Abstract
Background
Concerns remain whether robot-assisted radical cystectomy (RARC) compromises survival because of inadequate oncologic resection or alteration of recurrence patterns.
Objective
To describe recurrence patterns following open radical cystectomy (ORC) and RARC.
Design, setting, and participants
Retrospective review of 383 consecutive patients who underwent ORC (n = 120) or RARC (n = 263) at an academic institution from July 2001 to February 2014.
Intervention
ORC and RARC.
Outcome measurements and statistical analysis
Recurrence-free survival estimates were illustrated using the Kaplan-Meier method. Recurrence patterns (local vs distant and anatomic locations) within 2 yr of surgery were tabulated. Cox regression models were built to evaluate the effect of surgical technique on the risk of recurrence.
Results and limitations
The median follow-up time for patients without recurrence was 30 mo (interquartile range [IQR] 5–72) for ORC and 23 mo (IQR 9–48) for RARC (p = 0.6). Within 2 yr of surgery, there was no large difference in the number of local recurrences between ORC and RARC patients (15/65 [23%] vs 24/136 [18%]), and the distribution of local recurrences was similar between the two groups. Similarly, the number of distant recurrences did not differ between the groups (26/73 [36%] vs 43/147 [29%]). However, there were distinct patterns of distant recurrence. Extrapelvic lymph node locations were more frequent for RARC than ORC (10/43 [23%] vs 4/26 [15%]). Furthermore, peritoneal carcinomatosis was found in 9/43 (21%) RARC patients compared to 2/26 (8%) ORC patients. In multivariable analyses, RARC was not a predictor of recurrence. Limitations of the study include selection bias and a limited sample size.
Conclusions
Within limitations, we found that RARC is not an independent predictor of recurrence after surgery. Interestingly, extrapelvic lymph node locations and peritoneal carcinomatosis were more frequent in RARC than in ORC patients. Further validation is warranted to better understand the oncologic implications of RARC.
Patient summary
In this study, the locations of bladder cancer recurrences following conventional and robotic techniques for removal of the bladder are described. Although the numbers are small, the results show that the distribution of distant recurrences differs between the two techniques.
Keywords: Bladder cancer, Open radical cystectomy, Robot-assisted radical cystectomy, Recurrence, Local, Distant
1. Introduction
Open radical cystectomy (ORC) is the mainstay of therapy for patients with muscle-invasive and high-risk non–muscle-invasive bladder cancer (BCa) [1]. Following successful adoption of minimally invasive techniques in kidney and prostate surgery, the last few years have seen growing interest in robot-assisted radical cystectomy (RARC). In retrospective studies [2,3] and in the Memorial Sloan Kettering Cancer Center randomized trial [4], large differences in complication rates between RARC and ORC have not been observed.
However, the introduction of RARC into surgical practice has been accompanied by legitimate concerns regarding its oncologic efficacy [5]. To date, favorable outcomes in terms of positive surgical margin (PSM) rates and lymph node yield have been published [2,3,6]. Early oncologic outcomes appear to be acceptable [7–9]. Nevertheless, the use of RARC remains controversial and restricted to specialized centers [3,6,7,9]. Furthermore, there is anecdotal evidence of peritoneal seeding during minimally invasive surgery [10], and pneumoperitoneum may impact BCa cell seeding [11].
In light of the possibility of unusual recurrence locations after RARC, limited information is available for the robotic approach with regard to patterns of disease recurrence. In the present study we describe recurrence patterns in patients who underwent ORC and RARC.
2. Patients and methods
2.1. Patient population
Institutional review board approval was obtained to use data prospectively maintained in our BCa registry. A total of 411 RCs (136 ORC, 275 RARC) were performed at Weill Cornell Medical College by one surgeon from July 2001 to February 2014. Patients with non-bladder primary tumors (n = 24; 14 ORC, 10 RARC) and for whom RC had only palliative indication (n = 4; 2 ORC, 2 RARC) were excluded. A total of 383 patients (120 ORC, 263 RARC) remained for final analysis. Clinical stage was assigned based on a combination of specimen pathology at transurethral resection of the bladder tumor, evaluation during examination under anesthesia, and imaging studies. By definition, preoperative chemotherapy was administered to patients with clinically metastatic disease in lymph nodes and/or unresectable disease. In the case of a clinical response, surgical consolidation with RC and pelvic lymph node dissection (PLND) was offered. Neoadjuvant chemotherapy was proposed to patients with clinically nonmetastatic disease [12], and its use steadily increased during the study period. For instance, for patients with clinical T2–T4 disease, the rate of neoadjuvant therapy increased from 27% in 2001–2009 to 47% in 2010–2014. Adjuvant chemotherapy was proposed within 3 mo of surgery according to pathologic stage (T3–4, positive nodes) [12]. Institution of any chemotherapy was also discussed according to the patient’s performance status, and the decision was ultimately made at the discretion of the patient and the genitourinary medical oncologist. To reduce the effect of variable use of chemotherapy on outcome, all three chemotherapy regimens were grouped into a single variable, perioperative chemotherapy, in the analyses.
2.2. Surgical techniques
The standard techniques for ORC and RARC have been described previously [13,14]. In both techniques, the limits of the PLND were the upper border of the common iliac artery superiorly, Cooper’s ligament (including the node of Cloquet) inferiorly, the genitofemoral nerve laterally, and the bladder and sacral promontory medially. Although we attempt PLND in every RC candidate, this was not surgically feasible in eight patients because of prior pelvic irradiation, in two patients because PLND had been performed previously (in the context of nephroureterectomy in one case and an aborted RC attempt elsewhere in the other), and in five patients for other reasons (elderly morbid patients in three cases, one case of marked retroperitoneal desmoplastic reaction in the context of acute myelogenous leukemia and status after chemotherapy, and one case in which the tumor was unexpectedly found to be infiltrating adjacent structures in the pelvis).
2.3. Outcomes measures
2.3.1. Pathologic data
Bladder specimens were evaluated according to a standard pathology protocol. Pathologic data included histologic type, tumor grade according to the World Health Organization/International Society of Urological Pathology consensus classification [15], tumor and nodal stage according to the 2002 TNM classification [16], the presence of lymphovascular invasion, and surgical margin status. A soft-tissue PSM was defined as the presence of tumor at the bladder and urethral and/or ureteral margin.
2.3.2. Oncologic outcomes
During the entire study period, the follow-up protocol comprised history, physical examination, urine cytology, and laboratory measurements every 3–4 mo in the first year, semi-annually in the second year, and annually thereafter. Diagnostic imaging was performed at least annually or when clinically indicated. Documentation of events was based on clinical and radiologic findings, and categorized as the first evidence of local recurrence, distant recurrence, or secondary urothelial carcinoma. Local recurrences, by definition, occurred within the soft-tissue field of exenteration (cystectomy bed and PLND template). Distant recurrences were defined as those that occurred at any other site. Peritoneal carcinomatosis was diagnosed either by imaging (nodular or solid peritoneal masses, focal or nodular peritoneal thickening in abdominopelvic computed tomography [CT]) [17] or intraoperatively during surgery for abdominal symptoms. Histologic confirmation was obtained whenever possible.
2.3.3. Statistical analysis
The χ2 test (or Fisher’s exact test) and Mann-Whitney U test were used to compare baseline variables between the two groups. Kaplan-Meier curves were used to illustrate the probability of recurrence-free survival (RFS) for the entire cohort. Because our cohort was not balanced in terms of stage (Table 1), Kaplan-Meier curves for patients with pathologic stage T0/Ta/Tis/T1 (n = 181), T2–T4 (n = 202), N0/Nx (n = 301), and N1–N3 (n = 82) were generated to reduce the effect of selection bias. Recurrence patterns within 2 yr of surgery were also described. Each patient was followed to recurrence or 2 yr of follow-up, whichever came first. For descriptive purposes, percentages were calculated as: number of patients with event within 2 yr/(number of patients with event within 2 yr + number of patients without event and follow-up ≥2 yr). Finally, a multivariable Cox regression model including all patients of the cohort tested for the effect of surgical technique on the risk of recurrence, adjusting for patient age (continuous), female gender (yes/no), clinical stage (T0/Ta/Tis, T1, T2, T3, T4), perioperative (i.e. preoperative, neoadjuvant, or adjuvant) chemotherapy (yes/no), pathologic stage (T0/Ta/Tis, T1, T2, T3, T4), nodal stage (N0/Nx, N1-3), lymphovascular invasion (yes/no), and PSM (yes/no). Collinearity between predictors was evaluated before formulating the final multivariable model. Competing-risks survival regression was also performed to correct the univariate and multivariable hazard ratios for the competing event of death before recurrence [18]. All p values are two-sided, with statistical significance evaluated at the α = 0.05 level. We calculated 95% confidence intervals (CIs) to assess the precision of the estimates obtained. All analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC, USA).
Table 1.
Baseline characteristics of patients treated with open (ORC) and robot-assisted radical cystectomy (RARC)
| Parameter | ORC (n = 120) | RARC (n = 263) | p value |
|---|---|---|---|
| Age (yr) | 69 (63–75) | 72 (65–79) | 0.002 |
| Female gender | 35 (29) | 56 (21) | 0.1 |
| Ethnic origin | 0.5 | ||
| White | 104 (87) | 220 (84) | |
| Black | 8 (7) | 14 (5) | |
| Asian | 3 (3) | 14 (5) | |
| Other | 5 (4) | 15 (6) | |
| Body mass index (kg/m2) | 24 (24–28) | 25 (23–28) | 0.6 |
| History of smoking | 82 (68) | 148 (56) | 0.03 |
| ASA score >2 | 65 (54) | 138 (52) | 0.8 |
| Previous abdominal surgery | 47 (39) | 94 (36) | 0.5 |
| Previous pelvic radiotherapy | 11 (9) | 26 (10) | 0.8 |
| Preoperative creatinine (mg/dl) | 1.02 (0.90–3.62) | 1.09 (0.87–1.46) | 0.7 |
| eGFR (ml/min/1.73 m2) | 73 (25–177) | 60 (47–80) | 0.4 |
| Type of urinary diversion | 0.4 a | ||
| Ileal conduit | 63 (53) | 157 (60) | |
| CCUD | 22 (18) | 49 (18) | |
| OBS | 33 (28) | 55 (21) | |
| No diversion | 2 (2) | 2 (1) | |
| Clinical stage | 0.02 | ||
| T0/Ta/Tis | 6 (5) | 31 (12) | |
| T1 | 37 (31) | 62 (24) | |
| T2 | 54 (45) | 142 (54) | |
| T3 | 14 (12) | 20 (8) | |
| T4 | 9 (8) | 8 (3) | |
| Preoperative/neoadjuvant chemotherapy | 28 (23) | 62 (24) | >0.9 |
| Adjuvant chemotherapy | 21 (18) | 45 (17) | >0.9 |
| Histologic type | 0.2 a | ||
| UC | 107 (89) | 250 (95) | |
| SCC | 5 (4) | 5 (2) | |
| Adenocarcinoma | 5 (4) | 4 (2) | |
| Others | 3 (3) | 4 (2) | |
| Pathologic stage | 0.03 | ||
| T0/Ta/Tis | 31 (26) | 105 (40) | |
| T1 | 16 (13) | 29 (11) | |
| T2 | 22 (18) | 39 (15) | |
| T3 | 27 (23) | 61 (23) | |
| T4 | 24 (20) | 29 (11) | |
| High tumor grade | 114 (95) | 258 (98) | 0.1 |
| Lymphovascular invasion | 37 (31) | 61 (23) | 0.1 |
| Soft-tissue positive margin | 15 (13) | 16 (6) | 0.03 |
| Lymph nodes removed (n) | 20 (11–27) | 21 (13–28) | 0.3 |
| Pathologic nodal stage | 0.4 | ||
| Nx | 6 (5) | 9 (3) | |
| N0 | 84 (70) | 202 (77) | |
| N1 | 12 (10) | 18 (7) | |
| N2 | 15 (13) | 32 (12) | |
| N3 | 3 (3) | 2 (1) |
ASA = American Society of Anesthesiologists; eGFR = estimated glomerular filtration rate according to Chronic Kidney Disease Epidemiology Collaboration; CCUD = continent cutaneous urinary diversion; OBS = orthotopic bladder substitute; UC = urothelial carcinoma; SCC = squamous cell carcinoma.
Continuous data are presented as median (interquartile range) and categorical data as n (%). Percentages may not sum to 100% because of rounding.
Fisher’s exact test. All other p values calculated using χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively.
3. Results
3.1. Baseline and pathologic data
The distribution of pathologic stage was unbalanced between the groups (p = 0.03; Table 1). Pathologic stage T4 was found in 24 (20%) of 120 ORC patients and 29 (11%) of 263 RARC patients, while pathologic stage T0/Ta/Tis was found in 31 (26%) of 120 ORC patients and 105 (40%) of 263 RARC patients. This difference in stage distribution was reflected in the rates of PSM (p = 0.03).
3.2. Oncologic outcomes and patterns of recurrence
The median follow-up time for patients without recurrence was 30 mo (interquartile range [IQR] 5–72) for ORC patients and 23 mo (IQR 9–48 mo) for RARC patients (p = 0.6). At last follow-up, 99 patients had experienced a recurrence, 37 in the ORC group and 62 in the RARC group. Kaplan-Meier curves illustrating RFS probability after ORC and RARC for all patients and after stratification by tumor and nodal stage are shown in Figure 1.
Fig. 1.
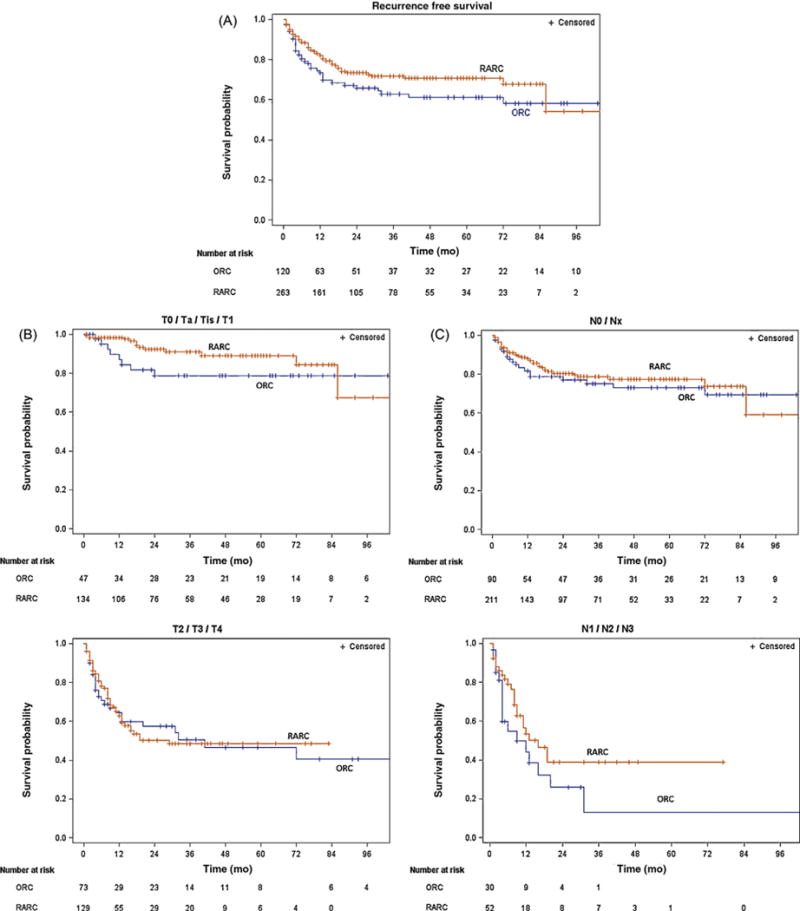
Kaplan-Meier estimates of recurrence-free survival probability according to surgical technique in (A) all 383 patients who underwent open radical cystectomy (ORC) or robot-assisted radical cystectomy (RARC); (B) non–muscle-invasive and muscle-invasive cases; and (C) node-negative and node-positive cases.
There was no large difference in the number of local recurrences within 2 yr between ORC and RARC patients (15/65 [23%] vs 24/136 [18%]), and the distribution of local recurrences was similar between the groups (Table 2). Similarly, the number of distant recurrences did not differ between ORC and RARC patients (26/73 [36%] vs 43/147 [29%]). However, there were distinct patterns of distant recurrence. Extrapelvic lymph node locations were more frequent in RARC than in ORC patients (4/26 [15%] ORC patients with distant recurrence vs 10/43 [23%] RARC patients with distant recurrence). In detail, all cases in the ORC group and seven cases in the RARC group were recurrences in the retroperitoneum. In addition, two recurrences in the RARC group were detected in the cervical chain and one in the mediastinum. Furthermore, peritoneal carcinomatosis was found in 2/26 (8%) ORC patients with distant recurrence, in contrast to 9/43 (21%) RARC patients with distant recurrence. In detail, five RARC patients had peritoneal carcinomatosis only, all diagnosed with abdominopelvic CT and histologically confirmed in three patients. Four RARC patients with multiple recurrence locations also had peritoneal carcinomatosis, confirmed histologically in one case. The two cases of peritoneal carcinomatosis in ORC patients were diagnosed by CT only. No port-site metastasis was documented in the RARC cohort.
Table 2.
Distribution of locations among patients with recurrence and secondary urothelial carcinomas within 2 yr after open (ORC) and robot-assisted radical cystectomy (RARC)
| Variable | ORC | RARC |
|---|---|---|
| Any recurrence a | 33/79 (42) | 57/158 (36) |
| Local recurrence a | 15/65 (23) | 24/136 (18) |
| Cystectomy bed | 11 (73) | 14 (58) |
| PLND template | 6 (40) | 12 (50) |
| Distant recurrence a | 26/73 (36) | 43/147 (29) |
| Lung | 9 (35) | 14 (33) |
| Liver | 9 (35) | 10 (23) |
| Bone | 12 (46) | 16 (37) |
| Extrapelvic lymph node | 4 (15) | 10 (23) |
| Peritoneal carcinomatosis | 2 (8) | 9 (21) |
| Other (brain, adrenal) | 3 (12) | 0 |
| Secondary urothelial carcinoma | 0 | 4 |
| Upper urinary tract | 0 | 3 (75) |
| Urethra | 0 | 1 (25) |
PLND = pelvic lymph node dissection.
Data are presented as n/N (%) or n (%). Percentages for recurrence locations do not sum because four patients had local recurrences located in both the cystectomy bed and the PLND template, and 22 patients had multiple distant recurrence locations.
For descriptive purposes, percentages were calculated as: number of patients with event within 2 yr/(number of patients with event within 2 yr + number of patients without event and follow-up ≥2 yr).
In the multivariable Cox regression model, RARC was not an independent predictor of recurrence after adjusting for patient age, gender, clinical stage, perioperative chemotherapy, pathologic stage, nodal stage, lymphovascular invasion, and PSM (hazard ratio 0.78, 95% CI 0.50–1.21; p = 0.2; Table 3). Accounting for the competing risk of death before recurrence using competing-risks survival regression did not alter the findings in the univariate and multivariable models (data not shown).
Table 3.
Multivariable Cox regression analysis of variables associated with recurrence after radical cystectomy
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Age (continuous) | 1.01 | 0.99–1.04 | 0.2 |
| Female gender | 1.11 | 0.70–1.76 | 0.7 |
| Clinical stage | |||
| T0/Ta/Tis | – | Referent | – |
| T1 | 0.35 | 0.12–1.03 | 0.057 |
| T2 | 0.41 | 0.15–1.14 | 0.09 |
| T3 | 0.59 | 0.19–1.84 | 0.37 |
| T4 | 0.44 | 0.12–1.66 | 0.22 |
| Technique | |||
| ORC | – | Referent | – |
| RARC | 0.78 | 0.50–1.21 | 0.2 |
| Perioperative chemotherapy | 3.27 | 2.01–5.32 | <0.0001 |
| Pathologic stage | |||
| T0/Ta/Tis | – | Referent | – |
| T1 | 1.20 | 0.43–3.36 | 0.7 |
| T2 | 2.36 | 1.02–5.47 | 0.04 |
| T3 | 4.92 | 2.34–10.32 | <0.0001 |
| T4 | 4.02 | 1.63–9.88 | 0.003 |
| Nodal stage | |||
| N0/Nx | – | Referent | – |
| N1–N3 | 1.39 | 0.83–2.36 | 0.2 |
| Lymphovascular invasion | 1.83 | 1.10–3.06 | 0.02 |
| Positive surgical margin | 1.18 | 0.60–2.31 | 0.6 |
HR = hazard ratio; CI = confidence interval; ORC = open radical cystectomy; RARC = robot-assisted radical cystectomy.
4. Discussion
Oncologic efficacy remains the standard that will ultimately verify the true value of RARC. Concerns about proper oncologic resection and the possibility that pneumoperitoneum enhances tumor cell dissemination prompted our precise analysis of recurrence patterns after ORC and RARC, while bearing in mind that selection bias accounted for baseline differences between the two groups.
In this study, we detected 15/65 (23%) and 24/136 (18%) local recurrences within 2 yr of surgery in ORC and RARC patients, respectively. In agreement with ORC series, patients who developed local recurrence in the current study usually did so within the first 18 mo after surgery [19–21]. Nevertheless, the relatively small number of patients does not preclude the possibility of differences between the two surgical techniques with regard to local recurrence. Importantly, selection bias was present, as patients undergoing ORC presented with higher clinical and pathologic stages.
The most interesting finding with regard to metastases is the distribution of distant recurrence locations between ORC and RARC. The most frequent locations remained the lungs, liver, and bone, which is consistent with the pattern of metastasis seen in autopsy studies and previous clinical series [21–24]. However, extrapelvic lymph node locations were more frequent for RARC than for ORC in patients with distant recurrence (10/43 [23%] vs 4/26 [15%]). It is often suggested that the maneuverability of the robotic system may limit the ability to perform a thorough extended PLND. However, we believe that our robot-assisted PLND technique adheres to the same oncologic standards and anatomic boundaries as our open technique. In this study, the median number of lymph nodes removed did not significantly differ between ORC and RARC (20 vs 21). Of course, it is still possible that factors related to the dissection technique are responsible for the current findings. Nevertheless, all of the extrapelvic lymph node recurrences were, by definition, outside the primary extended PLND template, regardless of whether an open or robotic approach was used. One possible explanation for the higher number of extrapelvic lymph node recurrences could be variant lymphatic dissemination as a result of the robotic technique. The true answer to this is not known, however, and the results remain intriguing.
Similarly, peritoneal carcinomatosis was more frequent in RARC patients than in ORC patients with distant recurrence (9/43 [21%] vs 2/26 [8%]). Laparoscopic surgery has been associated with a minimal risk of peritoneal tumor spread through the effect of pneumoperitoneum [10,11]. Peritoneal carcinomatosis is found in 16–19% of BCa subjects in autopsy and clinical studies, albeit most often in association with extensive metastases at other locations [22,23], while peritoneal carcinomatosis was the sole location in more than half of our cases. However, eight of nine RARC patients who developed peritoneal carcinomatosis had pathologic stage ≥T3, supporting the notion that peritoneal carcinomatosis was more a reflection of cancer biology than a surgical issue. Although the numbers are too few for robust conclusions, our findings warrant further investigations. If differences in recurrence patterns indeed exist between RARC and ORC, we do not believe that the cause is related to the experience level of the surgeon, as most RARC patients with extrapelvic lymph node metastasis or peritoneal carcinomatosis were operated on from 2008 to 2012. Furthermore, precautionary measures are taken to prevent spillage of any malignant cells into the operative field. Following division of the anterior urethral wall, the Foley catheter is immediately clipped with a large Hem-o-lok clip and then cut distal to the clip to maintain the integrity of the balloon. The posterior urethral wall is divided, and the bladder and the indwelling Foley catheter are immediately placed in an Endo Catch bag, thus avoiding urinary extravasation or tumor spillage, which were never observed in this cohort.
Of note, within 2 yr of surgery no secondary urothelial carcinoma was detected in the ORC group, while only four cases were diagnosed in the RARC group. Previous reports have demonstrated that the median time to secondary urothelial carcinomas ranges from 3.3 to 4.3 yr [25,26], and rates of urinary tract recurrences are as low as 1.7% at 5 yr [19]. In accordance with our study design, we reported events within 2 yr of surgery. Moreover, the ORC group represented a smaller patient sample than the RARC group. These factors likely explain our findings.
Previous reports evaluating RARC included patients with few comorbidities and low disease burden [14,27,28], which reflects the expected selection bias when a new surgical technique is introduced. Although in our experience these factors have become less important when discussing the surgical technique, selection bias certainly remains, and must be accounted for when drawing conclusions related to oncologic outcomes. The open approach remains the technique of choice in patients with larger tumors and unfavorable local tumor status, allowing easier intraoperative manipulation of specimens [29]. Although adjustment for baseline differences showed no evidence of an additional risk of recurrence in RARC patients, it should be kept in mind that multivariable analyses cannot account for all confounding factors in a data set. Moreover, the CI of the hazard ratio for recurrence is relatively wide, implying that an association between RARC and recurrence cannot be excluded with certainty. However, the fact that extrapelvic lymph node locations and peritoneal carcinomatosis were found more frequently in RARC patients, the cohort with less advanced disease, suggests that selection bias could also have attenuated differences. Potential variations in the use of perioperative chemotherapy are an additional limitation of the study. Taken together, our results are hypothesis-generating and should encourage discussions among urologists.
5. Conclusions
Within limitations, we found that RARC is not an independent predictor of recurrence after surgery. Interestingly, extrapelvic lymph node locations and peritoneal carcinomatosis were more frequent in RARC than in ORC patients. Further validation is warranted to better understand the oncologic implications of RARC.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Daniel P. Nguyen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Nguyen, Scherr.
Acquisition of data: Nguyen, Al Hussein Al Awamlh, Inoyatov, Ayangbesan.
Analysis and interpretation of data: Nguyen, Wu, Christos, Faltas, Scherr.
Drafting of the manuscript: Nguyen.
Critical revision of the manuscript for important intellectual content: Nguyen, Al Hussein Al Awamlh, Faltas, Wu, Christos, O’Malley, Scherr.
Statistical analysis: Nguyen, Wu, Christos.
Obtaining funding: Scherr.
Administrative, technical, or material support: Scherr.
Supervision: Nguyen, Scherr.
Other: None.
Financial disclosures: Daniel P. Nguyen certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Daniel P. Nguyen is supported by research grants from the Nuovo-Soldati Foundation, the Arnold U. und Susanne Huggenberger-Bischoff Foundation, the Bangerter Foundation, and the Swiss Urological Association (Switzerland). Douglas S. Scherr is supported in part by the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust. Xian Wu and Paul J. Christos were partly supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medical College (UL1-TR000457-06). The remaining authors have nothing to disclose.
References
- 1.Zehnder P, Studer UE, Skinner EC, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int. 2013;112:E51–8. doi: 10.1111/bju.12215. [DOI] [PubMed] [Google Scholar]
- 2.Ng CK, Kauffman EC, Lee MM, et al. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010;57:274–82. doi: 10.1016/j.eururo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Johar RS, Hayn MH, Stegemann AP, et al. Complications after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2013;64:52–7. doi: 10.1016/j.eururo.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Bochner BH, Sjoberg DD, Laudone VP. A randomized trial of robot-assisted laparoscopic radical cystectomy. N Engl J Med. 2014;371:389–90. doi: 10.1056/NEJMc1405213. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW. Editorial comment. Urology. 2012;79:1308. doi: 10.1016/j.urology.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 6.Smith AB, Raynor M, Amling CL, et al. Multi-institutional analysis of robotic radical cystectomy for bladder cancer: perioperative outcomes and complications in 227 patients. J Laparoendosc Adv Surg Tech A. 2012;22:17–21. doi: 10.1089/lap.2011.0326. [DOI] [PubMed] [Google Scholar]
- 7.Khan MS, Elhage O, Challacombe B, et al. Long-term outcomes of robot-assisted radical cystectomy for bladder cancer. Eur Urol. 2013;64:219–24. doi: 10.1016/j.eururo.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy: description of an evolved approach to radical cystectomy. Eur Urol. 2013;64:654–63. doi: 10.1016/j.eururo.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Xylinas E, Green DA, Otto B, et al. Robotic-assisted radical cystectomy with extracorporeal urinary diversion for urothelial carcinoma of the bladder: analysis of complications and oncologic outcomes in 175 patients with a median follow-up of 3 years. Urology. 2013;82:1323–9. doi: 10.1016/j.urology.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Shin YS, Doo AR, Kim MK, Jeong YB, Kim HJ. First case of peritoneal seeding of prostate cancer during robot-assisted laparoscopic radical prostatectomy. Can J Urol. 2012;19:6303–5. [PubMed] [Google Scholar]
- 11.Ost MC, Patel KP, Rastinehad AR, et al. Pneumoperitoneum with carbon dioxide inhibits macrophage tumor necrosis factor-alpha secretion: source of transitional-cell carcinoma port-site metastasis, with prophylactic irrigation strategies to decrease laparoscopic oncologic risks. J Endourol. 2008;22:105–12. doi: 10.1089/end.2007.9858. [DOI] [PubMed] [Google Scholar]
- 12.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 13.Stein JP, Skinner DG. Surgical atlas. Radical cystectomy. BJU Int. 2004;94:197–221. doi: 10.1111/j.1464-410X.2004.04981.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang GJ, Barocas DA, Raman JD, Scherr DS. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008;101:89–93. doi: 10.1111/j.1464-410X.2007.07212.x. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, editors. AJCC Cancer Staging Manual. 6. New York, NY: Springer; 2002. Urinary bladder; pp. 335–40. [Google Scholar]
- 17.Turkbey B, Basaran C, Karcaaltincaba M, et al. Peritoneal carcinomatosis in urinary bladder cancer. Clin Imaging. 2008;32:192–5. doi: 10.1016/j.clinimag.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Gray JP, Fine RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Volkmer BG, Kuefer R, Bartsch GC, Gust K, Hautmann RE. Oncological followup after radical cystectomy for bladder cancer—is there any benefit? J Urol. 2009;181:1587–93. doi: 10.1016/j.juro.2008.11.112. [DOI] [PubMed] [Google Scholar]
- 20.Dotan ZA, Kavanagh K, Yossepowitch O, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;176:2308–12. doi: 10.1016/j.juro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Yafi FA, Aprikian AG, Fradet Y, et al. Surveillance guidelines based on recurrence patterns after radical cystectomy for bladder cancer: the Canadian Bladder Cancer Network experience. BJU Int. 2012;110:1317–23. doi: 10.1111/j.1464-410X.2012.11133.x. [DOI] [PubMed] [Google Scholar]
- 22.Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2–4): an autopsy study on 367 patients. Urol Int. 1999;62:69–75. doi: 10.1159/000030361. [DOI] [PubMed] [Google Scholar]
- 23.Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. Am J Roentgenol. 2011;196:117–22. doi: 10.2214/AJR.10.5036. [DOI] [PubMed] [Google Scholar]
- 24.Giannarini G, Kessler TM, Thoeny HC, Nguyen DP, Meissner C, Studer UE. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486–94. doi: 10.1016/j.eururo.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson KM, Cai J, Miranda G, Skinner DG, Stein JP. Upper tract urothelial recurrence following radical cystectomy for transitional cell carcinoma of the bladder: an analysis of 1,069 patients with 10-year followup. J Urol. 2007;177:2088–94. doi: 10.1016/j.juro.2007.01.133. [DOI] [PubMed] [Google Scholar]
- 26.Umbreit EC, Crispen PL, Shimko MS, Farmer SA, Blute ML, Frank I. Multifactorial, site-specific recurrence model after radical cystectomy for urothelial carcinoma. Cancer. 2010;116:3399–407. doi: 10.1002/cncr.25202. [DOI] [PubMed] [Google Scholar]
- 27.Murphy DG, Challacombe BJ, Elhage O, et al. Robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008;54:570–80. doi: 10.1016/j.eururo.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Pruthi RS, Wallen EM. Is robotic radical cystectomy an appropriate treatment for bladder cancer? Short-term oncologic and clinical follow-up in 50 consecutive patients. Urology. 2008;72:617–20. doi: 10.1016/j.urology.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 29.Challacombe BJ, Bochner BH, Dasgupta P, et al. The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications. Eur Urol. 2011;60:767–75. doi: 10.1016/j.eururo.2011.05.012. [DOI] [PubMed] [Google Scholar]


