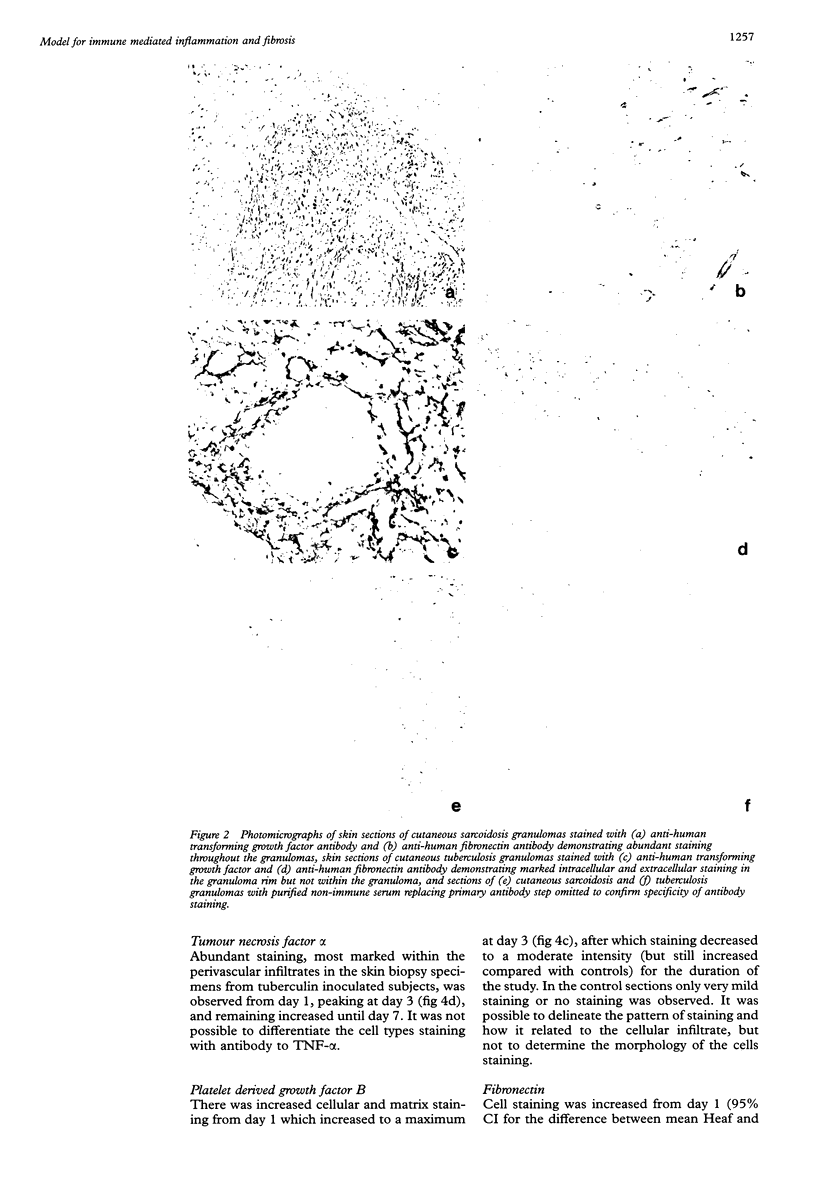
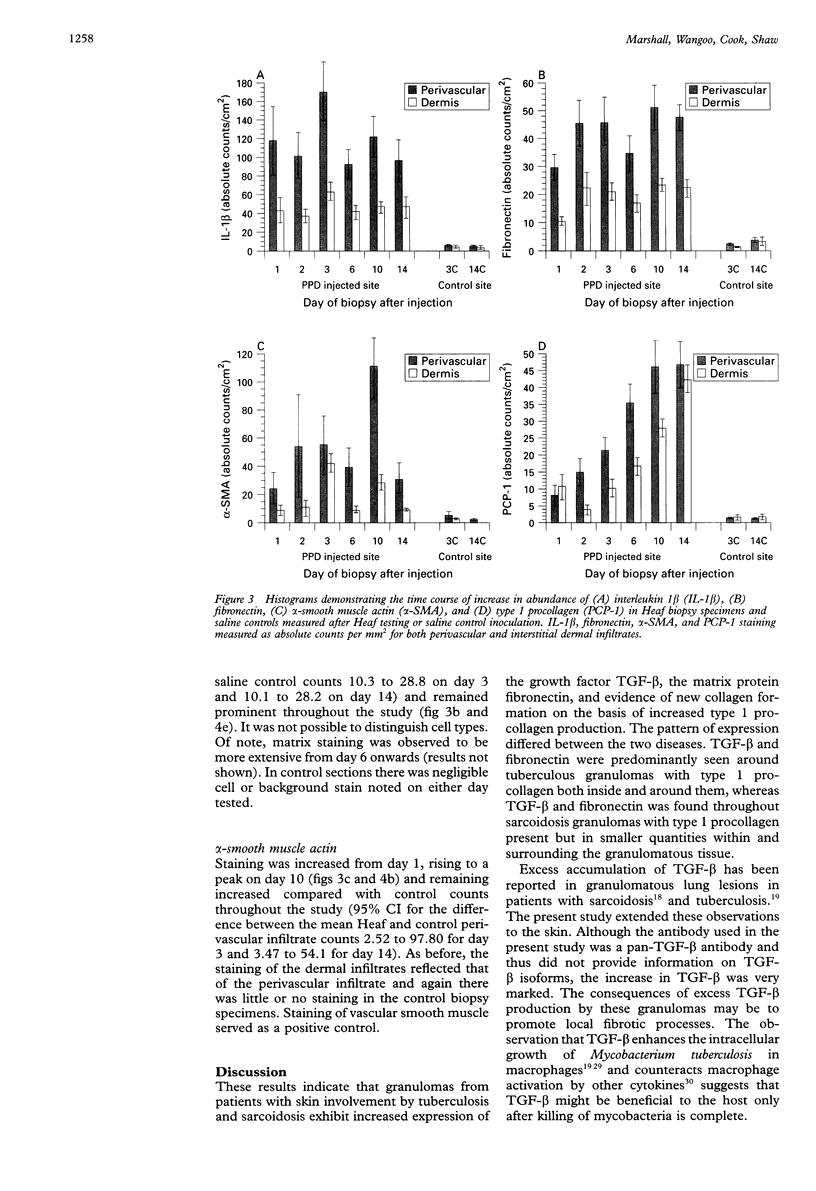
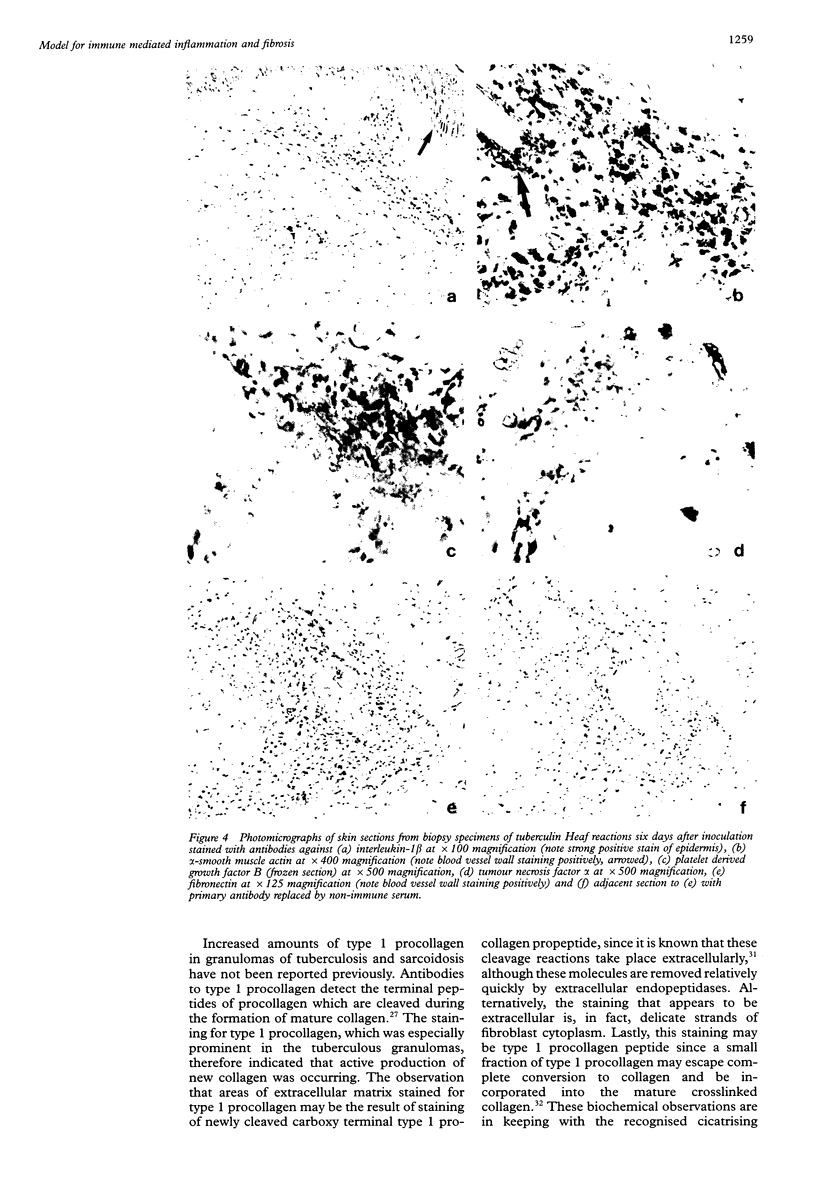
Abstract
BACKGROUND: Interactions between mononuclear cells, vascular endothelium, fibroblasts, and cytokines during the inflammatory reaction within a granuloma have the potential to contribute to the progression to fibrosis. METHODS: Biopsy specimens of six tuberculous and eight sarcoidosis skin lesions were examined by immunohistochemistry to seek evidence for the presence of inflammatory and fibrotic reactions in human granulomatous disease. Additionally, to understand how a T cell mediated delayed type hypersensitivity reaction--a component of chronic granulomatous inflammation--could progress to fibrosis, the human in vivo model of the cutaneous tuberculin Heaf reaction to purified protein derivative (PPD) was studied in a group of 48 subjects. RESULTS: Granulomas from tuberculous and sarcoidosis skin biopsy specimens were seen to contain cells with marked staining by antibodies to fibronectin, transforming growth factor beta (pan TGF-beta), and type 1 procollagen (PCP-1). Accentuated staining of extracellular matrix was seen both in the granulomas and in the peri-granulomatous regions. Less prominent staining was observed using antibodies against interleukin 1 beta (IL-1 beta) and alpha-smooth muscle actin (alpha-SMA). Biopsies of Heaf reactions revealed cells staining for IL-1 beta, tumour necrosis factor alpha (TNF-alpha), platelet derived growth factor B (PDGF-B), and fibronectin which were detected as early as day 1 and persisted throughout the 14 day study period. Cells staining for PCP-1 increased to greatest abundance at day 14. All these cytokines were present in low abundance in biopsy specimens from sites inoculated with saline only. CONCLUSIONS: Evidence is provided that granulomas in tuberculosis and sarcoidosis behave as active centres of fibrogenesis. Using the Heaf model, the temporal relationship between the early appearance of cytokines and the later increase in the collagen precursor PCP-1 linked the immune mediated chronic inflammatory response with subsequent fibrosis and suggested that the tuberculin Heaf reaction will serve as a model for studying the early events of granuloma formation in patients with tuberculosis and sarcoidosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade Z. A., Reed S. G., Roters S. B., Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984 Jan;114(1):137–148. [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. The role of cytokines in the formation of the schistosome egg granuloma. Immunobiology. 1994 Oct;191(4-5):441–450. doi: 10.1016/S0171-2985(11)80450-X. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Andrew E., Haskard D., Feldmann M., Maini R. N. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man. Clin Exp Immunol. 1992 Dec;90(3):522–529. doi: 10.1111/j.1365-2249.1992.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costabel U. The alveolitis of hypersensitivity pneumonitis. Eur Respir J. 1988 Jan;1(1):5–9. [PubMed] [Google Scholar]
- Cree I. A., Nurbhai S., Milne G., Beck J. S. Cell death in granulomata: the role of apoptosis. J Clin Pathol. 1987 Nov;40(11):1314–1319. doi: 10.1136/jcp.40.11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Roles of cytotoxic delayed-type hypersensitivity and macrophage-activating cell-mediated immunity in the pathogenesis of tuberculosis. Immunobiology. 1994 Oct;191(4-5):461–473. doi: 10.1016/S0171-2985(11)80452-3. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E., Turino G. M. Interleukin 1 secretion from human alveolar macrophages in lung disease. J Clin Immunol. 1986 Jul;6(4):326–333. doi: 10.1007/BF00917334. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Gibbs J. H., Ferguson J., Brown R. A., Kenicer K. J., Potts R. C., Coghill G., Swanson Beck J. Histometric study of the localisation of lymphocyte subsets and accessory cells in human Mantoux reactions. J Clin Pathol. 1984 Nov;37(11):1227–1234. doi: 10.1136/jcp.37.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey H. P., Canfield L. S., Kindler H. L., Angadi C. V., Tomasek J. J., Goodman J. W. Production of a fibronectin-associated lymphokine by cloned mouse T cells. J Immunol. 1988 Sep 1;141(5):1508–1515. [PubMed] [Google Scholar]
- Godfrey H. P. T cell fibronectin: an unexpected inflammatory lymphokine. Lymphokine Res. 1990 Fall;9(3):435–447. [PubMed] [Google Scholar]
- Grimaud J. A., Boros D. L., Takiya C., Mathew R. C., Emonard H. Collagen isotypes, laminin, and fibronectin in granulomas of the liver and intestines of Schistosoma mansoni-infected mice. Am J Trop Med Hyg. 1987 Sep;37(2):335–344. doi: 10.4269/ajtmh.1987.37.335. [DOI] [PubMed] [Google Scholar]
- Hirsch C. S., Yoneda T., Averill L., Ellner J. J., Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J Infect Dis. 1994 Nov;170(5):1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Ishioka S., Yamakido M. [Role of cytokines from BAL cells in granuloma formation]. Nihon Rinsho. 1994 Jun;52(6):1467–1472. [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991 Aug;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Kuhn C., McDonald J. A. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991 May;138(5):1257–1265. [PMC free article] [PubMed] [Google Scholar]
- Limper A. H., Colby T. V., Sanders M. S., Asakura S., Roche P. C., DeRemee R. A. Immunohistochemical localization of transforming growth factor-beta 1 in the nonnecrotizing granulomas of pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994 Jan;149(1):197–204. doi: 10.1164/ajrccm.149.1.8111583. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Matheke M. L., Crouch E., Koo M., Kuhn C., 3rd A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest. 1986 Nov;78(5):1237–1244. doi: 10.1172/JCI112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Kabe J., Noda M., Kobayashi N., Miura K. Physiologic and pathologic respiratory changes in delayed type hypersensitivity reaction in guinea pigs. Am Rev Respir Dis. 1971 Apr;103(4):509–515. doi: 10.1164/arrd.1971.103.4.509. [DOI] [PubMed] [Google Scholar]
- Myatt N., Coghill G., Morrison K., Jones D., Cree I. A. Detection of tumour necrosis factor alpha in sarcoidosis and tuberculosis granulomas using in situ hybridisation. J Clin Pathol. 1994 May;47(5):423–426. doi: 10.1136/jcp.47.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R. B., Bhutani L. K., Sharma A. K., Nath I. Fibronectin in leprosy lesions: observations using monoclonal antibodies to human fibronectin. Indian J Lepr. 1984 Jul-Sep;56(3):532–539. [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Sappino A. P., Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990 Mar 15;344(6263):245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Quismorio F. P., Jr Immunologic studies on cutaneous lesions in sarcoidosis. Clin Dermatol. 1986 Oct-Dec;4(4):54–61. doi: 10.1016/0738-081x(86)90034-9. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Chensue S. W., Hiserodt J. C., Higashi G. I., Kunkel S. L. Flow-cytometric evaluation of lymphocyte subpopulations in synchronously developing Schistosoma mansoni egg and Sephadex bead pulmonary granulomas. Am J Pathol. 1988 May;131(2):298–307. [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Benedict S. H., Clark R. A., King T. E., Jr Pathogenesis of pulmonary fibrosis in interstitial lung disease. Alveolar macrophage PDGF(B) gene activation and up-regulation by interferon gamma. Am Rev Respir Dis. 1991 Jan;143(1):167–173. doi: 10.1164/ajrccm/143.1.167. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Gogate P., Shiratsuchi H., Young T., Ellner J. J. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J Immunol. 1995 Jan 1;154(1):465–473. [PubMed] [Google Scholar]
- Torikata C., Villiger B., Kuhn C., 3rd, McDonald J. A. Ultrastructural distribution of fibronectin in normal and fibrotic human lung. Lab Invest. 1985 Apr;52(4):399–408. [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Wahl S. M. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med. 1994 Nov 1;180(5):1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangoo A., Cook H. T., Taylor G. M., Shaw R. J. Enhanced expression of type 1 procollagen and transforming growth factor-beta in tuberculin induced delayed type hypersensitivity. J Clin Pathol. 1995 Apr;48(4):339–345. doi: 10.1136/jcp.48.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangoo A., Taylor I. K., Haynes A. R., Shaw R. J. Up-regulation of alveolar macrophage platelet-derived growth factor-B (PDGF-B) mRNA by interferon-gamma from Mycobacterium tuberculosis antigen (PPD)-stimulated lymphocytes. Clin Exp Immunol. 1993 Oct;94(1):43–50. doi: 10.1111/j.1365-2249.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M. J., Ferns S. A., Hughes C. M., Sear C. H., Rudland P. S. Generation of cell types with myoepithelial and mesenchymal phenotypes during the conversion of rat mammary tumor epithelial stem cells into elongated cells. J Natl Cancer Inst. 1987 Jun;78(6):1191–1201. [PubMed] [Google Scholar]






