Abstract
Background and purpose
Neural mobilization techniques are used clinically to treat neuropathic pain and dysfunction. While selected studies report efficacy of these techniques, the mechanisms of benefit are speculative. The purpose of this study was to evaluate the effects of in vitro simulated stretch/relax neural mobilization cycles on fluid dispersion within sections of unembalmed cadaveric peripheral nerve tissue.
Methods
Bilateral sciatic nerve sections were harvested from six cadavers. Matched pairs of nerve sections were secured in a tissue tester and injected with a plasma/Toluidine Blue dye solution. Once the initial dye spread stabilized, the experimental nerve sections underwent 25 stretch/relaxation cycles (e.g. simulated neural mobilization) produced by a mechanical tissue tester. Post-test dye spread measurements were compared to pre-test measurements as well as control findings (no simulated mobilization). Data were analyzed using paired t-tests.
Results
Individual dye spread measurements were reliable [ICC(3,1) = 0·99]. The post-test intraneural fluid movement (dye spread) in the experimental section increased significantly with simulated neural mobilization compared to pre-test measurements (3·2±2·1 mm; P = 0·015) and control measurements (3·3±2·7 mm; P = 0·013).
Conclusion
Repetitive simulated neural mobilization, incorporating stretch/relax cycles, of excised cadaveric peripheral nerve tissue produced an increase in intraneural fluid dispersion. Neural mobilization may alter nerve tissue environment, promoting improved function and nerve health, by dispersing tissue fluid and diminishing intraneural swelling and/or pressure.
Keywords: Entrapment, Neuropathy, Nerve injury, Intraneural edema
This article is the first in a two-part series and the second part will feature in the next Issue of the Journal
Introduction
Peripheral nerve disorders, such as entrapment and compression syndromes, can result in changes to nerve tissue including decreased mobility,1–4 increased mechanosensitivity,3,5 reduced nerve conduction,6–8 nerve ischemia,9,10 inhibition of axonal transport,11–17 and intraneural edema.10,15,18,19 Clinicians incorporate neural mobilization techniques to both assess and treat peripheral nerve impairments and related neuropathic pain.20–28
Nerve injury commonly leads to intraneural edema18,19,29,30 and can dramatically affect nerve structure and function.18,31 Peripheral nerve tissue trauma, leading to edema, can be caused by compression,6,15,18,19,32 excessive tension,10,16,33 or vibration.17,18 Epineurial edema may be caused from relatively mild injury, whereas long standing compression may lead to endoneurial edema as a result of changes in the diffusion barriers of the perineurium and microvasculature.19 Without lymphatic vessels, the endoneurium is unable to drain this fluid accumulation,34 which leads to fibrosis, adhesions, and impaired intrafascicular gliding.10,31,34,35 Fibrosis may subsequently result in an intraneural thickening,36 as well as nerve enlargement and extraneural compression from adjacent structures.37,38 Even in the absence of severe axonal damage and significant morphological changes, somatosensory changes and nerve sheath inflammation may ensue, leading to neuropathic pain.39
Neural mobilization techniques are clinically implemented in order to diminish pain21–23,40,41 and restore neural mobility.42 Even though studies suggest improvement in patient symptoms as a result of these techniques,23–25,40,43,44 the mechanisms for symptom improvement are not clear. Similarly, while mobility studies for peripheral nerve tissue cite significant displacement during limb movement,25 other studies have challenged the idea that lower extremity movements produce substantial displacement proximally in the lumbosacral nerve roots when the foraminal ligaments remain intact.45,46 The observed clinical benefits40,42 of neural mobilization, therefore, could be due to factors other than lumbosacral nerve root displacement. These other factors might include changes in fluid dynamics, such as decreased intraneural edema40 and alterations in blood flow. Currently, however, there is a paucity of research evaluating the effects of neural mobilization on fluid movement in peripheral nerve tissue. It is unknown whether or not mechanical action, as in neural mobilization resulting in mild cyclic nerve strain, can create a passive mechanism for intraneural fluid movement and, if so, how much fluid movement occurs. Therefore, the purpose of the study was to investigate the effects of neural mobilization on intraneural fluid movement within peripheral nerve tissue in a controlled in vitro environment. It was hypothesized that neural mobilization would cause greater fluid movement in peripheral nerve sections compared to those peripheral nerve sections not undergoing neural mobilization.
Methods
Experimental design
A pre-test–post-test control group design using an in vitro cadaveric tissue model was used to examine the movement behavior (longitudinal spread or dispersion) of fluid injected into excised sciatic nerve sections pre- and post-neural mobilization and compared within-subject matched pair control nerve sections.
Dissection and specimens
Six right/left matched pair sections of sciatic nerves from unembalmed cadavers (three female and three male) were harvested and used in this study. The cadaver demographic information for the specimens has been previously reported.47 Cadavers were placed in a prone position and the skin, subcutaneous tissue, and fascia were reflected from the posterior thigh and muscles were moved or reflected to expose the sciatic nerve from the region spanning the piriformis muscle to the popliteal fossa. A 15-cm section of sciatic nerve was harvested and labeled as ‘right’ or ‘left’ within each matched pair (right and left sample of one cadaver). By random selection within each matched pair of nerve sections, one section (right or left limb) was designated as the control specimen and the other as the experimental specimen. Once harvested, sciatic nerve sections were frozen (−80°C) and stored until thawing before data collection. Because the focus was on the mechanical and not histological effects of mobilization on tissue fluid behavior, the histological state of autolysis was not examined. All cadaveric specimens were handled in accordance with State and university regulations.
Experimental set-up and testing
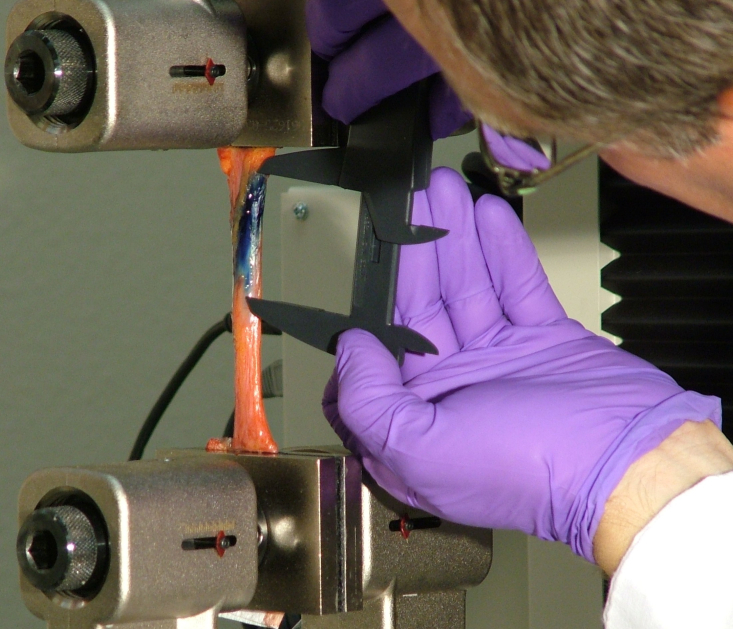
The frozen sciatic nerve sections were allowed to thaw until they reached room temperature (21·7°C). The excised sciatic nerve was secured in a biomaterials testing system (Insight Electromechanical Testing Systems, 10 kN Load Capacity Model; MTS Systems Corporation, Eden Prairie, MN, USA). The proximal and distal ends of the nerve section were wrapped with strips of durable paper towel to prevent slipping within the material tester clamps (advantage screw grips; MTS Systems Corporation). The proximal portion of the section was first clamped from above. Gravity was allowed to pretension the nerve section before the bottom clamp was tightened around the distal portion of the nerve section. The dimensions (initial length, width, and thickness) of the nerve section were recorded. These values were entered into the material tester in order to calculate the degree of elongation that would lead to the desired strain. The materials tester was set to provide repetitive stretch/relax cycles to the nerve segment at 6% strain. These stretch/relax cycles were designed to simulate clinically oriented neural mobilization strategies. While the authors understand that this in vitro method cannot be generalized to the in vivo condition, the term ‘neural mobilization’ will be used through the remainder of the manuscript to describe this simulated intervention. Additionally, from a clinical perspective, an absolute strain of 6% would not be expected to restrict blood flow or impair nerve fiber conduction since it is within the normal stress tolerance of the tissue.48 A dye mixture composed of human plasma and toluidine blue stock solution (2∶1-Plasma: 1% toluidine blue stock solution, 0·5 cc)49 was injected to simulate nerve edema/swelling. Human plasma was used in order to most closely mimic in vivo fluid dynamics. The toluidine blue was used for its gross visual properties. The dye solution was injected into the center of the proximal third of the sciatic nerve section just under the epineurium using steady pressure via a syringe (1 cc) and a 0·45×23 mm needle. The initial injection bolus created a longitudinal dye spread that was manually measured using a digital caliper (Digimax 6″ precision digital caliper; Wiha Tools.com LLC; Willi Hahn Corp., Monticello, MN, USA). Measurements were taken from the most distinct proximal and distal longitudinal borders of the dye spread (Fig. 1). Non-distinct borders were avoided during the measurements. Although these non-distinct borders could have indicated spread of the fluid into the deeper parts of the nerve section (radial dimension dye spread), the investigators remained conservative in the measurement of clear, distinct borders of the longitudinal dye spread to reduce subjectivity and potential bias. The investigator responsible for the measurements was the same throughout the data collection process and the reliability of measurements was confirmed before the experimental testing. Additionally, the investigator was blinded to the measurement results.
Figure 1.
The sciatic nerve section was clamped between the plates of the tissue tester. Dye was injected into the center of the proximal third of the nerve, just under the epineurium. Dye spread was measured with a digital caliper.
Pre-test measurements
After initial injection, dye spread was measured in five minute intervals until fluid motion, as indicated by the dye spread, stabilized. In order to maximize measurement reliability, three separate dye spread measurements were performed during each measurement period and the values were averaged. Dye spread was considered stable when two, successive, 5-minute interval averaged measurements, were within 0·5 mm of one another. Once the dye spread stabilized, the neural mobilizations (stretch/relax cycles) were initiated by the tester.
Intervention and post-test measurements in the experimental nerve sections
The materials tester was set to provide 25 mobilization (stretch/relax) cycles to 6% strain at a strain rate of 1 mm/s. Completion of 25 mobilization cycles required approximately five minutes; therefore, the rate of mobilization was approximately five cycles per minute. At the end of the 25 mobilization cycles, three separate dye spread measurements were obtained and averaged. Pilot testing was conducted incorporating proximal and distal pins within the nerve sections to establish that the nerve section resting length did not change as a result of the 25 mobilization cycles. These results confirm that the specimens were not permanently deformed.
Intervention and post-test measurements in the control nerve sections
The control nerve sections were secured in the testing apparatus and injected with fluid as previously described. After the initial dye spread stabilized, these control segments were exposed to a 5-minute waiting period without the mobilization intervention. Post-test measurements were obtained at the end of the 5-minute waiting period as previously described.
Statistical analysis
Intra-rater reliability of the dye-spread measurements was calculated using the Intra-class Correlation Coefficient (ICC) model 3·1. The mean, standard deviation, 95% confidence intervals (95% CI), and range (minimum and maximum values) were calculated for the dye-spread distance for pre-test and post-test conditions in both the control and experimental nerve sections.
The effects of neural mobilization on longitudinal dye spread was determined using paired t-tests, which compared: (1) pre-test with post-test in the control nerve sections; (2) pre-test with post-test in the experimental nerve sections; and (3) change from pre-test to post-test between control and experimental nerve sections. Paired t-tests were used for the control versus experimental comparison (instead of independent t-test) because the samples comprising the groups were within-subject, matched pairs. Alpha level (two-tailed) was set to 0·05. Values of effect size (partial eta-squared) and statistical power also were reported. Effect sizes were interpreted based on the following criteria: small ≤0·01, medium = 0·06, and large ≧0·14.50 All statistical analyses were conducted using SPSS (v21·0).
Results
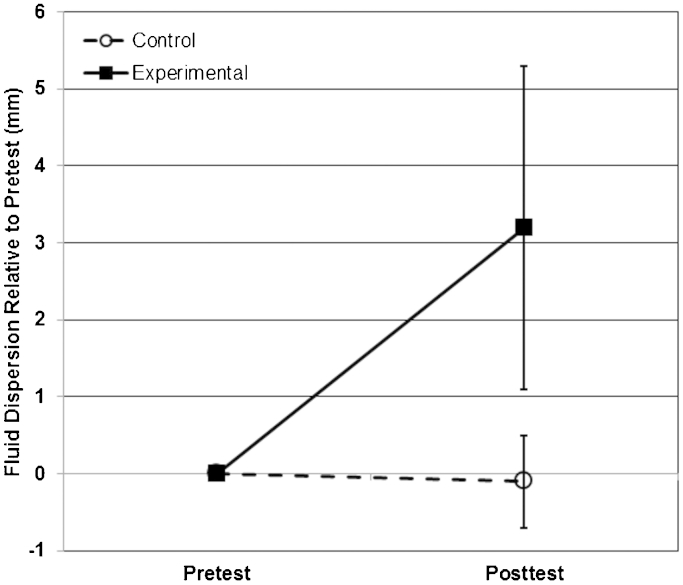
Individual dye-spread measurements were reliable (ICC = 0·99, 95% CI = 0·99–1·0). Neural mobilization increased fluid movement in the experimental nerve sections but not in the control sections. In the experimental nerve sections, fluid dispersion was 3·2±2·1 mm greater (t = 3·7, P = 0·015, partial eta-squared = 0·73, Power = 83%) after neural mobilization compared with before mobilization (Table 1 and Fig. 2). In the control nerve sections, fluid dispersion was 0·1 mm less but not significantly different (t = 0·3; P = 0·758, partial eta-squared = 0·02, Power = 6%) after the non-mobilization waiting period compared with before the waiting period (Table 1 and Fig. 2). Additionally, the change in fluid dispersion from pre-test to post-test was 3·3±2·1 mm greater (t = 3·78; P = 0·013, partial eta-squared = 0·74, Power = 85%) in the experimental nerve sections compared with the control sections (Table 1 and Fig. 2).
Table 1.
Descriptive results
| Control segment dye spread | Experimental segment dye spread | |||||
|---|---|---|---|---|---|---|
| Dye spread | Minimum and maximum | Mean±SD | 95% CI lower and upper bounds | Minimum and maximum | Mean±SD | 95% CI lower and upper bounds |
| Initial dye spread (mm) | 14·6–53·2 | 29·8±13·6 | 15·5–44·1 | 14·9–36·5 | 26·3±8·0 | 17·9–34·7 |
| Time to stabilize (minute) | 20·0–55·0 | 30·0±13·8 | 15·5–44·5 | 10·0–25·0 | 18·3±6·1 | 11·9–24·7 |
| Pre-test dye spread (mm) | 14·6–67·0 | 43·0±20·0 | 22·0–64·0 | 18·6–51·6 | 32·5±12·3 | 19·6–45·4 |
| Post-test dye spread (mm) | 14·3–66·8 | 42·9±20·2 | 21·7–64·1 | 21·4–54·4 | 35·7±11·9 | 23·2–48·2 |
| Dye spread length change (pre- to post-) (mm) | −1·0–0·7 | −0·1±0·6 | −0·7–0·5 | 1·2–7·4 | 3·2±2·1 | 0·9–5·4 |
| Dye spread percent change (%) | −2·4–1·1 | −0·1±1·3 | −1·5–1·3 | 5·1–32·0 | 11·8±10·6 | 0·7–22·9 |
Note:
Significantly (P⩽0·05) different from pre-test.
Significantly (P⩽0·05) different from control segment.
SD: standard deviation; CI: confidence interval.
Figure 2.
Mean dye spread measurements before (pre-test) and after (post-test) mobilization. The post-test experimental sections exhibited statistically significant dye spreads compared to the pre-test experimental and post-test control sections.
Discussion
Neural mobilization is a common and often effective treatment for peripheral nerve entrapment;20–24,26–28,51 however, mechanisms for clinical symptom improvement are unclear. This study examined and described the effects of neural mobilization on fluid dynamics of peripheral nerve tissue in vitro. The hypothesis that neural mobilization performed via a mechanical tissue tester would lead to significant dispersion of fluid within excised nerve tissue was supported. There was no significant longitudinal dispersion of the plasma/toluidine dye solution within the control section during the 5-minute ‘non-mobilization’ time window. Once the initial dye spread stabilized, mechanical intervention was required to produce further longitudinal dye spread. Significant longitudinal dye dispersion occurred within the experimental section during the 5-minute cyclical (stretch/relax) mobilization time, demonstrating an intervention effect. In addition, there was a significant difference of longitudinal dye spread between the post-test control section condition and the post-test experimental condition. This difference indicates that fluid dispersion occurred within peripheral nerve tissue as a result of neural mobilization in a cyclical (stretch/relax) manner, and not simply due to the force of gravity.
The use of excised cadaveric tissue created an environment where changes in dye spread could be attributed to the intraneural mechanical effects of passive neural mobilization instead of active physiological effects, such as blood flow, lymphatics, or axonal transport. The dye spread created from the effects of gravity was allowed to stabilize before the neural mobilization was performed. The lack of dye movement within the control sections during the ‘non-mobilization’ time period indicates that gravity did not further affect dye spread after initial stabilization had taken place.
The mechanisms that led to increased dye spread within the experimental (neural mobilization) sections cannot be known with complete certainty, but likely include the mechanical action and pressure gradient changes induced by the mechanical mobilization. Within this context, the physiological benefits of neural mobilization (often seen clinically) may be related to the repetitive movement of the nerve tissue instead of the mechanical abolition of scar tissue adhesions. Millesi et al.52 describe intrafascicular gliding and transverse contraction of nerve tissue with lengthening or stretching of nerve tissue. This transverse contraction from repetitive elongation/relaxation loading may create a change in intrafascicular pressure. It is possible that this change in intrafascicular pressure leads to a ‘pumping’ or ‘flushing’ of the intraneural fluid, with repetitive elongation/relaxation phases. Experimentally, this ‘pumping’ resulted in a movement of fluid, as indicated by the increased dye spread with neural mobilization. While not measured in this study, it is possible that the dispersal of excessive fluid (e.g. intraneural edema) as a result of neural mobilization could alter intraneural pressures, reducing the ‘miniature compartment syndrome’ effect described by Lundborg and colleagues18 and contributing to positive clinical responses.20–28,41
Increased intraneural pressures have been shown to be detrimental.9,19 Furthermore, in response to the use of repetitive motion, neural mobilization techniques may promote a healthy nerve environment by improving axonal transport and blood flow, and reducing detrimental chemical and mechanical components resulting from intraneural edema.3,14 These positive physiological responses may, in turn, limit demyelination,7,15,32 as well as changes in neural elasticity, fibrosis, and overall physical structure and function.11,29,31,32,52
Brown et al.47 reported similar results in their work regarding the tibial nerve at the ankle. The current study, however, incorporated a mechanical tester to deliver a specific, consistent, and repeatable amount of strain over a 5-minute period. The consistency of findings between these two studies suggests that the phenomenon of intraneural fluid movement due to passive mobilization using tension/relaxation cycles is physiologically possible within the normal range of nerve strain and may be a mechanism by which neural symptoms are alleviated clinically. If intraneural fluid was pumped out of the local region in cadaveric nerve sections through this passive mechanism and within a passive physiological environment, then it is possible that the benefits in live, physiologically active tissue, may be even greater when coupled with changes from dynamic functions, such as blood flow, lymphatic drainage, and axoplasmic transport.
The current study provides an in vitro method used to evaluate intraneural fluid dynamics in cadaveric peripheral nerve tissue. This method has also been shown to work in situ within peripheral tissue in the tibial nerve.47 While the passive environment of cadaveric tissue limits the ability to generalize these results to live patients; in vivo studies of this type are not feasible. Other than the tibial nerve, preliminary data suggest that a similar fluid dynamic behavior may be noted within other nerve tissues in other regions of the body in situ. Further studies are being conducted to exam the fluid dynamics of other nerve tissue such as lumbosacral roots, cervical roots, and upper extremity nerves. Cross-sectional neural fluid dispersion patterns were not studied in this investigation since the purpose was not to define and describe the pathways for fluid dispersion, but instead to determine whether fluid dispersion occurred at all in response to neural mobilizations. Such fluid spread patterns should be considered for future studies. Additionally, direct measurement of intraneural pressures during and after neural mobilization may provide insight about how changes in intraneural fluid affect the pressures within peripheral neural tissue.
Limitations to this cadaveric study include the inability to directly generalize the results found in this study as they were conducted on isolated sciatic nerve sections, removed from the cadaver specimen and therefore, that of a passive mechanical tissue system without the influence of active physiological systems. Future studies should assess the influence of normal physiological movements on in situ cadaveric specimens as well as the effects of blood flow and lymphatics on dispersing intraneural tissue fluid and diminishing intraneural swelling and/or pressure in animal models.
Conclusions
Repetitive stretch/relax mobilization of excised cadaveric peripheral (sciatic) nerve tissue sections induced a significant increase in intraneural fluid dispersion. This study indicates that in the in vitro state, mechanical input elicited from neural mobilization may alter the nerve tissue environment. While speculative at this point, this alteration of the nerve tissue environment may assist in promoting improved nerve health and function by dispersing intraneural swelling and decreasing intraneural pressure. While we cannot ascertain whether the effects of neural mobilization in a passive (cadaveric) system can be generalized to an active (live) physiological system, in the absence of active neural blood flow and lymphatic systems, it stands to reason that the results may in fact be more substantial in an active physiological system. This study contributes to the understanding of the possible underlying mechanisms of neural mobilization efficacy.
Disclaimer Statements
Contributors All authors were involved in the development of this manuscript to a degree that warrants authorship. Research design: Gilbert, James, Apte, Smith, Brismee, Sizer
Data Collection: Gilbert, James, Apte, Smith, Brown. Analysis: Gilbert, James, Smith, Brismee, Sizer. Manuscript development: Gilbert, James, Apte, Brown, Brismee, Sizer, Smith.
Funding This study was supported by a grant from the South Plains Foundation of Lubbock, Texas.
Conflicts of interest The authors declare they have no conflict of interest.
Ethics approval This study was conducted following the guidelines established by the Texas Anatomical Board and the Texas Tech University Health Sciences Center’s (TTUHSC) Institutional Review Board. The TTUHSC IRB does not require approval of cadaveric studies, but demands the following of the rules set forth by the Texas Anatomical Board.
Acknowledgements
The authors thank the Texas Tech University Health Sciences Center and the School of Allied Health Sciences for the use of Gross Anatomy and the Clinical Anatomy Research Labs, respectively. The authors also thank Claude Lobstein for his assistance during this project and the South Plains Foundation (Lubbock, Texas) for financial support of this study.
References
- 1.Greening J, Smart S, Leary R, Hall-Craggs M, O’Higgins P, Lynn B. Reduced movement of median nerve in carpal tunnel during wrist flexion in patients with non-specific arm pain. Lancet. 1999;354(9174):217–8. [DOI] [PubMed] [Google Scholar]
- 2.Greening J, Lynn B, Leary R, Warren L, O’Higgins P, Hall-Craggs M. The use of ultrasound imaging to demonstrate reduced movement of the median nerve during wrist flexion in patients with non-specific arm pain. J Hand Surg Br. 2001;26(5):401–6; discussion 407–8. [DOI] [PubMed] [Google Scholar]
- 3.Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115(3):248–53. [DOI] [PubMed] [Google Scholar]
- 4.Hough AD, Moore AP, Jones MP. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88(5):569–76. [DOI] [PubMed] [Google Scholar]
- 5.Dilley A, Lynn B, Pang SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain. 2005;117(3):462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown R, Pedowitz R, Rydevik B, Woo S, Hargens A, Massie J,. et al Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin Orthop Relat Res. 1993;296:288–94. [PubMed] [Google Scholar]
- 7.Dahlin LB, Shyu BC, Danielsen N, Andersson SA. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol Scand. 1989;136(1):97–105. [DOI] [PubMed] [Google Scholar]
- 8.Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR. Experimental stretch neuropathy. Changes in nerve conduction under tension. J Bone Joint Surg Br. 1992;74(1):126–9. [DOI] [PubMed] [Google Scholar]
- 9.Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975;57(7):938–48. [PubMed] [Google Scholar]
- 10.Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg Br. 1973;55(2):390–401. [PubMed] [Google Scholar]
- 11.Dahlin LB, Lundborg G. The neurone and its response to peripheral nerve compression. J Hand Surg Br. 1990;15(1):5–10. [DOI] [PubMed] [Google Scholar]
- 12.Dahlin LB, McLean WG. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurol Sci. 1986;72(1):19–30. [DOI] [PubMed] [Google Scholar]
- 13.Dahlin LB, Sjostrand J, McLean WG. Graded inhibition of retrograde axonal transport by compression of rabbit vagus nerve. J Neurol Sci. 1986;76(2–3):221–30. [DOI] [PubMed] [Google Scholar]
- 14.Dilley A, Bove GM. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol. 2008;586(2):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydevik B, McLean WG, Sjostrand J, Lundborg G. Blockage of axonal transport induced by acute, graded compression of the rabbit vagus nerve. J Neurol Neurosurg Psychiatry. 1980;43(8):690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanoue M, Yamaga M, Ide J, Takagi K. Acute stretching of peripheral nerves inhibits retrograde axonal transport. J Hand Surg Br. 1996;21(3):358–63. [DOI] [PubMed] [Google Scholar]
- 17.Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA. Vibration-induced disruption of retrograde axoplasmic transport in peripheral nerve. Muscle Nerve. 2005;32(4):521–6. [DOI] [PubMed] [Google Scholar]
- 18.Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a ‘miniature compartment syndrome’. J Neurol Neurosurg Psychiatry. 1983;46(12):1119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg. 1977;11(3):179–87. [DOI] [PubMed] [Google Scholar]
- 20.Alshami AM, Babri AS, Souvlis T, Coppieters MW. Biomechanical evaluation of two clinical tests for plantar heel pain: the dorsiflexion–eversion test for tarsal tunnel syndrome and the windlass test for plantar fasciitis. Foot Ankle Int. 2007;28(4):499–505. [DOI] [PubMed] [Google Scholar]
- 21.Butler DL. The sensitive nervous system. Adelaide: Noigroup; 2000. [Google Scholar]
- 22.Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006;11(4):279–86. [DOI] [PubMed] [Google Scholar]
- 23.Cleland JA, Hunt GC, Palmer JA. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: a single-case design. J Man Manip Ther. 2004;12:143–52. [Google Scholar]
- 24.Ekstrom RA, Holden K. Examination of and intervention for a patient with chronic lateral elbow pain with signs of nerve entrapment. Phys Ther. 2002;82(11):1077–86. [PubMed] [Google Scholar]
- 25.Ellis RF, Hing WA. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Man Manip Ther. 2008;16(1):8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitland GD. Negative disc exploration: positive canal signs. Aust J Physiother. 1979;25:129–34. [DOI] [PubMed] [Google Scholar]
- 27.Maitland GD. The slump test: examination and treatment. Aust J Physiother. 1985;1:215–9. [DOI] [PubMed] [Google Scholar]
- 28.Shacklock M. Neurodynamics. Physiotherapy. 1995;81:9–16. [Google Scholar]
- 29.Dahlin LB, Archer DR, McLean WG. Axonal transport and morphological changes following nerve compression. An experimental study in the rabbit vagus nerve. J Hand Surg Br. 1993;18(1):106–10. [DOI] [PubMed] [Google Scholar]
- 30.Lundborg G, Dahlin LB, Danielsen N, Hansson HA, Necking LE, Pyykko I. Intraneural edema following exposure to vibration. Scand J Work Environ Health. 1987;13(4):326–9. [DOI] [PubMed] [Google Scholar]
- 31.Abe Y, Doi K, Kawai S. An experimental model of peripheral nerve adhesion in rabbits. Br J Plast Surg. 2005;58(4):533–40. [DOI] [PubMed] [Google Scholar]
- 32.Rydevik B, Nordborg C. Changes in nerve function and nerve fibre structure induced by acute, graded compression. J Neurol Neurosurg Psychiatry. 1980;43(12):1070–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driscoll PJ, Glasby MA, Lawson GM. An in vivo study of peripheral nerves in continuity: biomechanical and physiological responses to elongation. J Orthop Res. 2002;20(2):370–5. [DOI] [PubMed] [Google Scholar]
- 34.Lundborg G, Dahlin LB. Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin. 1996;12(2):185–93. [PubMed] [Google Scholar]
- 35.Rydevik B, Lundborg G, Nordborg C. Intraneural tissue reactions induced by internal neurolysis. An experimental study on the blood–nerve barrier, connective tissues and nerve fibres of rabbit tibial nerve. Scand J Plast Reconstr Surg. 1976;10(1):3–8. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai M, Miyasaka Y. Neural fibrosis and the effect of neurolysis. J Bone Joint Surg Br. 1986;68(3):483–8. [DOI] [PubMed] [Google Scholar]
- 37.Martinoli C, Bianchi S, Gandolfo N, Valle M, Simonetti S, Derchi LE. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics. 2000;20:S199–213; discussion S213–7. [DOI] [PubMed] [Google Scholar]
- 38.Peer S, Kovacs P, Harpf C, Bodner G. High-resolution sonography of lower extremity peripheral nerves: anatomic correlation and spectrum of disease. J Ultrasound Med. 2002;21(3):315–22. [DOI] [PubMed] [Google Scholar]
- 39.Greening J, Lynn B. Minor peripheral nerve injuries: an underestimated source of pain? Man Ther. 1998;3(4):187–94. [Google Scholar]
- 40.Coppieters MW, Bartholomeeusen KE, Stappaerts KH. Incorporating nerve-gliding techniques in the conservative treatment of cubital tunnel syndrome. J Manipulative Physiol Ther. 2004;27(9):560–8. [DOI] [PubMed] [Google Scholar]
- 41.Shacklock M. Clinical Neurodynamics: a new system of musculoskeletal treatment. New York: Elsevier Butterworth-Heinemann; 2005. [Google Scholar]
- 42.Herrington L. Effect of different neurodynamic mobilizations techniques on knee extension range of motion in the slump position. J Man Manip Ther. 2006;14:101–7. [Google Scholar]
- 43.Ellis R, Hing W, Dilley A, McNair P. Reliability of measuring sciatic and tibial nerve movement with diagnostic ultrasound during a neural mobilisation technique. Ultrasound Med Biol. 2008;34(8):1209–16. [DOI] [PubMed] [Google Scholar]
- 44.Murphy DR, Hurwitz EL, Gregory AA, Clary R. A non-surgical approach to the management of lumbar spinal stenosis: a prospective observational cohort study. BMC Musculoskelet Disord. 2006;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert KK, Brismee JM, Collins DL, James CR, Shah RV, Sawyer SF,. et al Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 1. A novel measurement technique during straight leg raise in unembalmed cadavers. Spine (Phila Pa 1976). 2007;32(14):1513–20. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert KK, Brismee JM, Collins DL, James CR, Shah RV, Sawyer SF,. et al Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 2. A comparison of 2 straight leg raise conditions in unembalmed cadavers. Spine (Phila Pa 1976). 2007;32(14):1521–25. [DOI] [PubMed] [Google Scholar]
- 47.Brown CL, Gilbert KK, Brismee JM, Sizer PS, Roger James C, Smith MP. The effects of neurodynamic mobilization on fluid dispersion within the tibial nerve at the ankle: an unembalmed cadaveric study. J Man Manip Ther. 2011;19(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86(1):92–109. [DOI] [PubMed] [Google Scholar]
- 49.van Hoof T, Gomes GT, Audenaert E, Verstraete K, Kerckaert I, D’Herde K. 3D computerized model for measuring strain and displacement of the brachial plexus following placement of reverse shoulder prosthesis. Anat Rec (Hoboken). 2008;291(9):1173–85. [DOI] [PubMed] [Google Scholar]
- 50.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Upper Saddle River, NJ: Pearson/Prentice Hall; 2009. [Google Scholar]
- 51.Ellis RF, Hing WA. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Man Manip Ther. 2008;16(1):8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millesi H, Zoch G, Reihsner R. Mechanical properties of peripheral nerves. Clin Orthop Relat Res. 1995;314:76–83. [PubMed] [Google Scholar]