Abstract
The acute promyelocytic leukemia (APL) subtype of acute myeloid leukemia (AML) is characterized by chromosomal translocations that result in fusion proteins, including the promyelocytic leukemia-retinoic acid receptor, alpha fusion protein (PML-RARα). All-trans retinoic acid (atRA) treatment is the standard drug treatment for APL yielding cure rates >80% by activating transcription and proteasomal degradation of retinoic acid receptor, alpha (RARα). Whereas combination therapy with As2O3 has increased survival further, patients that experience relapse and are refractory to atRA and/or As2O3 is a clinically significant problem. BCL-2 family proteins regulate apoptosis and over-expression of anti-apoptotic B-cell leukemia/lymphoma 2 (BCL-2) family proteins has been associated with chemotherapeutic resistance in APL including impairment of the ability of atRA to induce growth arrest and differentiation. Here we investigated the novel BH3 domain mimetic, JY-1-106, which antagonizes the anti-apoptotic BCL-2 family members B-cell lymphoma-extra large (BCL-xL) and myeloid cell leukemia-1 (MCL-1) alone and in combination with retinoids including atRA, AM580 (RARα agonist), and SR11253 (RARγ antagonist). JY-1-106 reduced cell viability in HL-60 cells alone and in combination with retinoids. The combination of JY-1-106 and SR11253 had the greatest impact on cell viability by stimulating apoptosis. These studies indicate that dual BCL-xL/MCL-1 inhibitors and retinoids could work cooperatively in leukemia treatment.
Keywords: HL60, leukemia, BCL-xL, MCL-1, retinoic acid, apoptosis
Introduction
Leukemia is a hematological malignancy of which the acute myeloid leukemia (AML) type is estimated to yield 14,590 new cases and 10,370 deaths in 2013 [1]. Acute promyelocytic leukemia (APL) is a subtype of AML that makes up 5–10% of AML cases. APL is characterized by a chromosomal translocation, t(15:17), that results in the production of promyelocytic leukemia (PML) and retinoic acid receptor alpha (RARα) fusion proteins (PML-RARα) [2]. PML-RARα fusion proteins disrupt RARα signaling which is important to the differentiation of normal myeloid progenitor cells towards neutrophils [3]. All-trans retinoic acid (atRA), an active metabolite of vitamin A and high-affinity ligand that initiates RARα signaling, is the standard drug treatment for APL yielding cure rates exceeding 80% [3]. atRA activates PML-RARα dependent transcription and triggers proteasomal degradation of RARα [2, 4]. Arsenic trioxide (As2O3) has also been shown to have efficacy in APL treatment by inducing PML-RARα degradation by targeting the PML moiety and has shown success in treating APL both as a single agent therapeutic and in combination with atRA [4, 5]. Combination therapy with both atRA and As2O3 has increased patient survival rates to over 90% [4–7]. While atRA and As2O3 have dramatically increased patient survival, patients that relapse or are refractory to atRA and/or As2O3 remains a clinically significant problem [8].
The B-cell lymphoma-2 (BCL-2) family of proteins regulates apoptosis through both pro-apoptotic and anti-apoptotic proteins [9–15]. More particularly, protein–protein interactions between the BH3 domains of pro-apoptotic proteins (for example, BCL-2 antagonist/killer 1 (BAK1), BCL-2 associated X protein (BAX), BCL-2 associated death promoter (BAD), BCL2-11 or BCL2-like 11 (BIM), BH3 interacting domain death agonist (BID), phorbal-12-myristate-13-acetate-induced protein 1 (NOXA), P53 upregulated modulator of apoptosis (PUMA)) and the BH3-binding hydrophobic grooves on the surfaces of anti-apoptotic proteins (for example, BCL-2, B-cell lymphoma-extra large (BCL-xL), Myeloid cell leukemia-1 (MCL-1)) neutralize the cell killing function of the pro-apoptotic BCL-2 proteins [15]. Both BCL-xL and MCL-1 have been associated with chemotherapeutic resistance in cancer, including APL [10–12]. Notably, over-expression of MCL-1 impairs the ability of atRA to induce growth arrest and differentiation in APL [11]. High levels of BCL-xL protect cancer cell lines from cytotoxic agents and inactivation of BCL-xL potentiates apoptosis [13]. For these reasons, mimicry of the α-helical BH3 “death” domain of the pro-apoptotic BCL-2 proteins is presently an intense area of research towards expanding the armory of antineoplastics in a highly targeted manner [16,17]. Recently, BH3 domain mimetics that function as pan-BCL-2 antagonists, inhibiting BCL-2, BCL-xL, and MCL-1, have been developed based on an α-helix mimetic strategy that centers on a terphenyl scaffold [14]. Efforts to simplify the synthetic chemistry associated with the synthesis of terphenyl-based α-helix mimetics led to a related oligoamide-foldamer strategy [18]. The trisarylamide JY-1-106 is one such oligoamide-foldamer-based α-helix mimetic [19]. JY-1-106 disrupts interactions between both BCL-xL and MCL-1 with BAK1, leading to apoptosis through the mitochondrial pathway in human cancer cells, sensitizes tumor cells to conventional chemotherapeutic agents and inhibits tumor growth in a xenograft model of lung cancer [15].
BCL-2 was previously shown to cooperate with PML-RARα to block neutrophil differentiation and to initiate APL [20]. Mice co-expressing BCL-2 and PML-RARα developed leukemia more rapidly indicating that genetic alterations that inhibit apoptosis can exacerbate APL development [20]. As combination therapy with BH3 domain mimetics has been shown to be beneficial toward cell death and because atRA and other retinoids have been shown to impact APL, we investigated the atRA as well as RAR isoform-specific retinoids in combination with JY-1-106 in HL-60 human leukemia cells.
Materials and methods
Chemicals
The following chemicals were used: JY-1-106, synthesized by Mr. Jeremy L. Yap in the laboratory of Dr. S. Fletcher (University of Maryland, MD) as a BCL-xL/MCL-1 inhibitor; all-trans-retinoic acid (RA) (Sigma) as RAR pan-agonist; Am580, 4-[(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carboxamido]benzoic acid (Tocris Bioscience) as RARα agonist; SR11253 (or MM11253), 6-[2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1,3-dithiolan-2-yl] 2-naphthalenecarboxylic acid (Tocris Bioscience) as RARγ antagonist. Dimethyl sulfoxide (DMSO) (Sigma) was used as a delivery vehicle.
Cell culture
HL-60 cells were purchased from the American Type Culture Collection (ATCC). This cell line has been derived from peripheral blood cells of a 36-year old Caucasian female with acute promyelocytic leukemia (APL). The HL-60 cells grow as a suspension culture in Iscove’s minimal essential medium (Quality Biological Inc.) supplemented with 10–20% fetal bovine serum (FBS) (Invitrogen) and maintained in a humidified 5% CO2 incubator at 37°C as recommended by the ATCC. For all experiments, cells were used at a concentration of 1×106 cells/ml.
MTT proliferation assay
Cell viability was determined by the MTT assay, measuring the reduction of 3-(4, 5-dimethylthiasol-2-yl)-2, 4,-diphenyltetrazolium bromide (MTT) by mitochondrial succinate dehydrogenase. The MTT enters the cells and passes into the mitochondria where is reduced to an insoluble, colored, formazan product. The amount of color produced is directly proportional to the number of viable cells. Leukemic cells were incubated with various concentrations of JY-1-106 (2–20 μM) alone or with DMSO (as a vehicle) at different time point in 96-well plates. Cells treated with SR11253 200 nM, Am580 200 nM and RA 1 μM individually or together with JY-1-106 (12 μM) for 48 h. At time of assay, 10 μl of MTT (5 mg/ml in PBS) was added to each well and incubated for 3 h at 37°C. The medium was then carefully aspirated, and 100 μl of dimethyl sulfoxide (DMSO) was added to solubilize the colored formazan product, agitating the plates for 5 min on a shaker. The absorbance of each well was measured with a microtiter plate reader at a test wavelength of 570 nm with a reference wavelength of 690 nm. The optical density (OD) was calculated as the difference between the absorbance at the reference wavelength and the absorbance at the test wavelength. Percent viability was calculated as (OD of drug treated sample/OD of control)×100.
DAPI staining
The change in nuclear morphology was assessed by 4,6-diamidino-2-phenylindole (DAPI) staining. Cells with fragmented or condensed nuclei were defined as apoptotic cells. HL-60 were grown in 24-well plates, treated with 12μM JY-1-106 and 200 nM SR11253 individually or in combination at different time points and then placed on poly-L-lysine coated coverslip. Cells were fixed with 4% paraformaldehyde (ThermoScientific), stained with DAPI (Prolong Gold antifade reagent with DAPI, Invitrogen) and imaged using a fluorescent microscope. At least five visual fields were analyzed under fluorescence microscope for each sample and cells were quantified in each of the visual fields.
Quantitative Real-Time RT-PCR
Expression levels of target genes in HL-60 cells for all experimental conditions were determined quantitatively by real-time RT-PCR. Cells were grown in 10 cm dishes to 70–80% confluence, and exposed to 12μM JY-1-106 and 200 nM SR11253 individually or together for 48 h. Total RNA was isolated and purified by spin protocol using the RNeasy Mini kit (Qiagen, Inc.) and QIAshredder (Qiagen, Inc.) according to the manufacturer’s instructions. Two micrograms of total RNA were reverse-transcribed using components of a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Following reverse transcription, quantitative PCR amplification was performed on an StepOnePlus™ System (Applied Biosystems) using TaqMan Universal PCR Master Mix (Applied Biosystems), and gene-specific TaqMan PCR primers for RARα, RARγ, VEGF-A and b-actin (Applied Biosystems). Relative gene expression levels were normalized to the basal, untreated sample chosen as calibrator. Final results are expressed as folds of difference in gene expression relative to β-actin mRNA and calibrator, calculated following the ΔCt (cycle threshold) method, as follows: Relative expression (folds) = 2−(ΔCtsample − ΔCtcalibrator) where ΔCt values of the sample and calibrator were determined by subtracting the average Ct value of the β-actin mRNA reference gene from the average Ct value of the analyzed gene.
Ki-67 staining
Leukemic cell proliferation was assessed by Ki-67 staining. HL-60 were grown in 6-well plates and treated with 12μM JY-1-106 and 200 nM SR11253 individually or in combination for 48h. After treatments, 10μL of each cell suspension were loaded onto 1 well of 8-well slide coated with poly-L-Lysine. Cells were fixed with 4% paraformaldehyde and permeabilized by 0.1% Triton X-100 for 5 minutes. Slides were blocked with 10% goat serum, incubated with Rabbit anti Ki67 Antibody (Thermo Scientific) overnight in humidified chamber in cold room and then stained with Alexa Fluor® 555 Goat Anti-Rabbit antibody (Invitrogen) for 1 hour at room temperature in dark. One drop of Prolong Gold Antifade Reagent with DAPI (Invitrogen) was applied to each well. Images were taken by EVOS FL image system (Life technologies).
Western blot analysis
RARα and PML-RARα protein levels were detected by western blot analysis. HL-60 cells were grown in 6-well plates and treated with the following conditions for 48 hours: 1) DMSO as vehicle control, 2) 12μM JY-1-106, 3) 200nM SR11253, 4) 12μM JY-1-106 plus 200nM SR11253. After treatments, cells were collected by spinning down at 500xg for 10 minutes. Cell pellets were resuspended in 200μL RIPA buffer with protein inhibitor (cOmplete ULTRA Tablets, Mini, EDTA-free, EASYpack, Roche) and the lysates were centrifuged (17000xg 30 minutes at 4°C). Samples were separated by 4–20% SDS-PAGE and proteins were transferred to Immobilon-FL PVDF membrane (Millipore). Membrane was incubated with Odyssey Blocking Buffer (LI-COR) for 1 hour and then with Rabbit anti RARα antibodies (Biolegend) and β-Actin (8H10D10) Mouse mAb (Cell signaling) for 2 hours at room temperature. The membrane was washed 3 times with TBS-T and then incubated with IRDye 800CW Goat anti-Rabbit IgG (H + L) (LI-COR) and IRDye 680LT Goat anti-Mouse IgG (H + L) (LI-COR) for 1 hour. Images were taken and data were quantified by Odyssey® Fc system from LI-COR.
Statistical analysis
Statistical differences were determined by one-way analysis of variance (ANOVA) followed by Dunnet’s Multiple Comparison Test, and the results were expressed as mean ± SD from n independent experiments. Differences were considered statistically significant for P<0.05.
Results
The BCL-xL/MCL-1 inhibitor JY-1-106 affects cell viability in HL60 leukemia cells
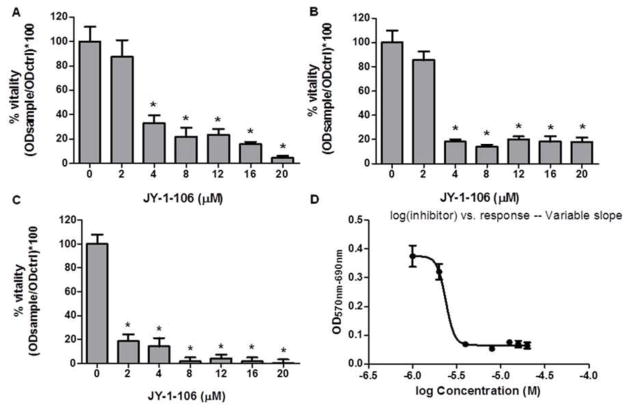
In order to examine the cytotoxic effect of JY-1-106, HL60 cells were cultured with 2 to 20 μM JY-1-106 for 24, 48, and 72 h and then cell viability was analyzed by MTT assay. Fig. 1 shows a significant reduction of cell viability in human leukemia cells. A strong cytotoxicity effect was demonstrated in HL60 cells after 24 h incubation of JY-1-106 at different concentrations (Fig. 1A). Cell survival declined drastically following an increase in the treatment time from 24 to 72 h in HL60 cells (Fig. 1A–C). Fig. 1D shows the typical dose-response for HL60 cells treated with JY-1-106 after 48 h with an IC50 of 2.4μM.
Fig. 1. The BCL-xL/MCL-1 inhibitor JY-1-106 affects human leukemia HL60 cell viability.
HL60 cells were treated with JY-1-106 at different concentrations and analyzed by MTT assay as described in Materials and methods. Statistical differences were determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test (n=4). A: 24 h B: 48 h C:72 h *p<0.0001 D: dose response curve after 48 h IC50= 2.4 uM.
Effect of combined BCL-xL/MCL-1 inhibitor JY-1-106 and RARs agonist/antagonist on cell viability in HL60 leukemia cells
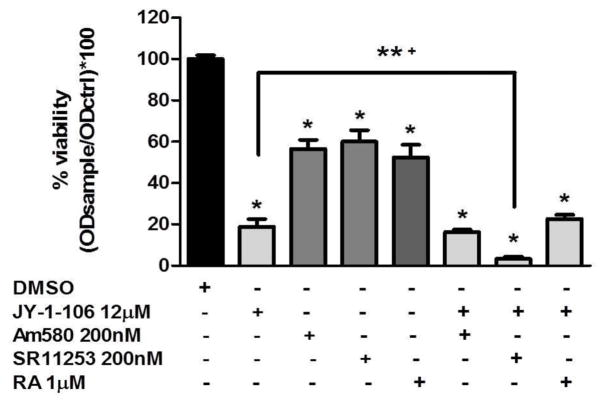
We tested the effects of RA (1μM, RAR pan-agonist), Am580 (200 nM, RARα agonist) and SR11253 (200 nM, RARγ antagonist) on human leukemia cell proliferation. As shown in Fig. 2, the effectiveness of all the compounds individually or in combination in reducing tumor cell viability is evident after 48 h incubation. A more pronounced effect as compared to the individual agent was observed with co-treatment of JY-1-106 12μM with SR11253 (≈95% cell growth inhibition). The combined treatment of the BCL-xL/MCL-1 inhibitor with the RARγ antagonist most strongly reduced the cell viability in HL60 leukemia cells. As such, we chose to further investigate the mechanism of action of combined JY-1-106 and SR11253 treatment.
Fig. 2. Effect of combined BCL-xL/MCL-1inhibitor JY-1-106 and RARs agonist/antagonist on cell viability in human leukemia cells.
HL60 cells were treated with 12μM JY-1-106 alone or in combination with 200nM Am580, 200nM SR11253 or 1μM RA after 48 h incubation and analyzed by MTT assay as described in Materials and methods. Statistical differences were determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test (n=4) * p<0.0001 **+ p<0.05.
The BCL-xL/MCL-1 inhibitor JY-1-106 in combination with SR11253 induces apoptosis in HL60 cells
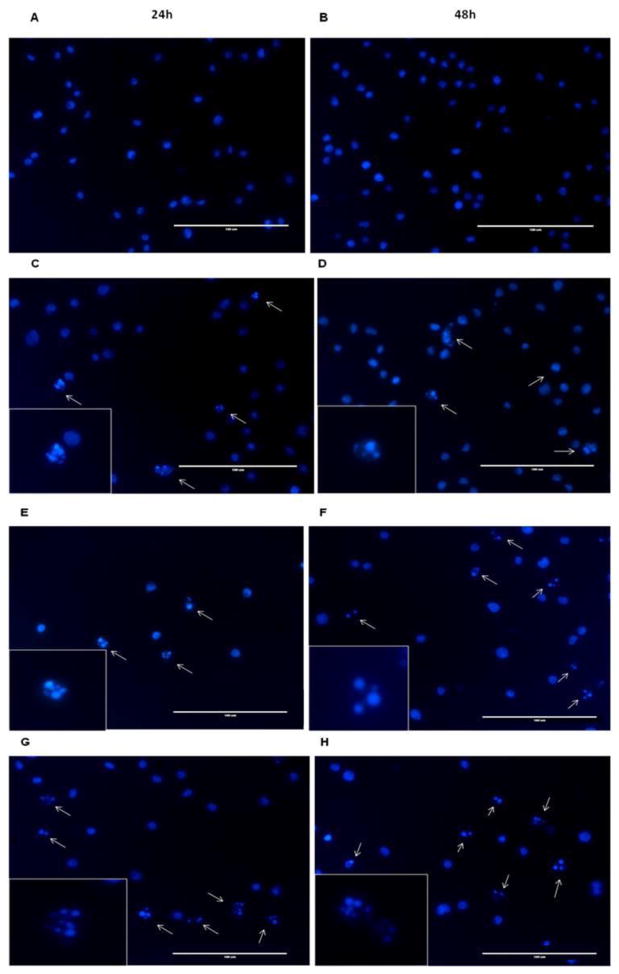
To determine whether the reduced cell viability involved apoptosis, we monitored the chromatin condensation of HL60 cells by DAPI staining. Cells treated with the BCL-xL/MCL-1 inhibitor JY-1-106 at 12μM and the RARγ antagonist SR11253 at 200nM strongly induces apoptosis between 24 and 48 h (Fig. 3). Statistical analysis indicates the number of apoptotic cells is significantly increased with either JY-1-106 or SR11253 with the greatest effect observed with the combination of JY-1-106 and SR11253 (Supplementary Fig. 1). The observed chromatin condensation indicates that the inhibition of cell growth in HL60 cells was associated with induction of apoptosis.
Fig. 3. DAPI staining in HL60 cells.
Morphology of apoptotic cell nuclei was observed by DAPI staining. HL60 cells were treated with (A–B) DMSO (vehicle), (C–D) 200nM SR11253, (E–F) 12μM JY-1-106, (G–H) 12μM JY-1-106 + 200nM SR11253 after 24/48 h and analyzed by a fluorescent microscope (40X magnification). The white arrows show the fragmented or condensed nuclei as apoptotic marker. The scale bar represents 100 μm. Figure is representative of three independent experiments.
RARα, RARγ and VEGF expression upon BCL-xL/MCL-1 inhibitor JY-1-106 and RARγ antagonist SR11253 in HL60 leukemia cells
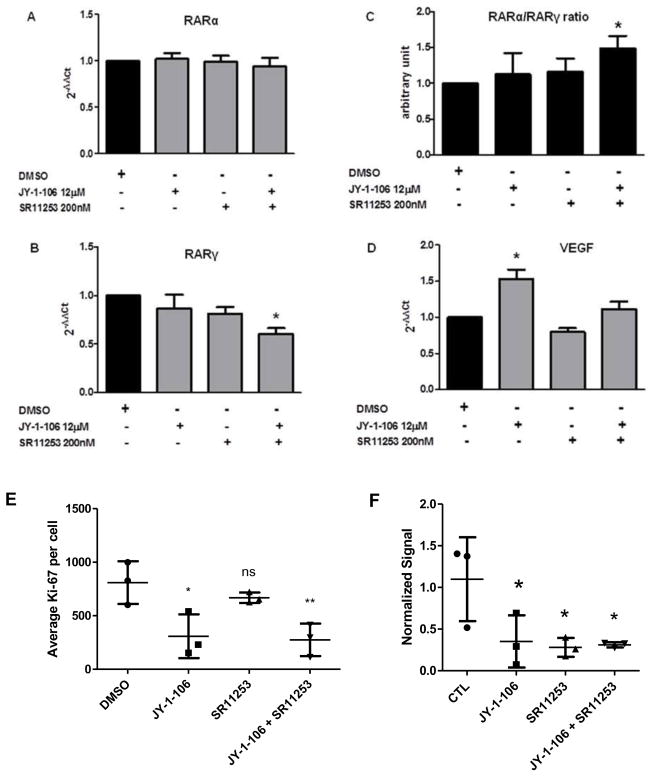
To further investigate the mechanism by which JY-1-106 and SR11253 impact apoptotic cell death, we examined the ability of the BCL-xL/MCL-1 inhibitor and the RARγ antagonist to modulate RARα, RARγ and VEGF expression in human leukemia cells. HL60 cells were treated with JY-1-106 (12μM) alone or in combination with SR11253 (200nM) for 48 h. RARα expression was not impacted by any of the treatment conditions, whereas RARγ was only significantly reduced with JY-1-106 + SR11253 treatment (Fig. 4A–B). As a result of the RARγ reduction, the RARα/RARγ ratio (Fig. 4C) was increased indicating that combination treatment with JY-1-106 and SR11253 makes the HL60 cells more responsive to apoptosis. We also observed that VEGF mRNA expression was up-regulated by JY-1-106 (12μM) whereas combination treatment with the BCL-xL/MCL-1 inhibitor and SR11253 (200nM) returned VEGF levels to that of control (Fig. 4D).
Fig. 4. Gene expression in HL60 cells.
HL60 cells were treated with 12μM JY-1-106 alone or in combination with 200nM SR11253 after 48 h incubation. The gene expression was perfomed as described in Materials and methods. Statistical differences were determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test (n=4) A: RARα not statistically significant; B: RARγ *p<0.05; C: RARα/RARγ ratio *p<0.05; D: VEGF *p<0.0001; E: Ki-67 quantification *p<0.05; F: PML-RARα quantification from Western Blot *p<0.05.
Because SR11253 antagonizes RARγ, which is pro-proliferative, cells were stained for Ki-67, a marker for proliferation. At 48h, SR11253 treatment resulted in a non-significant decrease in proliferation as compared to DMSO control. JY-1-106 inhibited proliferation significantly as compared to control with the combination of JY-1-106 and SR11253 yielding a similar inhibition to SR11253 alone (Fig. 4E, Supplementary Fig. 2). Additionally, we quantified the impact of SR11253 and JY-1-106 on PML-RARa protein levels. Each agent alone and in combination decreased protein expression of the PML-RARa fusion protein (Fig. 4F).
Discussion
The goal of these studies was to investigate the combination of BCL-xL/MCL-1 inhibition with atRA or retinoid receptor specific compounds in HL-60 APL cells. Overexpression of BCL-2 family members (i.e., BCL-xL and MCL-1) has been observed in many cancer types and has been shown to promote cancer cell survival, drug resistance, and defective apoptosis [21, 22]. MCL-1 and BCL-xL are anti-apoptotic members of the BCL-2 family. MCL-1 and BCL-xL have cellular expression that overlaps but is not identical [23, 24]. MCL-1 is also essential for survival of multipotent hematopoietic stem and progenitor cells and for the self-renewal capacity of pluripotent hematopoietic stem cells [25, 26]. Overexpression of MCL-1 has been associated with poor prognosis [27]. BCL-xL blocks the release of cytochrome c and the activation of caspases that induce apoptosis [12]. Inactivation of BCL-xL potentiates apoptosis [13].
JY-1-106 is a mimetic of the BH3 α-helical “death domain” of the pro-apoptotic BCL-2 proteins, such as BAK1 [28, 29]. In many cancers, including APL, excess BCL-xL and MCL-1 bind to the BH3 domain inactivating the function of pro-apoptotic BCL-2 proteins which promotes cell survival [10–13]. JY-1-106 inhibits BCL-xL and MCL-1 by binding the hydrophobic groove on the surfaces of those proteins which, in turn, sequesters the anti-apoptotic proteins through binding their hydrophobic groove that would normally bind BH3 domains. In this manner, JY-1-106 promotes apoptosis by disrupting the interaction of BCL-xL and MCL-1 with BAK1 in multiple cancer cell lines [15]. In this work, JY-1-106 effectively promoted apoptosis in HL60 cells, a hitherto unexplored cancer cell line (Fig. 1 – Fig. 3, Supplementary Fig. 1).
Because atRA and other vitamin A derived compounds are able to induce differentiation and apoptosis and because BCL-xL/MCL-1 inhibition can induce apoptosis in APL patients and in HL-60 cells, we sought to investigate the potential that these two therapeutic classes could work cooperatively in HL-60 cells to obtain a greater benefit [3, 4, 14, 15, 30, 31]. We tested atRA at a pharmacological dose (1 μM) as well as Am580, an RARα agonist, and SR11253, an RARγ antagonist, each at 200 nM due to their high affinity for their respective RAR isotypes [32, 33].
One limitation of atRA therapy is that atRA is a promiscuous ligand for binding to RAR receptors which can cause side effects [34]. In addition to inducing its beneficial effects, atRA treatment has also been shown to increase expression of MCL-1 and BCL-xL in APL [11, 35]. Studies have shown that down-regulation of MCL-1 sensitized cells to atRA mediated differentiation and apoptosis and blocking BCL-xL potentiated the action of atRA [11, 35]. Inhibition of MCL-1 has also been shown to inhibit c-Jun N-terminal kinase (JNK) which has been shown to be beneficial in potentiating the effects of atRA and As2O3 [11, 36]. As such, the combination of JY-1-106 (or other BH3 mimetics) and atRA may be beneficial towards sensitizing atRA resistant APL cells.
Another strategy for patients that experience atRA side effects or atRA syndrome is the use of retinoid receptor specific compounds [34, 37]. RARα specific compounds have been suggested to circumvent atRA toxicity [3]. Ligand activation of RARα is important to myeloid cell differentiation and treatment of HL-60 cells with a RARα agonist was sufficient to induce differentiation towards neutrophils [38, 39]. Activation of RARγ favors cell proliferation and survival as opposed to differentiation [40]. As such, RARγ antagonists interfere with cell growth [41]. Here, RARα agonist AM580 or RARγ antagonist SR11253 had similar reductions in cell viability (Fig. 2). The combination of JY-1-106 and RARγ antagonist SR11253 was most effective at inhibiting cell viability (Fig. 2). As such, we focused our efforts to characterize the combination of BH3 mimetics and retinoid compounds on the combination of JY-1-106 with SR11253 in evaluating the induction of apoptosis in HL-60 cells (Fig. 3) The fluorescence microscopy images of DAPI staining show a higher percentage of apoptotic cells with the combination of JY-1-106 and SR11253 as compared to either agent alone (Fig. 3, Supplementary Fig. 1). Altering the ratio of RARα/RARγ by pharmacologically activating RARα and/or inhibiting RARγ was shown to be favorable to cell death and reduced cell growth in vitro [42]. The combination of JY-1-106 and SR11253 had a significantly reduced RARγ level which shifted the RARα/RARγ ratio to favor cell death (Fig. 4).
An increase in VEGF production and angiogenesis has been observed in APL where atRA treatment reduces VEGF production and suppresses angiogenesis [43]. In this work, JY-1-106 increased VEGF levels while co-treatment with SR11253 returned VEGF levels to that of control (Fig. 4). Whereas combination treatment with JY-1-106 and SR11253 alleviated any increase in angiogenesis by reducing VEGF over expression, additional anti-angiogenic agents could be considered.
Based upon the quantification of the number of apoptotic cells, the combination of SR11253 with JY-1-106 seems to accelerate apoptosis with SR11253 with JY-1-106 at 24 h showing a similar degree of apoptosis as JY-1-106 alone at 48 h (Fig. 3). SR11253 combined with JY-1-106 does not yield an additional significant increase in apoptosis at 48 h as compared to 24 h. Yet cell viability (Fig. 2) indicates that the combination of SR11253 and JY-1-106 at 48 h shows a significant decrease in cell viability as compared to either JY-1-106 or SR11253 alone. Ki-67 staining data in Fig 4E and Supplementary Fig. 2 shows that inhibition of cell proliferation is an additional mechanism that contributes to the reduction in cell viability observed with the SR11253 and JY-1-106 combination. Furthermore, the impact of JY-1-106 and SR11253 on p27, a marker of cell cycle arrest, may contribute to the decrease in cell viability. JY-1-106 disrupts the BCL-xL protein interaction with BAK1 yielding more free BCL-xL which facilitates cell quiescence in G0 upregulating p27 [44]. RARγ anatagonism by SR11253 or RARγ silencing also increases level of p27 [42]. As such, the combination of SR11253 and JY-1-106 may have an additive effect on p27.
Activation of apoptosis and differentiation are essential elements in APL treatment and degradation of PML-RARα is required to fully clear the disease [44]. Recent work by Ablain et al. (2013) identified uncoupled retinoids (etretinate, etretinate’s active metabolite acitretin, or NRX195183) that activated RARα- or PML-RARα-dependent transcription but failed to degrade RARα or PML-RARα proteins. These retinoids elicited terminal differentiation and transcriptional activity, but the differentiated APL blasts retained PML-RARα expression which could re-initiate APL in secondary transplants [45]. As such, these compounds failed to abolish the leukemia-initiating activity of PML-RARα and demonstrated that differentiation alone is inadequate for APL eradication. Whereas our combination of JY-1-106 and SR11253 showed the greatest reduction in cell viability (Fig. 2), and also reduced the PML-RARα protein level (Fig. 4F), HL60 cells represent one patient-derived cell line. Other patient-derived cell lines will need to be tested to expand the utility of these findings. Due to the common use of atRA in patient care and the ability of atRA to also reduce PML-RARα, need for PML-RARα, the combination of JY-1-106 with atRA, which even though it displayed less of a reduction in cell viability than JY-1-106 with SR11253, might prove more practically effective. The combination of JY1-106 and atRA still had a greater impact on reducing cell viability than atRA alone, thus JY-1-106 combination therapy may prove useful in potentiating the effects of atRA.
Conclusions
Inhibition of the anti-apoptotic function of BCL-xL/MCL-1 represents a novel and promising strategy to potentiate the ability of atRA to induce apoptosis and differentiation as well as to overcome the resistance of cancers to chemotherapy. Our results indicate that dual Bcl-xL/Mcl-1 inhibitors and retinoids could work cooperatively in leukemia treatment.
Supplementary Material
Highlights.
Novel Bcl-xL / Mcl-1 inhibitor JY-1-106 reduces HL60 cell viability
JY-1-106 was investigated in combination with retinoic acid, AM580, and SR11253
AM580 is an RARα agonist; SR11253 is an RARγ antagonist
Combined use of JY-1-106 / SR11253 exhibited the greatest cell viability reduction
JY-1-106 alone or in combination with retinoids induces apoptosis
Acknowledgments
This work was supported by new faculty startup funds from the University of Maryland, School of Pharmacy (M.A.K.); U.S. National Institute of Allergy and Infectious Diseases contract HHSN272202000046C (M.A.K.); and the University of Maryland, School of Pharmacy Mass Spectrometry Facility: SOP1841-IQB2014 (M.A.K.). M.P. was supported by Regional Operative Programme (ROP) Calabria ESF 2007/2013–IV Axis Human Capital - Operative Objective M2-Action d.5 Postdoctoral Fellowship, in collaboration with University of Calabria (CS), Italy.
Abbreviations
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- atRA
all-trans retinoic acid
- BAD
BCL-2 associated death promoter
- BAK
BCL-2 antagonist/killer 1
- BAX
BCL-2 associated X protein
- BCL-2
B-cell lymphoma-2
- BCL-xL
B-cell lymphoma-extra large
- BID
BH3 interacting domain death agonist
- BIM
BCL211 or BCL2-like 11
- JNK
c-Jun N-terminal kinase
- MCL-1
Myeloid cell leukemia-1
- NOXA
phorbal-12-myristate-13-acetate-induced protein 1
- PML
promyelocytic leukemia
- PML-RARα
promyelocytic leukemia-retinoic acid receptor, alpha fusion protein
- PUMA
P53 upregulated modulator of apoptosis
- RAR
Retinoic Acid Receptor
- Am580
4-[(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carboxamido]benzoic acid
- SR11253
6-[2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1,3-dithiolan-2-yl]2-naphthalene carboxylic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.www.cancer.gov/statisitcs
- 2.Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–215. [PubMed] [Google Scholar]
- 3.Brown G, Hughes P. Retinoid differentiation therapy for common types of acute myeloid leukemia. Leuk Res Treatment. 2012;2012:939021. doi: 10.1155/2012/939021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Thé H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–83. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 5.Lallemand-Breitenbach V, Zhu J, Chen Z, de Thé H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18:36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, Li JM, Tang W, Zhao WL, Wu W, Sun HP, Chen QS, Chen B, Zhou GB, Zelent A, Waxman S, Wang ZY, Chen SJ, Chen Z. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106:3342–7. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH, Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lübbert M, Hänel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M, Ciceri F, Finizio O, Venditti A, Fabbiano F, Döhner K, Sauer M, Ganser A, Amadori S, Mandelli F, Döhner H, Ehninger G, Schlenk RF, Platzbecker U Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 8.Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int J Hematol. 2013;97:717–25. doi: 10.1007/s12185-013-1354-4. [DOI] [PubMed] [Google Scholar]
- 9.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 10.Schimmer AD, Hedley DW, Penn LZ, Minden MD. Receptor- and mitochondrial-mediated apoptosis in acute leukemia: a translational view. Blood. 2001 Dec;98:3541–53. doi: 10.1182/blood.v98.13.3541. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Ikezoe T, Nishioka C, Yokoyama A. Over-expression of Mcl-1 impairs the ability of ATRA to induce growth arrest and differentiation in acute promyelocytic leukemia cells. Apoptosis. 2013;18:1403–15. doi: 10.1007/s10495-013-0872-0. [DOI] [PubMed] [Google Scholar]
- 12.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–7. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 13.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–10. [PubMed] [Google Scholar]
- 14.Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, Rodriguez JM, Becerril J, Berndt N, Hamilton AD, Wang HG, Sebti SM. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J Biol Chem. 2011;286:9382–92. doi: 10.1074/jbc.M110.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X, Yap JL, Newell-Rogers MK, Peddaboina C, Jiang W, Papaconstantinou HT, Jupitor D, Rai A, Jung KY, Tubin RP, Yu W, Vanommeslaeghe K, Wilder PT, MacKerell AD, Jr, Fletcher S, Smythe RW. The novel BH3 α-helix mimetic JY-1-106 induces apoptosis in a subset of cancer cells (lung cancer, colon cancer and mesothelioma) by disrupting Bcl-xL and Mcl-1 protein-protein interactions with Bak. Mol Cancer. 2013;12:42. doi: 10.1186/1476-4598-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith KC, Hogarty MD. Small-molecule BH3 mimetics to antagonize Bcl-2-homolog survival functions in cancer. Curr Opin Investig Drugs. 2009;10:559–71. [PubMed] [Google Scholar]
- 17.Bodur C, Basaga H. Bcl-2 inhibitors: emerging drugs in cancer therapy. Curr Med Chem. 2012;19:1804–20. doi: 10.2174/092986712800099839. [DOI] [PubMed] [Google Scholar]
- 18.Ernst JT, Becerril J, Park HS, Yin H, Hamilton AD. Design and application of an alpha-helix-mimetic scaffold based on an oligoamide-foldamer strategy: antagonism of the Bak BH3/Bcl-xL complex. Angew Chem Int Ed Engl. 2003;42:535–9. doi: 10.1002/anie.200390154. [DOI] [PubMed] [Google Scholar]
- 19.Yap JL, Cao X, Vanommeslaeghe K, Jung KY, Peddaboina C, Wilder PT, Nan A, MacKerell AD, Jr, Smythe WR, Fletcher S. Relaxation of the rigid backbone of an oligoamide-foldamer-based α-helix mimetic: identification of potent Bcl-xL inhibitors. Org Biomol Chem. 2012;10:2928–33. doi: 10.1039/c2ob07125h. [DOI] [PubMed] [Google Scholar]
- 20.Kogan SC, Brown DE, Shultz DB, Truong BT, Lallemand-Breitenbach V, Guillemin MC, Lagasse E, Weissman IL, Bishop JM. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARalpha) to block neutrophil differentiation and initiate acute leukemia. J Exp Med. 2001;193:531–43. doi: 10.1084/jem.193.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 22.Shangary S, Johnson DE. Recent advances in the development of anticancer agents targeting cell death inhibitors in the Bcl-2 protein family. Leukemia. 2003;17:1470–81. doi: 10.1038/sj.leu.2403029. [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128:1173–84. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia L, Macey MG, Yin Y, Newland AC, Kelsey SM. Subcellular distribution and redistribution of Bcl-2 family proteins in human leukemia cells undergoing apoptosis. Blood. 1999;93:2353–9. [PubMed] [Google Scholar]
- 25.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–4. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 26.Campbell CJ, Lee JB, Levadoux-Martin M, Wynder T, Xenocostas A, Leber B, Bhatia M. The human stem cell hierarchy is defined by a functional dependence on Mcl-1 for self-renewal capacity. Blood. 2010;116:1433–42. doi: 10.1182/blood-2009-12-258095. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, Reed JC. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998;91:991–1000. [PubMed] [Google Scholar]
- 28.Yap JL, Cao X, Vanommeslaeghe K, Jung KY, Peddaboina C, Wilder PT, Nan A, MacKerell AD, Jr, Smythe WR, Fletcher S. Relaxation of the rigid backbone of an oligoamide-foldamer-based α-helix mimetic: identification of potent Bcl-xL inhibitors. Org Biomol Chem. 2012;10:2928–33. doi: 10.1039/c2ob07125h. [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Yap JL, Newell-Rogers MK, Peddaboina C, Jiang W, Papaconstantinou HT, Jupitor D, Rai A, Jung KY, Tubin RP, Yu W, Vanommeslaeghe K, Wilder PT, MacKerell AD, Jr, Fletcher S, Smythe RW. The novel BH3 α-helix mimetic JY-1-106 induces apoptosis in a subset of cancer cells (lung cancer, colon cancer and mesothelioma) by disrupting Bcl-xL and Mcl-1 protein-protein interactions with Bak. Mol Cancer. 2013;12:42. doi: 10.1186/1476-4598-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980;77:2936–40. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tallman MS. New agents for the treatment of acute myeloid leukemia. Best Pract Res Clin Haematol. 2006;19:311–20. doi: 10.1016/j.beha.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Delescluse C, Cavey MT, Martin B, Bernard BA, Reichert U, Maignan J, Darmon M, Shroot B. Selective high affinity retinoic acid receptor alpha or beta-gamma ligands. Mol Pharmacol. 1991;40:556–62. [PubMed] [Google Scholar]
- 33.Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem. 2002;9:623–37. doi: 10.2174/0929867023370789. [DOI] [PubMed] [Google Scholar]
- 34.Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP., Jr The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292–6. doi: 10.7326/0003-4819-117-4-292. [DOI] [PubMed] [Google Scholar]
- 35.Xia L, Wurmbach E, Waxman S, Jing Y. Upregulation of Bfl-1/A1 in leukemia cells undergoing differentiation by all-trans retinoic acid treatment attenuates chemotherapeutic agent-induced apoptosis. Leukemia. 2006;20:1009–16. doi: 10.1038/sj.leu.2404198. [DOI] [PubMed] [Google Scholar]
- 36.Huang HS, Liu ZM, Hong DY. Blockage of JNK pathway enhances arsenic trioxide-induced apoptosis in human keratinocytes. Toxicol Appl Pharmacol. 2010;244:234–41. doi: 10.1016/j.taap.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 37.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H, Burnett AK, Lo-Coco F. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 38.Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001;97:1314–20. doi: 10.1182/blood.v97.5.1314. [DOI] [PubMed] [Google Scholar]
- 39.Hughes PJ, Zhao Y, Chandraratna RA, Brown G. Retinoid-mediated stimulation of steroid sulfatase activity in myeloid leukemic cell lines requires RARalpha and RXR and involves the phosphoinositide 3-kinase and ERK-MAP kinase pathways. J Cell Biochem. 2006;97:327–50. doi: 10.1002/jcb.20579. [DOI] [PubMed] [Google Scholar]
- 40.Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203:1283–93. doi: 10.1084/jem.20052105. Erratum in: J Exp Med. 2006; 203: 1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keedwell RG, Zhao Y, Hammond LA, Wen K, Qin S, Atangan LI, Shurland DL, Wallace DM, Bird R, Reitmair A, Chandraratna RA, Brown G. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br J Cancer. 2004;91:580–8. doi: 10.1038/sj.bjc.6602024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosch A, Bertran SP, Lu Y, Garcia A, Jones AM, Dawson MI, Farias EF. Reversal by RARα agonist Am580 of c-Myc-induced imbalance in RARα/RARγ expression during MMTV-Myc tumorigenesis. Breast Cancer Res. 2012;14:R121. doi: 10.1186/bcr3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kini AR, Peterson LA, Tallman MS, Lingen MW. Angiogenesis in acute promyelocytic leukemia: induction by vascular endothelial growth factor and inhibition by all-trans retinoic acid. Blood. 2001;97:3919–24. doi: 10.1182/blood.v97.12.3919. [DOI] [PubMed] [Google Scholar]
- 44.Janumyan Y, Cui Q, Yan L, Sansam CG, Valentin M, Yang E. G0 Function of BCL2 and BCL-xL Requires BAX, BAK, and p27 Phosphorylation by Mirk, Revealing a Novel Role of BAX and BAK in Quiescence Regulation. J. Biol. Chem. 2008;283:34108–34120. doi: 10.1074/jbc.M806294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ablain J, Leiva M, Peres L, Fonsart J, Anthony E, de Thé H. Uncoupling RARA transcriptional activation and degradation clarifies the bases for APL response to therapies. J Exp Med. 2013;210:647–53. doi: 10.1084/jem.20122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.