage, restrictions on driving a car, full arm motion, and sexual activity. It is important to note that although there was agreement on the use of gauze dressings around drains, the use of drains themselves was not recommended. Indeed, 7 participants agreed with recommending gauze dressing at the drain site, but 13 stated they did not use drains. The authors agree that certain techniques work well for some surgeons, but specific postoperative techniques are generally less important than intraoperative technique for final outcome.
Strengths and Limitations
The current study had several important strengths and limitations. The recommendations reported here represent a unique view of real-world clinical practice approaches to breast augmentation with Natrelle 410 implants based on the experiences of an international group of surgeons. In this study, the participants were asked to consider not only the technical aspects of breast augmentation but also management approaches beyond the surgical procedure. Limitations of the study methods are described in Hedén et al 2015.17 Briefly, they include the lack of universally accepted consensus thresholds for the Delphi method14,27 and the possible influence of the iterative process inherent in the Delphi method on the responses provided in the Recommendations Survey.14 In addition, regional practice differences among the survey participants may not have been captured: strong support for 2 differing approaches, for example, could result in a lack of consensus for an item. Recommendations offered by this group of surgeons might differ from those of clinicians with a different breadth of experience or those with patient populations that differ in demographic characteristics and geographic distribution.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL PRACTICE
The Delphi method identified consensus recommendations on a broad range of intraoperative and postoperative management techniques for primary breast augmentation with Natrelle 410 implants. These recommendations can serve as a guide for assessment and refinement of a surgeon’s current procedures with the Natrelle 410 implant. Future areas of consensus-building for intraoperative technique and postoperative management for the Natrelle 410 implant include surgical techniques for positioning the inframammary fold scar and for optimizing the pocket size, postoperative restrictions, and best practices for minimizing the risk of complications.
The Natrelle 410 implant has been shown to be a predictable implant with advantages over smooth, round, and non–form-stable devices that deliver good aesthetic results and a low incidence of complications.7–9,28 As with any medical device, success can best be achieved through a well-defined process of patient selection, preoperative education, precise surgical technique, and a clear postoperative protocol. Given the wide range of specific features in any clinical case, the surgeon must always choose the course best suited to the individual patient. However, the consensus recommendations generated in this modified Delphi method provide surgeons with a framework for intraoperative technique and postoperative care that will contribute to good clinical outcomes with Natrelle 410 for their primary breast augmentation patients.
ACKNOWLEDGMENTS
We thank Svetlana Pidasheva, PhD, and Damien Bates, MD, PhD, FRACS, who were employees of Allergan at the time of this study, for their contributions to the study design. We also thank Dr. Bates for data interpretation. Writing and editorial assistance was provided to the authors by Kathleen M. Dorries, PhD, of Peloton Advantage, Parsippany, N.J., and was funded by Allergan, Irvine, Calif. All authors meet the International Committee of Medical Journal Editors authorship criteria. Neither honoraria nor other form of payments were made for authorship.
Footnotes
Disclosure: Dr. Maxwell has received royalties from Allergan and serves as a consultant for Allergan and LifeCell. Dr. Brown serves as a consultant for Allergan and as a speaker for LifeCell. Dr. Hedén serves as a consultant and receives honoraria for lectures and travel from Allergan. Dr. Luan serves as a consultant for Allergan. Dr. Munhoz serves as a consultant for Allergan. Dr. Carter was an employee of Allergan at the time of this research. This study was sponsored by Allergan, Irvine, Calif. The Article Processing Charge was paid for by Allergan.
REFERENCES
- 1.Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. 2005;116:2005–2016. [PubMed] [Google Scholar]
- 2.Hedén P. Breast augmentation with anatomic, high- cohesiveness silicone gel implants (European experience). In: Spear SL, Willey SC, Robb GL, et al., editors. In: Surgery of the Breast: Principles and Art. 3rd ed. Philadelphia, Pa.: Lippincott, Williams and Wilkins; 2011. pp. 1322–1345. [Google Scholar]
- 3.Adams WP, Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg. 2012;130:597e–611e. doi: 10.1097/PRS.0b013e318262f607. [DOI] [PubMed] [Google Scholar]
- 4.Spear SL, Bulan EJ, Venturi ML. Breast augmentation. Plast Reconstr Surg. 2006;118:188S–196S. doi: 10.1097/01.PRS.0000135945.02642.8B. [DOI] [PubMed] [Google Scholar]
- 5.Adams WP., Jr . Breast Augmentation: McGraw-Hill Plastic Surgery Atlas. New York, N.Y.: McGraw-Hill; 2011. [Google Scholar]
- 6.Pelosi MA, III, Pelosi MA., II Breast augmentation. Obstet Gynecol Clin North Am. 2010;37:533–546, viii. doi: 10.1016/j.ogc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Bengtson BP, Van Natta BW, Murphy DK, et al. Style 410 U.S. Core Clinical Study Group. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):40S–48S. doi: 10.1097/01.prs.0000286666.29101.11. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32:709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell GP, Van Natta BW, Bengtson BP, et al. Presented at the Annual Meeting of the American Society for Aesthetic Plastic Surgery, San Francisco, Calif., April 24–29; 2014. Ten-year results from the Natrelle® 410 anatomical form stable silicone breast implant core study [oral presentation] [Google Scholar]
- 10.Tebbetts JB. A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics. Plast Reconstr Surg. 2002;109:1396–1409; discussion 1410–1415. doi: 10.1097/00006534-200204010-00030. [DOI] [PubMed] [Google Scholar]
- 11.Berry MG, Cucchiara V, Davies DM. Breast augmentation: part III—preoperative considerations and planning. J Plast Reconstr Aesthet Surg. 2011;64:1401–1409. doi: 10.1016/j.bjps.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Hedén P, Jernbeck J, Hober M. Breast augmentation with anatomical cohesive gel implants: the world’s largest current experience. Clin Plast Surg. 2001;28:531–552. [PubMed] [Google Scholar]
- 13.Barone FE, Perry L, Keller T, et al. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;90:77–86. doi: 10.1097/00006534-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12:1–8. [Google Scholar]
- 15.Dalkey N, Helmer O. An experimental application of the Delphi Method to the use of experts. Manag Sci. 1963;9:458–467. [Google Scholar]
- 16.Cardoso MJ, Cardoso J, Santos AC, et al. Factors determining esthetic outcome after breast cancer conservative treatment. Breast J. 2007;13:140–146. doi: 10.1111/j.1524-4741.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 17.Heden P, Brown MH, Luan J. Delphi Study consensus recommendations: patient selection and preoperative planning measurements for Natrelle 410. Plast Reconstr Surg Glob Open. 2015;3 doi: 10.1097/GOX.0000000000000510. doi: 0.1097/GOX.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;118:46S––52S. doi: 10.1097/01.prs.0000185671.51993.7e. [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo DA. Breast augmentation: choosing the optimal incision, implant, and pocket plane. Plast Reconstr Surg. 2000;105:2202–2216; discussion 2217–2218. doi: 10.1097/00006534-200005000-00047. [DOI] [PubMed] [Google Scholar]
- 20.Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116:768–779; discussion 780–781. doi: 10.1097/01.prs.0000176259.66948.e7. [DOI] [PubMed] [Google Scholar]
- 21.Tebbetts JB. Dual plane breast augmentation: optimizing implant-soft-tissue relationships in a wide range of breast types. Plast Reconstr Surg. 2006;118:81S––98S. doi: 10.1097/00006534-200612001-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;133:567e–583e. doi: 10.1097/PRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 23.Hammond DC. Technique and results using MemoryShape implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2014;134:16S–26S. doi: 10.1097/PRS.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 24.Weum S, de Weerd L, Kristiansen B. Form stability of the Style 410 anatomically shaped cohesive silicone gel-filled breast implant in subglandular breast augmentation evaluated with magnetic resonance imaging. Plast Reconstr Surg. 2011;127:409–413. doi: 10.1097/PRS.0b013e3181f95aba. [DOI] [PubMed] [Google Scholar]
- 25.Munhoz AM, Fells K, Arruda E, et al. Subfascial transaxillary breast augmentation without endoscopic assistance: technical aspects and outcome. Aesthetic Plast Surg. 2006;30:503–512. doi: 10.1007/s00266-006-0017-8. [DOI] [PubMed] [Google Scholar]
- 26.Panettiere P, Marchetti L, Accorsi D. Rotation of anatomic prostheses: a possible cause of breast deformity. Aesthetic Plast Surg. 2004;28:348–353. doi: 10.1007/s00266-004-0068-7. [DOI] [PubMed] [Google Scholar]
- 27.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Hedén P, Bronz G, Elberg JJ, et al. Long-term safety and effectiveness of style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg. 2009;33:430–436; discussion 437–438. doi: 10.1007/s00266-009-9360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


