Abstract
Study Design
Systematic literature review.
Objective
To evaluate the diagnostic validity of manual examination techniques used to diagnose cervicogenic headache (CGH).
Background
Cervicogenic headache is a specific type of headache that originates from the cervical spine and is typically chronic in nature. Diagnostic criteria for CGH have been established by the International Headache Society (IHS) and are cited extensively in the literature. Diagnosis of CGH through manual examination is a more recent practice. To our knowledge, no systematic review of manual diagnosis of CGH has been performed.
Methods
Searches of electronic databases (CINAHL, Cochrane Library, Medline, PEDro, Scopus, and SPORTDiscus) were conducted for research studies from July 2003 to February 2014. The GRADE approach was used to determine the quality of each paper.
Results
Twelve papers that fulfilled the inclusion and exclusion criteria were identified (12 observational studies). The level of evidence ranged from very low to low, and recommendations for use of specific manual techniques ranged from weak to strong.
Conclusions
Despite low levels of evidence, manual examination of the cervical spine appears to aid the diagnostic process related to CGH and can be implemented by both experienced and inexperienced examiners.
Keywords: Cervicogenic headache, Cervical headache, Diagnosis, Manual examination, Physical examination
Introduction
Cervicogenic headache (CGH) is a classification of headache in which pain is referred from the cervical spine.1 This category of headache is typically chronic, presented as unilateral cephalgia, and is believed to be caused by musculoskeletal dysfunction of the neck.2 The convergence of sensory fibers from the upper three cervical spinal nerves and trigeminal nerve at the trigeminocervical nucleus has been proposed to be the mechanism by which pain from the cervical spine is referred to the face and head.1,2 It has also been suggested that the spinal accessory nerve is involved in this mechanism of pain referral, as spinal accessory nerve fibers join with the upper cervical nerve roots before they reach the descending tract of the trigeminal nerve.1
Cervicogenic headache is a common form of headache, which is estimated to affect 2·5% of the general population and 17·8% of people who suffer from frequent headache.3 Middle-aged patients and particularly women are more likely to have CGH.3 Common clinical characteristics of CGH include unilateral headache without signs of side shift (pain consistently on the same side of the head); pain that is exacerbated with neck movements or abnormal postures; pain produced with pressure applied over the supero-posterior ipsilateral neck; ipsilateral neck, shoulder, or arm pain; and restricted cervical spine range of motion (ROM).4
Cervicogenic headaches are typically identified through clinical or interventional diagnosis.5 Clinical diagnosis involves the classification of headache using specific criteria developed by the International Headache Society (IHS) that are based on history, temporal pattern, and aggravating features of the headache.6 Interventional diagnosis utilizes pharmaceuticals to establish a cervical source of pain.5 Fluoroscopic guidance is used to administer controlled nerve blocks into cervical joints.5 Complete relief of headache following nerve block supports a cervical source of pain.5 Manual examination also assists in making a clinical diagnosis. Manual examination of the upper cervical joints typically involves assessment of cervical ROM to determine the mobility of the cervical spine. Individuals with CGH have been found to exhibit painful dysfunction of the upper three cervical segments.6 Common clinical diagnostic techniques used include the flexion-rotation test (FRT),7–12 cervical AROM,6,9,13–16 passive accessory intervertebral movement (PAIM/PAIVM),12,17 passive physiological intervertebral movement (PPIM/PPIVM),12,17 cervical muscle strength,13–16 cross-sectional area (CSA) measurements of cervical extensor muscles,13,14 cranio-cervical flexion test (CCFT),6,13,14,16 palpation for trigger points,15 pain pressure threshold,6 and cervical kinesthetic sense/joint position sense.6,13,14,16
A review of the literature showed that the FRT is frequently used in making the diagnosis of CGH.7–12 The FRT is a manual examination technique with high sensitivity and specificity.7,8,10,12 It is performed with the patient in supine by passively taking the cervical spine into full flexion. End-range cervical flexion imparts ligamentous tension that impedes movement at vertebral segments below C2.7 Maintaining the flexion position, the patient’s head is then rotated to each side until the patient reports pain or the operator determines that end of motion has been achieved. It is then determined if a restriction in ROM is present.12 In cases of CGH, the FRT usually reveals a unilateral ROM restriction on the symptomatic side.7 This test is considered positive if the estimated ROM was reduced by more than 10° from the anticipated normal range of 44°.9
Magnetic resonance imaging (MRI) conducted simultaneously with the FRT has revealed that, in vivo, movement occurs primarily at the C1/C2 level.8 The aim of the FRT is to bias the C1/C2 segment to determine if a pathology is present, as this cervical level has been suggested to be the primary segment involved in patients with CGH.17 Hall and Robinson9 concluded that C1/C2 was the primary symptomatic segment in all patients with a positive FRT. Similarly, a study by Hall et al.17 identified C1/C2 as the most common symptomatic segment in 63% of patients with CGH.
The diagnostic criteria used to classify headaches of various origins have symptom overlap between headache types.6 Consequently, distinguishing CGH from other headache types may be challenging. Also, symptoms of CGH may be overshadowed by symptoms from migraine or tension-type headaches. Therefore, patients with CGH combined with another form of headache may not be receiving treatment, such as manual therapy, that would address their CGH symptoms.6 This makes it imperative that screening for CGH be conducted in all patients with headache. However, there is a dearth of information regarding the physical examination in diagnosing CGH. Therefore, the purpose of this systematic review of the literature was to examine the clinical utility of manual examination techniques in the diagnosis of CGH and to identify areas of future research.
Methods
The PRISMA 2009 checklist was used to assure that all relevant elements of a systematic literature review were included.18
Search strategy
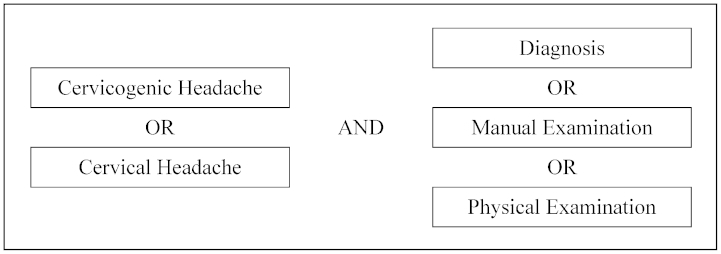
A literature search was performed to identify all research studies that addressed the diagnosis of CGH. The CINAHL, Cochrane Library, Medline, PEDro, Scopus, and SPORTDiscus databases were searched using the following key terms and phrases: cervicogenic headache, cervical headache, diagnosis, manual examination, and physical examination. Intra-group terms were combined using the search term ‘OR’ while inter-group terms were combined using ‘AND’ (Fig. 1). Search results from the different databases were stored and organized using RefWorks.19
Figure 1.
Search terms.
Selection criteria
Searches were limited using specific inclusion and exclusion criteria. Studies were included in this systematic literature review if they were published in the English language between July 2003 and February 2014, included patients age 18 and over, a main focus of the article was CGH, and the study evaluated physical examination. Publications were excluded if they included patients with cancer or those who had had surgical interventions of the head, neck, or thoracic spine. In addition, articles were excluded if the primary focus of the paper was injection, diagnostic imaging, or the use of pharmaceuticals.
Article assessment
In order to determine the quality of the articles, the six authors met to discuss and evaluate each article individually. At group meetings, each paper was assessed using the grades of recommendation, assessment, development, and evaluation (GRADE) approach.20–23 This method of grading the quality and strength of articles provides an inclusive approach for evaluating and developing clinical recommendations for using diagnostic tests. If disagreements or inconsistencies existed between reviewers regarding the grading, they were discussed and a consensus was reached.
Results
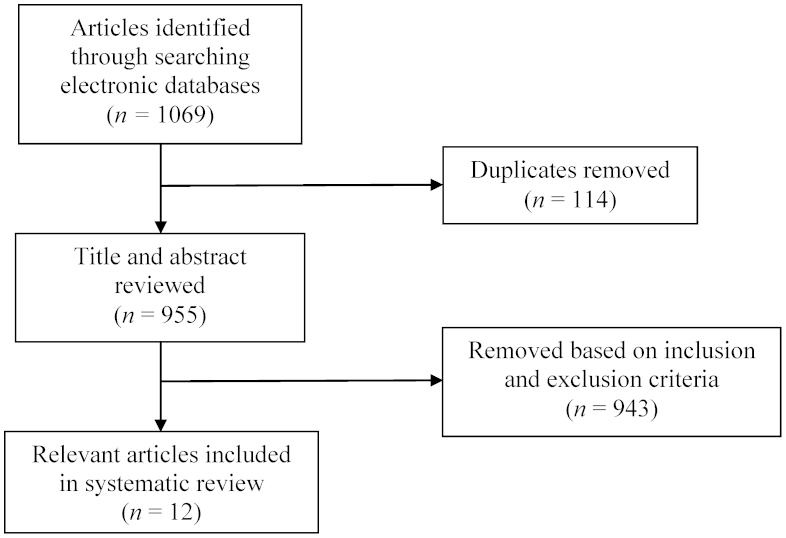
The initial search of the six databases generated a total of 1,069 articles. Nine hundred and 55 remained after duplicates were removed (Fig. 2). The titles and abstracts of these remaining articles were reviewed based on inclusion and exclusion criteria, and 943 articles were removed. Twelve articles on the diagnosis of CGH remained for inclusion in this systematic review.
Figure 2.
Flow diagram for article identification, screening, and selection.
The 12 papers selected for discussion in this systematic review were all observational studies.6–17 Evidence to support the various diagnostic tools ranged from low to very low quality. Recommendations for use of a particular diagnostic tool ranged from strong to weak. No disagreements or inconsistencies among reviewers arose during the grading process of the papers. Individual grades and relevant assessment criteria are presented in Table 1. In all of the articles, recruited patients were diagnosed with CGH according to the IHS criteria. A summary of the combined patient characteristics and methods of manual diagnosis can be found in Table 2.
Table 1.
Grades of recommendation, assessment, development, and evaluation evidence profile: manual diagnosis techniques for cervicogenic headache
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L |
| Hall et al.9: The FRT and active cervical mobility – a comparative measurement study in CGH | |||||||||||
| 1 | O | Yes (−1)a,b,c | No | No | No | No (U) | 56 | 28 | No difference in AROM between CGH and asymptomatic controls (P>0·05). Higher incidence of positive FRT in patients with CGH (P < 0·001). | VL | (+) |
| Hall et al.:10 Intertester reliability and diagnostic validity of the cervical FRT | |||||||||||
| 1 | O | No | No | No | No | No (U) | 64 | 22 | Diagnostic accuracy of FRT for experienced examiners 89%, sensitivity 90%, and specificity 88%. Agreement between experienced and 2 inexperienced examiners was 88 and 83% (P<0·005). | L | (+) |
| Hall et al.8: The influence of lower cervical joint pain on range of motion and interpretation of the FRT | |||||||||||
| 1 | O | No | No | No | No | No (U) | 24 | 0 | Mean difference in ROM of 11·5° (95% CI, 16·8–6·2) less in CGH group when compared to lower cervical facet joint pain group (P<0·01). Diagnostic accuracy of FRT 90% (P<0·01). | L | (++) |
| Hall et al.7: The relationship between CGH and impairment determined by the FRT | |||||||||||
| 1 | O | No | No | No | No | No (U) | 92 | 20 | FRT sensitivity 78%, specificity 85%, mean FRT ROM was 5·9° (95% CI, 7·4–4·5) less in patients with CGH at time of testing (P<0·001). 52% of variance in FRT ROM due to headache intensity, severity, and duration. | L | (+) |
| Hall et al.17: Reliability of manual examination and frequency of symptomatic cervical motion segment dysfunction in CGH | |||||||||||
| 1 | O | No | No | No | No | No (U) | 80 | 20 | C1/C2 was the most common symptomatic segment in 63% of cases of CGH (P<0·001). Interrater reliably 0·68 using an unadjusted Kappa coefficient. | L | (+) |
| Ogince et al.12: The diagnostic validity of the cervical FRT in C1/2-related CGH | |||||||||||
| 1 | O | No | No | No | No | No (U) | 58 | 35 | Sensitivity and specificity were 91 and 90%, respectively (P<0·001); 32° was the cut-off score for a positive CFRT. Subjects included patients with C1/C2 CGH, migraine with aura, and asymptomatic controls. ROM for FRT determined by firm end feel. | L | (++) |
| Hall et al.11: Comparative analysis and diagnostic accuracy of the cervical FRT | |||||||||||
| 1 | O | No | No | No | No | No (U) | 60 | 40 | CGH was correctly diagnosed with the FRT 85% of the time (P<0·001); 30° was the cut-off score for a positive FRT; 44% of the variance in FRT motion was explained by ‘neck movement or positions provoke headache’ and ‘neck symptoms precede headache’. | L | (++) |
| Jull et al.13: Cervical musculoskeletal impairment in frequent intermittent headache. Part 1: Subjects with single headaches | |||||||||||
| 1 | O | No | No | No | No | No (U) | 130 | 57 | CGH can be discriminated from other headache types by the use of a pattern of restricted motion, palpably painful upper cervical joint dysfunction, and impairment in the CCFT with a sensitivity and specificity of 100 and 94%, respectively. | M | (++) |
| 1 | O | Yes (−1)d | No | No | No | No (U) | 165 | 57 | Physical examination (cervical extension, upper cervical segmental palpation, and CCFT) can identify patients with CGH who also have other concurrent headache types (36 out of 108 patients). | VL | (+) |
| Huber et al.15: Reinvestigation of the dysfunction in neck and shoulder girdle muscles as the reason for CGH among office workers | |||||||||||
| 1 | O | Yes (−1)e | No | No | No | No (U) | 60 | 20 | Trigger points were predominantly found in the trapezius muscle on the ipsilateral side of CGH. Number of trigger points was highly correlated with intensity of headache for both supervised and untreated patients (P = 0·001). | VL | (+) |
| Uthaikhup et al.16: Cervical musculoskeletal impairment is common in elders with headache | |||||||||||
| 1 | O | No | No | No | No | No (U) | 151 | 44 | All patients were between 60 and 75 years old. Patients were tested regardless of the presence (42%) or absence of headache. Cervical musculoskeletal dysfunction found by manual examination may not distinguish elders with CGH from other headache. types. | L | (+) |
| Zito et al.6: Clinical tests of musculoskeletal dysfunction in the diagnosis of CGH | |||||||||||
| 1 | O | No | No | No | No | No (U) | 77 | 25 | Patients were all females between 18 and 34 years old. Painful palpation of C1/C2 segments and decreased pectoralis minor muscle length distinguished patients with CGH from patients with migraine and controls (80% sensitivity). | L | (+) |
CCFT: cranio-cervical flexion test; FRT: flexion-rotation test; ROM: range of motion; A: number of studies; B: design; RT: Randomized trial; O: Observational; C: limitations; No: no serious limitations, Yes: serious; D: inconsistency; E: indirectness; F: imprecision; G: publication bias; U: undetected; H: number of tested patients; I: number of controls; J: absolute effect and 95% confidence interval; K: quality; H: High; M: Moderate; L: Low; VL: Very low; L: recommendation; (++): Strong for; (+): Weak.
Randomization process unknown.
Time interval between testing not stated.
Possible bias due to segment identification by second examiner.
Use of questionnaire may not have identified all patients with CGH.
Diagnosis made by EMG, not manual examination.
Table 2.
Patient characteristics and methods of manual diagnosis
| Authors | Number of patients (% female) | Age range | Mean age (±SD) | Pain duration mean years (±SD) | Manual diagnosis technique |
|---|---|---|---|---|---|
| Hall et al.9 | 56 (71·4%) | 29–56 | CGH group: 43·3 (11·5), Asymptomatic group: 43 (13·5) | 8·9 (9) | Cervical AROM, FRT |
| Hall et al.10 | 64 (81·3%) | 18–66 | Group A: CGH with C1/2 dysfunction: 37 (13), Group B: CGH without C1/2 dysfunction: 38 (13), Group C: Asymptomatic: 38 (13) | 4·9 (3·4) | FRT |
| Hall et al.8 | 24 (45·8%) | 26–63 | CGH group: 42·2 (12·2), CFP group: 52·1 (5·5) | CGH: 4·5 (3·2) CFP: 5·9 (2·3) | FRT |
| Hall et al.7 | 92 (unclear) | 21–66 | CGH group: 39 (12·8), Control group: 35 (9·2) | 7·07 (7·3) | FRT |
| Hall et al.17 | 80 (65%) | 18–63 | CGH group: 33 (8·5), Asymptomatic group: 34 (11·5) | 4·5 (3·1) | Unilateral PAIM, PPIM |
| Ogince et al.12 | 58 (65·5%) | 18–66 | CGH group: 46, Asymptomatic group: 40, Migraine with aura group: 37 | Not provided | FRT, PAIVM, PPIVM |
| Hall et al.11 | 60 (63·3%) | 18–63 | CGH group: 35 (10·9), Migraine group: 30 (6·5), MHF group: 33 (9·4) | CGH: 4·8 (2·8) Migraine: 9·1 (4·8) MHF: 5·7 (3·9) | FRT |
| Jull et al.13 | 130 (64·6%) | 23–55 | CGH group: females 38·2 (9·5) and males 43·1 (12·3), migraine group: females 42·1 (10·2) and males 37·6 (14·2), tension-type: females 40·1 (10·3) and males 38·1 (11·3), controls: females 37·0 (12·0) and males 38·6 (10·5) | CGH: 9·3 (7·3) Migraine: 17·4 (12·2), Tension-type: 12·9 (9·3) | Cervical ROM, cervical muscle strength, CSA of cervical extensor muscles, CCFT, cervical kinesthetic sense |
| Amiri et al.14 | 165 (73%) | 26–49 | CGH group: 37·9 (1·7), Non-CGH group: 37·1 (9·1), Control group: 37·4 (11·2) | CGH: 15·1 (8·2) Non-CGH: 15·9 (10·2) | Cervical ROM, cervical muscle strength, CSA of cervical extensor muscles, CCFT, cervical kinesthetic sense |
| Huber et al.15 | 60 (75%) | 25–55 | Females: 37·8 (8·6), males 39·5 (10·3) | All patients: 1 | Cervical ROM, palpation for trigger points, muscle strength |
| Uthaikhup et al.16 | 162 (59·8%) | 60–75 | Headache group: 65·9 (4·6), control group: 66·4 (4·1) | 26·4 (13·3) | Cervical ROM, cervical manual palpation, joint position sense, CCFT, cranio-cervical flexor and extensor strength |
| Zito et al.6 | 77 (100%) | 18–35 | CGH group: 25·3 (3·9), migraine with aura group: 22·9 (3·5), control group 22·9 (3·5) | 9 months to >10 years | Cervical ROM, pressure pain thresholds, muscle length, CCFT, cervical kinesthetic sense |
CGH, cervicogenic headache; AROM, active range of motion; FRT, flexion-rotation test; CFP, cervical facet pain; PAIM/PAIVM, passive accessory intervertebral movement; PPIM/PPIVM, passive physiological intervertebral movement; MHF, multiple headache forms; CSA, cross-sectional area; CCFT, cranio-cervical flexion test.
Flexion-rotation test
Six of the 12 articles examined the use of the FRT as a diagnostic tool for CGH.7–12 Hall and Robinson9 found no statistically significant difference in active cervical ROM between the CGH group and asymptomatic controls. However, there was a significant difference in FRT measurements with a greater restriction in rotation toward the symptomatic side in the CGH group. The FRT was positive in all patients with C1/C2 as the primary symptomatic segment and negative in patients with a symptomatic segment other than C1/C2 and in asymptomatic controls.
Hall et al.8,10 examined the interrater reliability between experienced and inexperienced examiners. In Hall et al.,10 when the FRT was performed by two experienced examiners, it had a diagnostic accuracy of 89%, sensitivity of 90%, and specificity of 88% in determining the presence of CGH with C1/C2 as the dysfunctional level (positive likelihood ratios of 9 and 6, negative likelihood ratios of 0·11 and 0·12). While the two inexperienced examiners recorded larger ranges of motion, there was no significant difference between their findings compared to the two experienced examiners (sensitivity 83%, specificity 88%, positive likelihood ratios of 10 and 5, negative likelihood ratios of 0·18 and 0·2). Hall et al.8 found that the FRT was 90% accurate (sensitivity 75%, specificity 92%) when experienced and inexperienced raters compared patients with CGH to those with lower cervical facet pain (CFP). The mean difference in ROM between the two groups was 11·5° less in the CGH group.
Hall et al.7 found a positive FRT in 78% of patients from the CGH group and 15% of patients from the asymptomatic group, giving a sensitivity and specificity of 78 and 85%, respectively. Researchers found a statistically significant association between headache severity and ROM toward the more restricted side, with duration, frequency, and intensity being the most significant predictors of ROM. The presence of a headache did not affect the interpretation of a positive or negative test; however, range was reduced by 5·9° toward the side of the headache in patients who were symptomatic at the time of examination.
Ogince et al.12 found that patients with CGH had a side-to-side cervical rotation differential of 19° (P<0·001) compared to patients having migraine with aura and asymptomatic patients. The FRT was found to have good clinical utility (sensitivity 91%, specificity 90%, diagnostic accuracy 91%). It was also found that severity of CGH was not related to loss of ROM. Hall et al.11 found that 85% of the time CGH was correctly differentiated from other headache types (sensitivity 70%, specificity 70%, positive likelihood ratio 2·33, negative likelihood ratio 0·43).
Passive accessory and physiological movements
Hall et al.17 and Ogince et al.12 used unilateral PAIM/PAIVM and PPIM/PPIVM to determine the frequency that each or multiple segments in the upper cervical spine above the C4 vertebra were the principal source of pain in patients with CGH. The authors found the dominant symptomatic segments to be C1/C2 and C2/C3. Substantial interrater reliability, as defined by Landis and Koch,24 was found with manual examination techniques.
Clusters of tests
Jull et al.13 found significant differences in active cervical ROM (extension and bilateral rotation), palpable joint dysfunction, cervical muscle strength (flexion and extension), and the CCFT in patients with CGH when compared to patients with migraine, tension-type headache, and asymptomatic controls (all P<0·001). The combination of palpably painful joint dysfunction at C0–C4, limited cervical spine extension, and increased sternocleidomastoid muscle activity in the CCFT yielded 100% sensitivity and 94% specificity in distinguishing CGH. Amiri et al.14 examined the same parameters as in Jull et al.13 but examined patients who were excluded from the Jull et al.13 study because they had concurrent headache types. They determined that the cluster of upper cervical dysfunction, restricted cervical motion, and deep cervical flexor weakness contributed to greater confidence in making the diagnosis of CGH.
In Zito et al.,6 when the control group and patients with migraines with aura were compared to patients with CGH, 80% of patients with CGH were correctly identified based on C1/C2 findings and shortened length of the pectoralis minor muscle. Patients with migraine headache did not have muscle tightness of the pectoralis minor. In addition, there was tightness found in several other muscles, namely the upper trapezius, levator scapulae, scalenes, and sub-occipital extensors, regardless of headache type. However, the frequency of tightness was greater in patients with CGH.
Huber et al.15 found decreases in all cervical movements in patients with CGH, with the most limited being flexion. Trigger points were also investigated and were found to be predominant in the trapezius muscle of the ipsilateral side. The number of trigger points was highly correlated with intensity of headache (P = 0·001) for all patients with CGH.
Due to a higher prevalence of elders with cervical musculoskeletal dysfunction (CMD), Uthaikhup et al.16 investigated the relationship between CMD and CGH. Patients were grouped into two clusters. One cluster included patients with less cervical extension ROM and greater occurrence of C1/C2 joint dysfunction. It was later determined that this cluster contained the majority of patients with CGH. Although this cluster had a higher incidence of CMD, it was not found to be unique to patients with CGH.
Discussion
The purpose of this systematic literature review was to examine the clinical utility of manual diagnostic tools for CGH. To our knowledge, this review is the first of its kind to assess this topic. The evidence presented in this review included 12 papers that performed the FRT and other manual examination techniques.6–17 While only observational studies were included in this review, their results suggest that clinicians may utilize various manual techniques to assist in the diagnosis of CGH.
Six articles7–12 discussed in this review utilized the FRT as the primary diagnostic tool for CGH. When looking at studies on CGH, the FRT was a common test used for differential diagnosis and evaluation of headaches. However, it is essential that the FRT be performed correctly. If the FRT is executed properly it is less likely to engage the lower cervical spine. Consequently, it is important to achieve end-range cervical flexion in order to bias the C1/C2 segments prior to performing the upper cervical rotation component. If end-range flexion is not achieved, the test may engage the lower cervical spine possibly resulting in a false negative test. Magnetic resonance imaging has confirmed that the lower cervical spine’s available ROM is taken up fully during end-range flexion, which isolates the rotation of the head during the FRT to the C1/C2 segment.8 In the studies reviewed, the majority found that passive ROM during the FRT was significantly less predominant in the trapezius muscle of the side ipsilateral to the headache.7–10 The usefulness, sensitivity, and specificity of the FRT did not seem to be affected in patients who had a headache at the time measurements were taken.7,8 However, Hall et al.11 felt that the presence of headache was relevant and only tested patients on headache free days. It is important to note that a lower diagnostic accuracy was found when differentiating CGH from migraine without aura and multiple headache forms (MHF). Hall et al.11 stated that a negative FRT test does not rule out CGH as other cervical segments besides the upper cervical spine may produce headache.
Also there is conflicting evidence on the value of active cervical range of motion in making the diagnosis of CGH. In some studies, active range of motion was limited in patients with CGH.6,13–16 Extension was limited in four of the studies,6,13,14,16 flexion in two studies,6,15 and rotation in two studies.13,16 However, Hall et al.9 concluded that there was no difference in active cervical ROM in patients with CGH compared to those who were asymptomatic. Consequently, it is unclear whether active cervical ROM is consistently restricted in patients with CGH. Compensatory movement at various spinal levels may affect the test.
In addition to observing AROM in considering the diagnosis of CGH, several studies12–14,17 discussed the importance of manual examination in identifying the dominant symptomatic segment involved in CGH. PAIM and PPIM manual examination techniques have been found to have substantial interrater reliability between examiners.17 Researchers have also found that the majority of patients with CGH had C1/C2 as the primary dysfunctional level, which is the targeted level in the FRT. As stated in Hall et al.,17 it is unclear as to why the C1/C2 segment is the most frequently symptomatic segment. One possible explanation is that the increased rotation available at the C1/C2 segment compared to the remaining cervical spine makes it more likely to produce CGH than the rest of the cervical spine.9,17 However, Jull et al.13 found that patients with CGH had joint dysfunction from C0–C4. Given this finding, it appears possible that the FRT may not be helpful in identifying all patients with CGH.
Another consideration is the relationship between degenerative joint disease and CGH. Degenerative joint disease may not play a major role in CGH, because cervical degeneration occurs more commonly in lower cervical segments compared with upper cervical segments.17 Therefore, it is unlikely that osteoarthritis is a major contributing factor to CGH. Furthermore, some patients with CGH are younger adults, reducing the possibility of osteoarthritis as a cause of CGH.25 Uthaikhup et al.16 supported this contention when they found that despite the prevalence of CMD in the elderly population, it is not unique to patients with CGH.
In discussing the anatomical factors that potentially contribute to CGH, it is important to direct attention not only to C1/C2, but to the inferior cervical segments as well as soft tissue structures surrounding the cervical spine. Despite the prevalence of C1/C2 segmental dysfunction in CGH, there is evidence that patients with CGH may also have dysfunction in the form of trigger points.6 Zito et al.6 examined pressure pain thresholds (PPTs) in patients with CGH and patients with migraine with aura and found that both headache groups had a significantly lower PPT over the transverse process of C4. Because of similar findings in the migraine group, this finding may not be specific to CGH. However, it does suggest the possibility that trigger points are a contributing factor in CGH. For example, Huber et al.15 found an increased incidence of trigger points in the trapezius on the side ipsilateral to the headache in patients with CGH.
The demographics of patients included in this review can be found in Table 2. Researchers recruited patients included in the 12 studies by a matter of convenience and placed them in groups based on the IHS criteria. The majority of patients included in the reviewed studies were middle-aged females. Due to the large number of females included in these studies, the generalizability of the results to males with CGH may be limited because none of the studies differentiated the statistics between females and males in the studies.
When considering the FRT data, it is important to note that patients who could not tolerate the FRT were excluded from all of the six studies discussing the FRT.7–12 None of the studies reported how many patients were lost due to intolerance to the test. This may be problematic because the patients excluded from the studies potentially could have had a positive FRT as pain is one of the criteria for a positive test. In addition, studies involving the presence of upper limb symptoms were excluded from our search in an attempt to eliminate the effects of cervical directional preference and disorders, such as cervical radiculopathy. One paper8 did include patients with upper limb symptoms; however, this was not addressed in their outcomes.
As a particular point of interest, all of the studies included in this systematic review discussed the high interrater reliability of both the FRT and manual examination in the diagnosis of CGH. All of the studies that included analysis of the sensitivity (70–91%) and specificity (70–92%) of the FRT found it able to accurately identify the presence or absence of CGH.7,8,10–12 The range of positive likelihood ratios for the FRT was 2·33–10·65 and the range of negative likelihood ratios was 0·095–0·43.7,8,10–12 Overall, the diagnostic accuracy of the FRT was found to be between 89 and 90%,8,10 which makes the FRT a valuable test for inclusion in assessments. Also, in two articles, researchers found that the examiner’s level of experience did not affect the results of the FRT.8,10 Despite the larger ranges recorded by inexperienced examiners, the sensitivity, specificity, and inter-examiner agreement of positive test identification were relatively high. These two studies showed that the FRT can be confidently utilized and interpreted by all examiners regardless of their level of experience.
Areas for future research include further investigation of patient position during the FRT. A previous study performed the FRT with patients in the seated position; however, researchers found the mean normal passive ROM of C1/C2 to be 38° in asymptomatic patients, which is lower than the normal 44°.26 Healthcare professionals may find it more efficient to perform the FRT in a seated position. Therefore, studies could examine the impact of administering the FRT in a seated position versus supine on patients with and without CGH. Also, further studies should address the diagnosis of CGH when C1/C2 is not the primary symptomatic segment. Patients with CGH may have pain arising from a mid or lower cervical level, potentially resulting in a negative FRT.17 Another area for research could explore the extent to which gender impacts the results of the FRT, as this was not analyzed in the 12 articles.
Limitations
This review was limited to articles in English and published from July 2003 to February 2014. Additionally, it was required that all patients be at least 18 years of age. Articles may have been missed secondary to these search criteria.
Also this literature search only yielded observational studies, which are generally considered to be low levels of evidence.20–23 Additionally, in one of the 12 studies included,9 the exact methodology was unclear, rendering the results questionable.
Another consideration was the inconsistency of how end range was determined in the FRT. Some studies used firm end range or pain provocation7–9,11 while others used only a firm end range to determine FRT ROM.10,12
Conclusions
This systematic literature review revealed low levels of evidence using the GRADE system in assessing papers discussing manual examination of CGH. These low levels were based on the fact that the included studies were all observational. Despite the low level of evidence, many of the manual examination techniques used for the diagnosis of CGH appear to be helpful in the diagnostic process. For example, the FRT is simple to perform and has good sensitivity and specificity. There is also evidence to suggest that passive cervical joint mobilizations to determine the primary symptomatic segment may provide useful information, as the findings correlate well with the FRT.
Disclaimer Statements
Contributors Each of the six co-authors played a major role in developing this project, conducting the literature searches, discussing and grading each paper, and writing the final manuscript.
Funding None.
Conflicts of interest None of the authors have any conflicts of interest with any of the material presented in this paper.
Ethics approval None required.
References
- 1.Biondi DM. Cervicogenic headache: a review of diagnostic and treatment strategies. J Am Osteopath Assoc. 2005;105:S16–22. [PubMed] [Google Scholar]
- 2.Rana MV. Managing and treating headache of cervicogenic origin. Med Clin North Am. 2013;97:267–80. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20–59 years olds. Spine (Phila Pa 1976). 1995;20:1884–8. [DOI] [PubMed] [Google Scholar]
- 4.Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. the cervicogenic headache international study group. Headache. 1998;38:442–5. [DOI] [PubMed] [Google Scholar]
- 5.Bogduk N, Govind J. Cervicogenic headache: An assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009;8:959–68. [DOI] [PubMed] [Google Scholar]
- 6.Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man Ther. 2006;11:118–29. [DOI] [PubMed] [Google Scholar]
- 7.Hall TM, Briffa K, Hopper D, Robinson KW. The relationship between cervicogenic headache and impairment determined by the flexion-rotation test. J Manipulative Physiol Ther. 2010;33:666–71. [DOI] [PubMed] [Google Scholar]
- 8.Hall T, Briffa K, Hopper D. The influence of lower cervical joint pain on range of motion and interpretation of the flexion-rotation test. J Man Manip Ther. 2010;18:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall T, Robinson K. The flexion-rotation test and active cervical mobility – a comparative measurement study in cervicogenic headache. Man Ther. 2004;9:197–202. [DOI] [PubMed] [Google Scholar]
- 10.Hall TM, Robinson KW, Fujinawa O, Akasaka K, Pyne EA. Intertester reliability and diagnostic validity of the cervical flexion-rotation test. J Manipulative Physiol Ther. 2008;31:293–300. [DOI] [PubMed] [Google Scholar]
- 11.Hall TM, Briffa K, Hopper D, Robinson K. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010;11:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogince M, Hall T, Robinson K, Blackmore AM. The diagnostic validity of the cervical flexion-rotation test in C1/2-related cervicogenic headache. Man Ther. 2007;12:256–62. [DOI] [PubMed] [Google Scholar]
- 13.Jull G, Amiri M, Bullock-Saxton J, Darnell R, Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 1: subjects with single headaches. Cephalalgia. 2007;27:793–802. [DOI] [PubMed] [Google Scholar]
- 14.Amiri M, Jull G, Bullock-Saxton J, Darnell R, Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. part 2: Subjects with concurrent headache types. Cephalalgia. 2007;27:891–8. [DOI] [PubMed] [Google Scholar]
- 15.Huber J, Lisinski P, Polowczyk A. Reinvestigation of the dysfunction in neck and shoulder girdle muscles as the reason of cervicogenic headache among office workers. Disabil Rehabil. 2013;35:793–802. [DOI] [PubMed] [Google Scholar]
- 16.Uthaikhup S, Sterling M, Jull G. Cervical musculoskeletal impairment is common in elders with headache. Man Ther. 2009;14:636–41. [DOI] [PubMed] [Google Scholar]
- 17.Hall T, Briffa K, Hopper D, Robinson K. Reliability of manual examination and frequency of symptomatic cervical motion segment dysfunction in cervicogenic headache. Man Ther. 2010;15:542–6. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP,. et al The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RefWorks http://www.refworks.com. Updated 2009. Accessed July, 2013. [Google Scholar]
- 20.Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW,. et al Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–77. [DOI] [PubMed] [Google Scholar]
- 21.Brozek JL, Akl EA, Jaeschke R, Lang DM, Bossuyt P, Glasziou P,. et al Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy. 2009;64:1109–16. [DOI] [PubMed] [Google Scholar]
- 22.Brozek JL, Akl EA, Compalati E,. Kreis J, Terracciano L, Fiocchi A. et al Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66:588–95. [DOI] [PubMed] [Google Scholar]
- 23.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S,. et al Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 25.Taylor M, Twomey L. Functional and applied anatomy of the cervical spine. Physical therapy of the cervical and thoracic spine, 3rd edn. Edinburgh: Churchill Livingstone; 2002. p. 1–25. [Google Scholar]
- 26.Dvorak J, Antinnes JA, Panjabi M, Loustalot D, Bonomo M. Age and gender related normal motion of the cervical spine. Spine (Phila Pa 1976). 1992;17:S393–8. [DOI] [PubMed] [Google Scholar]