Abstract
Neurophysiological measurements of the response to prepulse and startle stimuli have been suggested to represent an important endophenotype for both substance dependence and other select psychiatric disorders. We have previously shown, in young adult Mexican Americans (MA), that presentation of a short delay acoustic prepulse, prior to the startle stimuli can elicit a late negative component at about 400 msec (N4S), in the event-related potential (ERP), recorded from frontal cortical areas. In the present study we investigated whether genetic factors associated with this endophenotype could be identified. The study included 420 (age 18 – 30 years) MA men (n=170) and women (n=250). DNA was genotyped using an Affymetrix Axiom Exome1A chip. An association analysis revealed that the CCKAR and CCKBR (cholecystokinin A and B receptor) genes each had a nearby variant that showed suggestive significance with the amplitude of the N4S component to prepulse stimuli. The neurotransmitter cholecystokinin (CCK), along with its receptors, CCKAR and CCKBR, have been previously associated with psychiatric disorders, suggesting that variants near these genes may play a role in the prepulse/startle response in this cohort.
Keywords: Mexican Americans, startle response, EEG, ERP, alcohol dependence
The identification of neurophysiological endophenotypes associated with psychiatric disorders may help in determining the causal relationship between clinical phenomena associated with the disorder and basic molecular processes that are in large part determined by genetic factors. One psychophysiological measure that has been used as a potential endophenotype for a number of psychiatric disorders is the acoustic startle reflex (ASR) and prepulse inhibition of the startle (PPI). The startle reflex is a constellation of responses usually indexed by eye blink responses (Swerdlow et al., 1992), but also by electrophysiological recordings from cortical areas that may index cognitive responses to startle (Ehlers et al., 2011; Ford et al., 1999; Ford et al., 1994; Putnam & Roth, 1990). Prepulse inhibition of the startle (PPI) refers to the fact that if a weak stimulus is presented prior to the presentation of the startle stimuli (prepulse) the response to the startle is reduced in amplitude. It has been suggested that prepulse inhibition is an index of automatic sensorimotor gating (Geyer & Swerdlow, 2001). In prepulse facilitation (PPF), the response to the startle is enhanced by short or long delay prepulses. PPF has been suggested to reflect a combination of alerting, attention and/ or arousal (Filion et al., 1998; Hsieh et al., 2006; Ludewig et al., 2003).
The anatomical substrates of the neurobehavioral responses (ASR/PPI/PPF) to the presentation of the startle stimuli have been extensively investigated in clinical and preclinical studies (Braff, Geyer, Light, et al., 2001; Braff, Geyer, & Swerdlow, 2001; Kumari et al., 2005; Swerdlow et al., 1994). Startle responses involve a complex neural network extending from brainstem nuclei via the thalamus to higher order cortical areas that may regulate cognitive responses to startle (Campbell et al., 2007; Fendt et al., 2001; Kumari et al., 2005; Neuner et al., 2010; Schall et al., 1999). There is some evidence that the cognitive response to ASR/PPI may share a common underlying neurophysiology with some behavioral and clinical measures of cognition that require response inhibition (Filion et al., 1999). For instance, both performance on 3 the Wisconsin Card Sorting Task and PPI of startle have been suggested to reflect prefrontal cortical function and dysfunction (Filion et al., 1999; Swerdlow & Geyer, 1999). Impairments in frontal lobe function and associated behaviors, such as executive functioning, have been an important theoretical construct in the understanding a number of behavioral disorders, such as schizophrenia (Bagney et al., 2013; Chan et al., 2014; Eisenberg & Berman, 2010; Holmen et al., 2012; Owens et al., 2010), bipolar disorders (Erol et al., 2014; Kulkarni et al., 2010; Yen et al., 2009; Zimmerman et al., 2006) and substance use disorders (Fernandez-Serrano et al., 2010; Gierski et al., 2013; Loeber et al., 2009; Maurage et al., 2014; van der Plas et al., 2009; Zorko et al., 2004). If frontal cortical responses to the startle stimuli index some aspect of frontal lobe functioning involving higher cognition, then psychiatric disorders postulated to involve some aspects of frontal lobe dysfunction should also have startle deficits such as anxiety disorders (De Pascalis et al., 2013), schizophrenia (De Koning et al., 2014; Swerdlow et al., 2014), post-traumatic stress disorder (Grillon et al., 1996), and alcoholism (Ehlers et al., 2011; Marin et al., 2012).
The present investigation used a startle paradigm with short delay prepulse-plus-startle stimuli, that elicits a large frontal negative slow wave designated the N4S component (Ehlers et al., 2011). In the present study, we used this endophenotype in an association analysis using an Affymetrix Axiom Exome1A array, to explore potential genetic factors underlying the N4S component response to prepulse startle stimuli in a young adult Mexican American cohort that has been previously well characterized clinically (Criado & Ehlers, 2007; Criado et al., 2014; Ehlers et al., 2009, 2010; Ehlers et al., 2012; Ehlers & Phillips, 2007; Ehlers et al., 2011; Ehlers et al., 2014).
MATERIALS AND METHODS
Sample ascertainment
To investigate risk and protective factors for the prepulse inhibition and startle response in a select population of Mexican American young adults, we investigated a cohort of 420 (age 18 – 30) Mexican American men (n=170) and women (n=250). Participants were recruited using a commercial mailing list that provided the addresses of individuals with Hispanic surnames in 11 zip codes in San Diego County. The mailed invitation stated that potential participants must be of Mexican American heritage, between the ages of 18 and 30 years, residing in the United States legally, and able to read and write in English. Based on a phone interview, participants were excluded if they were pregnant, were nursing, or currently had a major medical or neurological disorder or head injury. All participants identified as having over 20% Hispanic heritage, with 92% reporting over 50% Hispanic heritage. The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), was used to make lifetime substance use and other psychiatric disorder diagnoses according to DSM-III-R and DSM-IV criteria. There have been several studies that have evaluated the concurrent diagnostic validity of the SSAGA across alcohol and drug dependencies, major depression, anxiety disorders, and antisocial personality disorder (Bucholz et al., 1994; Hesselbrock et al., 1999). These findings indicate that the SSAGA is a highly reliable and valid instrument for use in studies of psychiatric disorders, including substance dependence. The protocol for the study was approved by the Institutional Review Board (IRB) at The Scripps Research Institute, and written consent was obtained for all participants. Participants were asked to refrain from alcohol and drug usage for 24 hours prior to the testing.
Startle ERP collection and analysis
Recordings were obtained from participants who were seated on a hospital bed in a sound-attenuated room. Acoustic startle stimuli were presented binaurally through headphones. The behavioral response to the startle (eye blink) is recorded using electrodes placed below and lateral to the eye as described (Braff, Geyer, & Swerdlow, 2001). The auditory stimuli consist of 45 trials. These trials include randomly presented startle stimuli (115 dB white noise burst for 40 msec n=30) and prepulse-startle stimuli (85 dB white noise burst for 20 msec-duration) immediately (<5 msec) followed by the startle (115 dB white noise burst for 40 msec n=15). Each individual startle and/or prepulse startle trial is separated by an interval of 15 seconds. Background white noise was presented for the entire session at a level of 60 dB. The behavioral variables assessed included: ASR magnitude on startle trials and prepulse trials as determined by quantification of the eye blink response as described below.
Seven channels of ERP data (FZ, CZ, PZ, F3, F4, F7, and F8, referenced to linked ear lobes with a forehead ground, international 10–20 system) were obtained using gold-plated electrodes with impedance held below 5 KΩ. Frontal electrodes were emphasized in the montage as previous data had suggested that ERP decrements in frontal areas distinguished subjects with a risk for alcohol dependence (Bauer, 1997). An electrode placed left lateral infraorbitally and reference to the left earlobe was used to monitor both horizontal and vertical eye movements. ERP signals were amplified (time constant 0.3 s, 35 Hz low pass) using a Nihon Kohden EEG machine and were transferred on-line to a PC. The combined gain of the EEG amplifiers and the analog-to-digital multiplexer amplifier was 50K.
The eye blink and ERP trials were simultaneously digitized at a rate of 256 Hz (bandwidth 0.5–35 Hz). Individual trials where the EEG or eye blink exceeded ±250 microvolts (<5% of the trials) were eliminated before averaging. The N4S component of the ERP was quantified using a computerized peak detection routine that identifies baseline-to-peak amplitudes (in μV) within the specified latency window (350–500 msec). The eye blink amplitude was also assessed using this routine. The latency window for the eye blink was 50–120 msec. The baseline was determined by averaging the 150 ms of pre-stimulus activity obtained for each trial. The routine is user-driven and each peak detection must be verified by the user. All peaks were quantified by one investigator, and verified by a second investigator, both of whom were blind to participants’ characteristics.
The N4S component of the ERP was also evaluated to determine if it was altered as a function of alcohol dependence, antisocial personality disorder/conduct disorder (ASPD/CD), affective/anxiety disorders (ANYAXAF), and any other drug dependence (AnyDrugDep). In this analysis, regionally averaged N4S component responses to startle and prepulse/startle were compared between those participants with and without the psychiatric disorders using ANCOVA (co-varying for gender).
Sample preparation and genotyping
For all subjects, DNA was extracted from blood samples, followed by genotyping using an Affymetrix Exome1A chip. The DNA samples were prepared and the exome chip genotyping was performed on the Affymetrix Axiom Exome 1A Array according to the Affymetrix Axiom 2.0 Assay Manual Workflow documentation. The Affymetrix Exome 1A chip contains 247,222 markers. Variant quality from the exome chip genotyping was initially assessed according to Affymetrix best practices (Affymetrix, 2011). Plink version 1.07 (Purcell et al., 2007) was used to calculate Hardy-Weinberg (HWE) p-values on the set of unrelated samples, followed by the removal of 653 variants with an HWE p < 10−10.
Association analysis
PLINK was used to test for genome-wide association for the N4S component of the event-related potential (ERP) in response to acoustic startle stimuli following the presentation of a prepulse. PLINK was run with linear regression model parameters and with one million permutations. Gender and age were included as covariates. To determine the effect of extreme outliers in the phenotypic values, custom R code was written to generate winsorized phenotype values at 5% and 95% cutoffs, which were then used as the phenotype values in PLINK. Manhattan plots were generated using Manhattan R library (Stephen Turner, http://gettinggeneticsdone.blogspot.com/2011/04/annotated-manhattan-plots-and-qq-plots.html). Annotations of the variants were obtained from the Affymetrix Exome 1A chip description file. Multiple test correction p-value thresholds were calculated for the Affymetrix Exome1A chip using the Genetic Type 1 Error Calculator (GEC) software (Li et al., 2012). The UCSC Genome Browser (Kent et al., 2002) was also utilized to visualize the genomic region containing the significant SNPs.
In order to determine if the SNPs that were significantly associated with the pre-pulse response phenotype were shared with the alcohol dependence trait, we used the multivariate version of the PLINK software (Ferreira & Purcell, 2009) with covariates age and gender.
LD analysis
In order to check for linkage disequilibrium across the CCKAR and CCKBR gene regions, PLINK was utilized to extract a subset of variants for analysis based on the physical position of these genes on chromosomes 4 and 11, respectively. In particular, a region of chromosome 4 from genomic location 25,450,000–28,000,000 and chromosome 11 from genomic location 6,100,000–6,500,000, were extracted from the data set. Haploview (Barrett et al., 2005) was used to calculate the LD statistics and visualize the haplotype block structure of the gene regions.
RESULTS
Demographics of the Mexican American population
The demographics for the full sample of individuals (N=420) that were included in the association analysis are shown in Table 1. The subjects were a mean age of 23.6 (range 18 – 30) years at the time of interview, with 40% of the sample being male and 60% of the sample being female. Participants had a mean of 13.3 years of education (SD=1.8), and a mean income of $30,000-$49,000. Using self-reported ancestry based on grandparent origin, 92% of the participants reported at least 50% Hispanic heritage. The mean BMI was 27 (SD=7, range 17 – 64). Approximately 29% (N=123) of the participants were diagnosed with alcohol dependence according to DSM-III-R guidelines, indicating that these subjects had symptoms from 3 or more symptom groups, out of 9 possible symptom groups. Eleven percent (N=45) of the participants were diagnosed with conduct disorder or antisocial personality disorder/conduct disorder. Thirty-one percent (N=132) of the participants has a lifetime diagnosis of affective and/or anxiety disorder. Twenty-seven percent (N=115) of the participants had a diagnosis of another drug disorder (nicotine, cannabis, hallucinogens, stimulants, sedatives, opioids).
Table 1.
Demographics for Mexican American study participants
| Male | Female | Total | |
|---|---|---|---|
| Participants | 170 (40%) | 250 (60%) | 420 |
| Age: mean(sd), [range] | 23.7 (3.9), [18–30] | 23.5 (3.8), [18–30] | 23.6 (3.8), [18–30] |
| Self-reported MA heritage >= 50% | 90.60% | 92.60% | 91.60% |
| BMI: mean(sd), [range] | 27.6 (6.0) [17.3–52.3] | 27.2 (7.4) [16.9–64.4] | 27.3 (6.9) [16.9–64.4] |
| Education in years: mean(sd), [range] | 13.2 (1.7), [9–17] | 13.4 (1.8), [7–18] | 13.3 (1.8), [7–18] |
| Income: mean(sd), [range] | 4.8 (2.1), [1–9] | 4.2 (2.1), [0–9] | 4.4 (2.2) [0–9] |
| Alcohol Dependence, # of subject affected | 63 [63/170 = 37%] | 60 [60/250 = 24%] | 123 [123/420 = 29.3%) |
| Pre-pulse inhibition: mean(sd), [range] | 13.07 (8.38), [0.01–50.63] | 15.48 (9.18), [0.01–60.12] | 14.51 (8.93), [0.01–60.12] |
| Startle: mean(sd), [range] | 12.28 (6.70), [0.01–36.28] | 15.12 (8.00), [0.01–47.09] | 13.97 (7.62), [0.01–47.09] |
The mean N4S amplitude was evaluated using ANCOVA (co-varying with gender) as a function of alcohol dependence, ASPD/CD, any affective and/or anxiety disorder, and any other drug dependency. Participants with a lifetime diagnosis of alcohol dependence had significantly increased amplitude N4S responses to prepulse/startle stimuli as compared to participants with no alcohol dependence diagnoses (F=6.535; p=0.011). There were no significant associations between the N4S amplitude to prepulse stimuli phenotypic trait and ASPD/CD, ANYAXAF, or AnyDrugDep diagnoses.
Association analysis
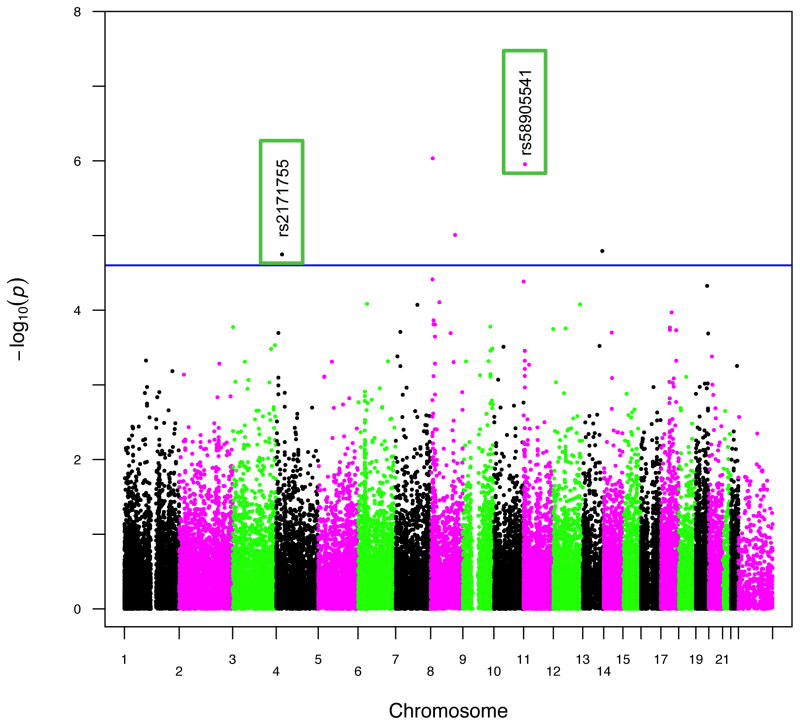
Figure 1 contains the Manhattan plot for the amplitude of the N4S response to prepulse stimuli phenotypic trait tested in the association analysis across the entire genome, using covariates age and gender, and applying a minor allele cutoff of 0.01. Although there were 5 total variants which exhibited p-values <= E-05 after association, as shown in Table 2, there were only two variants (rs2171755 and rs58905541) that showed suggestive significance and possessed plausible gene functions for this phenotype. One protective variant (rs2171755; NC_000004.12:g.26502338T>C) is located 12kb upstream from the CCKAR gene in a MIRb class SINE repetitive element, and is common in our sample, with an allele frequency of 0.36. The rs58905541 variant is a risk variant (NC_000011.10:g.6296157C>T) located 24kb downstream from the CCKBR gene in a DNaseI hypersensitivity cluster, with an allele frequency of 0.018. The variant retained its significance through permutation and winsorization and the exclusion of covariates age and gender. The association values for these two variants, along with the allele and genotype frequencies, are shown in Table 3. The minor allele frequencies of these variants in the 1000 Genomes project was obtained from the dbSNP website (http://www.ncbi.nlm.nih.gov/SNP/).
Figure 1. Manhattan plot for prepulse phenotype.
Manhattan plot across all chromosomes, using covariates age and gender. Minor allele frequency cutoff of 0.01 applied to the plot. Suggestive significance line calculated from GEC software. Green rectangles in plot highlight SNPs rs2171755 and rs58905541.
Table 2.
Significant variants for prepulse phenotype
| Chr | Position | dbSNP RS ID | Association p-value | MAF | Location of nearest gene | Description of nearest gene |
|---|---|---|---|---|---|---|
| 4 | 26503960 | rs2171755 | 1.79E-05 | 0.3607 | 12kb upstream, CCKAR | cholecystokinin A receptor |
| 8 | 9649769 | rs7830613 | 9.25E-07 | 0.4485 | 9kb downstream, TNKS | tankyrase, TRF1-interacting ankyrin-related ADP-ribose polymerase |
| 8 | 111264112 | rs1403843 | 9.83E-06 | 0.4613 | 83kb downstream, CSMD3 | CUB and Sushi multiple domains 3 |
| 11 | 6317387 | rs58905541 | 1.11E-06 | 0.0176 | 24kb downstream, CCKBR | cholecystokinin B receptor |
| 13 | 108518444 | rs1771137 | 1.61E-05 | 0.1518 | FAM155A | family with sequence similarity 155 member A |
Table 3.
Allele frequencies, genotype frequencies, and association analysis results
| dbSNP RS ID | rs2171755 | rs58905541 |
|---|---|---|
| Minor Allele | C | T |
| 1000GENOMES AF (ALL) | 0.455 | 0.129 |
| 1000GENOMES AF (AMR) | 0.403 | 0.044 |
| Present Study Sample | ||
| Overall MAF | 0.361 | 0.018 |
| Genotype counts | 59/185/176 | 0/15/405 |
| Frequency | 0.1405/0.4405/0.419 | 0/0.03571/0.9643 |
| HWE p-value | 0.3463 | 1 |
| Association analysis with no covars | ||
| Beta +/− StdError | −2.512 +/− 0.6165 | 11.56 +/− 2.283 |
| T-stat | −4.0750 | 5.0650 |
| P-value | 5.517E-05 | 6.15E-07 |
| Association following winsorization | ||
| Beta +/− StdError | −1.947 +/− 0.5209 | 8.738 +/− 1.934 |
| T-stat | −3.7380 | 4.518 |
| P-value | 2.116E-04 | 8.14E-06 |
| Permutation testing | ||
| Number of permutations performed | 901,000 | 1,000,000 |
| Empirical adaptive P-value | 4.44E-05 | 1.30E-05 |
| Association analysis with covars | ||
| (Unscaled Beta, P-value) | ||
| Additive | −2.658, 1.789E-05 | 11.24, 1.114E-06 |
| Sex | 2.675, 2.155E-03 | 2.167, 1.207E-02 |
| Age | −0.07525, 0.5007 | −0.06604, 0.5519 |
| (Scaled Beta, P-value) | ||
| Additive | −0.2068, 1.789E-05 | 0.2337, 1.114E-06 |
| Sex | 0.1471, 2.155E-03 | 0.1192, 1.207E-02 |
| Age | −0.03204, 0.5007 | −0.02812, 0.5519 |
The minor allele frequencies from the 1000 Genomes project, 1000GENOMES AF (ALL=total subjects and AMR=Admixed American subjects) were obtained from the 1000Genomes website (http://www.1000genomes.org/).
Abbreviations: MAF=minor allele frequency; HWE=Hardy-Weinberg equilibrium.
From the multivariate association analysis of the N4S response to prepulse stimuli phenotypic trait and alcohol dependence diagnosis phenotype, we found that the inclusion of the alcohol dependence phenotype did not significantly alter the association of the SNPs with the prepulse phenotype. That is, the p-values were significant whether we used univariate PLINK (rs2171755 p-value=1.79E-05; rs58905541 p-value=1.11E-06), or multivariate PLINK (rs2171755 p-value=9.36E-05; rs58905541 p-value=5.77E-06). Additionally, the weights determined by multivariate PLINK demonstrated that the prepulse phenotype had a strong correlation to the SNPs (rs2171755 weight for prepulse = 0.995; rs58905541 weight for prepulse = 0.999), while the alcohol dependence phenotype had a very weak correlation (rs2171755 weight for alcohol dependence = 0.026; rs58905541 weight for alcohol dependence = 0.109). These results suggest that the prepulse phenotype is driving the significance of the association for these two SNPs.
Multiple test correction
Multiple test correction p-value thresholds were calculated for the Affymetrix Axiom Exome1A chip using the Genetic Type 1 Error Calculator (GEC) software (Li et al., 2012), and the thresholds generated were thereby used to determine that the variants could be characterized to possess suggestive significance. In particular, for the Affymetrix Axiom Exome1A chip at a minor allele frequency of 0.01 or greater, the threshold for a suggestive p-value was calculated as 2.53E-05, significant p-value as 1.27E-06, and highly significant p-value as 2.53E-08.
LD analysis
The linkage disequilibrium (LD) was calculated across the CCKAR and CCKBR gene regions. The average D’ value across the SNP pairs in the CCKAR region was 0.77 in this data set. No other SNPs on the Affymetrix chip were found to be in high LD with the variant near the CCKAR gene. The average D’ value across the SNP pairs in the CCKBR region was 0.85 in this data set. While no SNP was found to be in complete LD with rs58905541, the SNP with highest LD was rs1462983 (D’=0.837, r2=0.023, LOD=2.19, CI=0.37–0.95). This SNP was located in OR56B4 (olfactory receptor, family 56, subfamily B, member 4) and possessed a positive beta value, suggesting it is a risk factor. However, rs1462932 was not significantly associated with the prepulse inhibition response phenotype in our sample when using PLINK for the analysis.
DISCUSSION
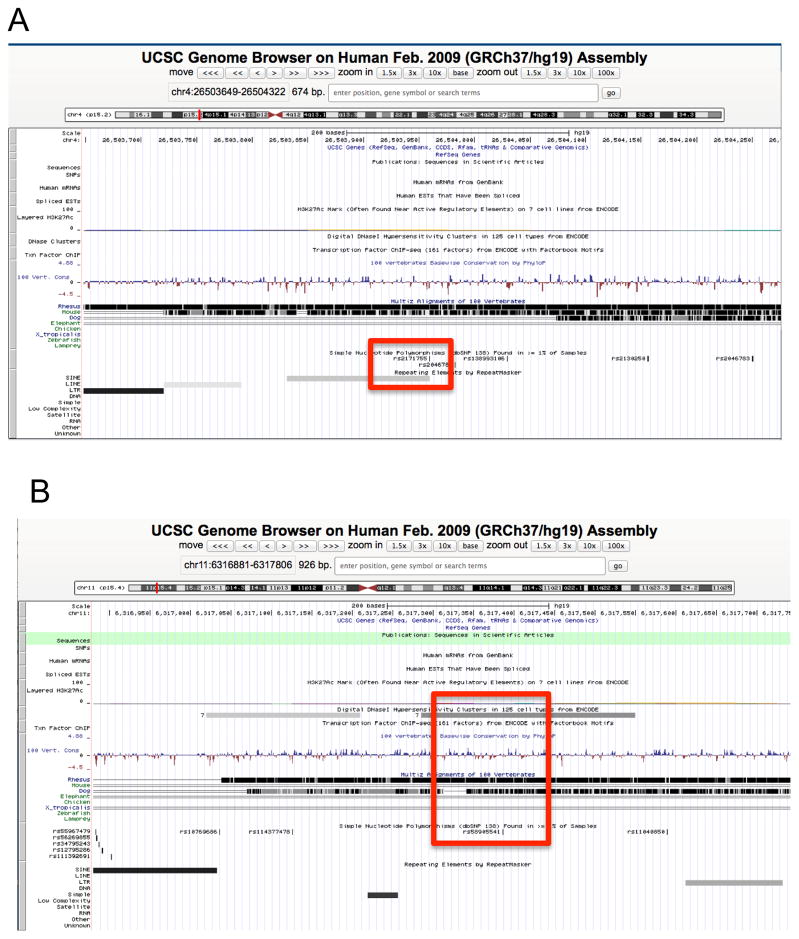
The present study confirmed previous observations in this population of an increase in prepulse facilitation of the N4S ERP component to the acoustic prepulse stimuli (Ehlers et al., 2011). In the present study, we used this endophenotype in an association analysis using an Affymetrix Axiom Exome1A array, to explore potential genetic factors underlying the N4S component response to prepulse startle stimuli in this young adult Mexican American cohort. The results of the present study suggest that variants located near CCKAR and CCKBR (cholecystokinin A and B receptor) genes are suggestive to be associated with the N4S ERP response to prepulse startle stimuli in this Mexican American cohort. The rs2171755 variant (NC_000004.12:g.26502338T>C) is located 12kb upstream from the CCKAR gene in a MIRb class SINE repetitive element. Interestingly, a variant in a MIRb class SINE repetitive element has been reported to be associated with human cognition (Gosso et al., 2007). The rs58905541 variant (NC_000011.10:g.6296157C>T) is located 24kb downstream from the CCKBR gene in a DNaseI hypersensitivity cluster. Variants in regulatory regions of the genome, such as DNase I hypersensitive sites, have been found to be associated with a number of diseases (Consortium, 2012; Maurano et al., 2012). Additionally, both SNPs show phylogenetic conservation in the UCSC Genome Browser alignment tracks in Figure 2. Since it has been reported that phenotype-associated variants occur more frequently in evolutionarily constrained regions of the genome (Parker et al., 2009), it suggests that these SNPs are likely to be real and functional. Therefore, it is feasible that the non-coding variants found in this present study could potentially play a role in the N4S ERP response to prepulse stimuli.
Figure 2. UCSC Genome Browser view of the SNP locations.
This figure shows the genomic location of the SNPs rs2171755 and rs58905541.
The neurotransmitter cholecystokinin (CCK) is widely present in the human body and in the central nervous system, where it modulates the dopaminergic system (Crawley & Corwin, 1994). Because of its potential modulation of the dopaminergic system and associated reward pathways, CCK has been investigated in a number of studies as a candidate gene for substance dependence and other behavioral disorders. CCK, along with its two receptors, CCKAR and CCKBR, have been previously associated with anxiety and panic disorders (Maron et al., 2008; Wilson et al., 2012), schizophrenia (Christoforou et al., 2007; Sanjuan et al., 2004), nicotine dependence (Takimoto et al., 2005), and alcohol dependence (Miyasaka et al., 2004; Okubo & Harada, 2001; Okubo et al., 2002). Although one study reported no association between CCK and CCKBR with alcohol dependence (Vanakoski et al., 2001), the study was performed using a Finnish population and did not examine the same significant SNPs that were determined in our study. Besides possible neurological influences of CCK on alcohol addiction, it has also been found that CCK through activation of CCKA receptors protects the gastric mucosa against ethanol-induced gastric damage in rats (Konturek et al., 1995). In the context of alcoholism, variation in CCK activity in the gut could allow for the ingestion of greater or lesser amounts of alcohol, which in turn could influence liability towards alcoholism.
Further evidence of the role of CCK in the regulation of startle responses is supported by studies where the administration of CCK-related peptides has been found to influence the startle response in rats and humans. In particular, infusion of CCK has been found to enhance the acoustic startle response in rats (Feifel et al., 2001; Feifel & Swerdlow, 1997; Fendt et al., 1995), and some CCK antagonists attenuate startle responses (Feifel et al., 1999; Josselyn et al., 1995). In human subjects, CCK infusion has also been demonstrated to increase eye-blink startle as well as produce a mild increase in anxiety and heart rate as well as increases in plasma concentrations of ACTH, cortisol, prolactin and growth hormone (Shlik et al., 1999). Since CCK peptides have been demonstrated to enhance anxiety, several studies have investigated the role of the CCK system in anxiety and panic disorders, using CCK-related peptide administration as a challenge method (Koszycki et al., 2012; Maron et al., 2008). These CCK challenge studies were able to explore genetic factors in panic disorders by performing candidate gene analyses to find alleles that may be associated with greater sensitivity to the CCK peptides. One study found an association of the tryptophan hydroxylase gene isomer 2 (TPH2) with subjects experiencing panic attacks after CCK infusion (Maron et al., 2008). Another study found an association to the CCKBR gene in subjects with greater pre-challenge anxiety (Koszycki et al., 2012). Additional studies have utilized CCK-peptide infusion to determine its effects on EEG and ERP measures in healthy human subjects (Knott et al., 2002; Knott et al., 2003). CCK-4 infusion was found to delay the latencies of N100 and P200 components of the ERP that was elicited during an auditory oddball task (Knott et al., 2002). During EEG recording of resting subjects, CCK-4 infusion was also found to increase asymmetry and reduce coherence of the slow-wave activity at mid-temporal recording sites (Knott et al., 2003). Taken together, these studies suggest a plausible role for CCK variants in the regulation of brain activity and behavior.
Impairments in frontal lobe function and associated behaviors such as executive functioning have been suggested to underlie anxiety disorders (Castaneda et al., 2008) as well as a number of other behavioral disorders such as schizophrenia (Bagney et al., 2013; Chan et al., 2014; Eisenberg & Berman, 2010; Holmen et al., 2012; Owens et al., 2010), bipolar disorders (Erol et al., 2014; Kulkarni et al., 2010; Yen et al., 2009; Zimmerman et al., 2006) and substance use disorders (Fernandez-Serrano et al., 2010; Gierski et al., 2013; Loeber et al., 2009; Maurage et al., 2014; van der Plas et al., 2009; Zorko et al., 2004). Interestingly, schizophrenia and bipolar disorders also demonstrate significant clinical comorbidity with substance use disorders (Regier et al., 1990) and may also share genetic susceptibility factors (Schizophrenia Working Group of the Psychiatric Genomics, 2014; Schuckit et al., 2003). There is also some data to suggest that they may share common endophenotypes such as deficits in some aspects of responses to startle (Kohl et al., 2013), although this hypothesis requires further testing and confirmation.
In summary, in the present study, associations between the N4S ERP response to acoustic prepulse startle stimuli, were determined using association analyses. Our results suggest that variants located in regulatory non-coding regions near the cholecystokinin A and B receptors may play a role in the prepulse/startle response. However, the results of this study should be interpreted in the context of several limitations. First, the findings may not generalize to the general American population of mixed heritage or all Mexican Americans, or all Hispanic young adult Americans. Over half of the participants in the present were women and thus findings many not generalize to previous studies that have focused on samples of entirely male participants. Second, the study was limited to young adults between the ages of 18 and 30 years, and the sample size may limit the interpretation of the results. Despite these limitations, this report represents an important step in an ongoing investigation to determine risk and protective factors associated with development of substance use disorders in this select Mexican American population.
Supplementary Material
Acknowledgments
We would like to thank and acknowledge the following people for their role in 1) the genotyping effort: Chris Bizon, Scott Chasse, Piotr Mieczkowski, Ewa Patrycja Malc, Joshua Sailsbery, and Phil Owens; and 2) for recruiting participants and collecting the clinical data: David Gilder, Susan Lopez, and Linda Corey.
FINANCIAL SUPPORT
Funding for this study was provided by grants from the National Institutes of Health (NIH); from the National Institute on Alcoholism and Alcohol Abuse (NIAAA) P60 AA006420 (CLE) and the National Institute of Drug Abuse (NIDA) 5 R01 DA030976 (CLE, IRG, KCW, & NJS). NIAAA, NCMHD and NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. NJS and his lab are supported in part by NIH grants 5 UL1 RR025774, R21 AI085374, 5 U01 DA024417, 5 R01 HL089655, 5 R01 AG035020, 1 R01 MH093500, 2 U19 AI063603, 2 U19 AG023122, 5 P01 AG027734, 1 R21 DA033813.
Footnotes
CONFLICT OF INTEREST
NJS is a founder and stock holder in Cypher Genomics and paid consultant for the following companies: Human Longevity, Inc., MD Revolution, and Click Therapeutics.
All of the authors declare that they have no conflicts of interests.
ETHICAL STANDARDS
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- Affymetrix. Affymetrix: Best Practice Supplement to Axiom Genotyping Solution Data Analysis User Guide Rev. 1. 2011;1:1–33. [Google Scholar]
- Bagney A, Rodriguez-Jimenez R, Martinez-Gras I, Sanchez-Morla EM, Santos JL, Jimenez-Arriero MA, … Parg Negative Symptoms and Executive Function in Schizophrenia: Does Their Relationship Change with Illness Duration? Psychopathology. 2013;46(4):241–248. doi: 10.1159/000342345. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug and Alcohol Dependence. 1997;44(1):1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophrenia Research. 2001;49(1–2):171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, …Schall U. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. The European journal of neuroscience. 2007;26(8):2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders. 2008;106(1–2):1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chan SKW, Chan KKS, Hui CL, Wong GHY, Chang WC, Lee EHM, …Chen EYH. Correlates of insight with symptomatology and executive function in patients with first-episode schizophrenia-spectrum disorder: A longitudinal perspective. Psychiatry Research. 2014;216(2):177–184. doi: 10.1016/J.Psychres.2013.11.028. [DOI] [PubMed] [Google Scholar]
- Christoforou A, Le Hellard S, Thomson PA, Morris SW, Tenesa A, Pickard BS, …Evans KL. Association analysis of the chromosome 4p15-p16 candidate region for bipolar disorder and schizophrenia. Molecular Psychiatry. 2007;12(11):1011–1025. doi: 10.1038/sj.mp.4002003. [DOI] [PubMed] [Google Scholar]
- Encode Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15(4):731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Electrophysiological responses to affective stimuli in Mexican Americans: Relationship to alcohol dependence and personality traits. Pharmacology, biochemistry, and behavior. 2007;88(2):148–157. doi: 10.1016/j.pbb.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Gizer IR, Edenberg HJ, Ehlers CL. CHRNA5 and CHRNA3 variants and level of neuroticism in young adult Mexican American men and women. Twin Res Hum Genet. 2014;17(2):80–88. doi: 10.1017/thg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning MB, Bloemen OJ, Van Duin ED, Booij J, Abel KM, De Haan L, … Van Amelsvoort TA. Pre-pulse inhibition and striatal dopamine in subjects at an ultra-high risk for psychosis. J Psychopharmacol. 2014;28(6):553–560. doi: 10.1177/0269881113519507. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Cozzuto G, Russo E. Effects of personality trait emotionality on acoustic startle response and prepulse inhibition including N100 and P200 event-related potential. Clinical Neurophysiology. 2013;124(2):292–305. doi: 10.1016/j.clinph.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Criado JR, Caetano R. Acculturation stress, anxiety disorders, and alcohol dependence in a select population of young adult Mexican Americans. Journal of Addiction Medicine. 2009;3(4):227–233. doi: 10.1097/ADM.0b013e3181ab6db7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Criado JR, Caetano R. Sleep quality and alcohol-use disorders in a select population of young-adult Mexican Americans. J Stud Alcohol Drugs. 2010;71(6):879–884. doi: 10.15288/jsad.2010.71.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liang T, Gizer IR. ADH and ALDH polymorphisms and alcohol dependence in Mexican and Native Americans. American Journal of Drug and Alcohol Abuse. 2012;38(5):389–394. doi: 10.3109/00952990.2012.694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. Association of EEG alpha variants and alpha power with alcohol dependence in Mexican American young adults. Alcohol. 2007;41(1):13–20. doi: 10.1016/j.alcohol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Criado JR, Gilder DA. N4 component responses to pre-pulse startle stimuli in young adults: relationship to alcohol dependence. Psychiatry Research. 2011;188(2):237–244. doi: 10.1016/j.psychres.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Stouffer GM, Gilder DA. Associations between a history of binge drinking during adolescence and self-reported responses to alcohol in young adult native and mexican americans. Alcoholism, Clinical and Experimental Research. 2014;38(7):2039–2047. doi: 10.1111/acer.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, Berman KF. Executive Function, Neural Circuitry, and Genetic Mechanisms in Schizophrenia. Neuropsychopharmacology. 2010;35(1):258–277. doi: 10.1038/Npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Kosger F, Putgul G, Ersoy B. Ventral prefrontal executive function impairment as a potential endophenotypic marker for bipolar disorder. Nordic Journal of Psychiatry. 2014;68(1):18–23. doi: 10.3109/08039488.2012.756062. [DOI] [PubMed] [Google Scholar]
- Feifel D, Priebe K, Shilling PD. Startle and sensorimotor gating in rats lacking CCK-A receptors. Neuropsychopharmacology. 2001;24(6):663–670. doi: 10.1016/S0893-133X(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. Journal of Pharmacology and Experimental Therapeutics. 1999;288(2):710–713. [PubMed] [Google Scholar]
- Feifel D, Swerdlow NR. The modulation of sensorimotor gating deficits by mesolimbic cholecystokinin. Neuroscience Letters. 1997;229(1):5–8. doi: 10.1016/s0304-3940(97)00409-6. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Kungel M, Schnitzler HU. Cholecystokinin enhances the acoustic startle response in rats. Neuroreport. 1995;6(15):2081–2084. doi: 10.1097/00001756-199510010-00030. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156(2–3):216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24(9):1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, Purcell SM. A multivariate test of association. Bioinformatics. 2009;25(1):132–133. doi: 10.1093/bioinformatics/btn563. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biological Psychology. 1998;47(1):1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Filion DL, Kelly KA, Hazlett EA. Behavioral analogies of short lead interval startle inhibition. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge, UK: Cambridge University Press; 1999. pp. 269–283. [Google Scholar]
- Ford JM, Roth WT, Menon V, Pfefferbaum A. Failures of automatic and strategic processing in schizophrenia: comparisons of event-related brain potential and startle blink modification. Schizophrenia Research. 1999;37(2):149–163. doi: 10.1016/s0920-9964(98)00148-0. [DOI] [PubMed] [Google Scholar]
- Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biological Psychiatry. 1994;35(2):96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Current Protocols in Neuroscience. 2001;Chapter 8(Unit 8):7. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, …Limosin F. Executive functions in adult offspring of alcohol-dependent probands: toward a cognitive endophenotype? Alcoholism, Clinical and Experimental Research. 2013;37(Suppl 1):E356–363. doi: 10.1111/j.1530-0277.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- Gosso FM, de Geus EJ, Polderman TJ, Boomsma DI, Posthuma D, Heutink P. Exploring the functional role of the CHRM2 gene in human cognition: results from a dense genotyping and brain expression study. BMC Medical Genetics. 2007;8:66. doi: 10.1186/1471-2350-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Research. 1996;64(3):169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Holmen A, Juuhl-Langseth M, Thormodsen R, Ueland T, Agartz I, Sundet K, …Melle I. Executive function in early- and adult onset schizophrenia. Schizophrenia Research. 2012;142(1–3):177–182. doi: 10.1016/J.Schres.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Swerdlow NR, Braff DL. Effects of background and prepulse characteristics on prepulse inhibition and facilitation: implications for neuropsychiatric research. Biological Psychiatry. 2006;59(6):555–559. doi: 10.1016/j.biopsych.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Frankland PW, Petrisano S, Bush DE, Yeomans JS, Vaccarino FJ. The CCKB antagonist, L-365,260, attenuates fear-potentiated startle. Peptides. 1995;16(7):1313–1315. doi: 10.1016/0196-9781(95)02013-m. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Research. 2002;12(6):996–1006. doi: 10.1101/gr.229102. Article published online before print in May 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott VJ, Mahoney C, Gunnarsson T, Bradwejn J, Shlik J. Acute cholecystokinin effects on event-related potentials in healthy volunteers. Hum Psychopharmacol. 2002;17(6):285–291. doi: 10.1002/hup.417. [DOI] [PubMed] [Google Scholar]
- Knott V, Mahoney C, Bradwejn J, Shlik J, Gunnarsson T. Effects of acute cholecystokinin infusion on hemispheric EEG asymmetry and coherence in healthy volunteers. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(1):179–184. doi: 10.1016/S0278-5846(02)00350-0. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. Journal of Psychiatric Research. 2013;47(4):445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Konturek SJ, Brzozowski T, Pytko-Polonczyk J, Drozdowicz D. Exogenous and endogenous cholecystokinin protects gastric mucosa against the damage caused by ethanol in rats. European Journal of Pharmacology. 1995;273(1–2):57–62. doi: 10.1016/0014-2999(94)00674-v. [DOI] [PubMed] [Google Scholar]
- Koszycki D, Prichard Z, Fiocco AJ, Shlik J, Kennedy JL, Bradwejn J. CCK-B receptor gene and response to cholecystokinin-tetrapeptide in healthy volunteers. Peptides. 2012;35(1):9–13. doi: 10.1016/j.peptides.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Jain S, Reddy YCJ, Kumar KJ, Kandavel T. Impairment of verbal learning and memory and executive function in unaffected siblings of probands with bipolar disorder. Bipolar Disorders. 2010;12(6):647–656. doi: 10.1111/J.1399-5618.2010.00857.X. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Zachariah E, Galea A, Aasen I, Ettinger U, …Sharma T. Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans. Neuroimage. 2005;26(4):1052–1058. doi: 10.1016/j.neuroimage.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Li MX, Yeung JM, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Human Genetics. 2012;131(5):747–756. doi: 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Vollstadt-Klein S, von der Goltz C, Flor H, Mann K, Kiefer F. Attentional bias in alcohol-dependent patients: the role of chronicity and executive functioning. Addiction Biology. 2009;14(2):194–203. doi: 10.1111/j.1369-1600.2009.00146.x. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biological Psychology. 2003;63(3):311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Marin M, Ponce G, Martinez-Gras I, Koeneke A, Curivil P, Jimenez-Arriero MA, Rubio G. Impairments of prepulse inhibition of the startle response in abstinent alcoholic male patients. Alcohol and Alcoholism. 2012;47(5):545–551. doi: 10.1093/alcalc/ags055. [DOI] [PubMed] [Google Scholar]
- Maron E, Toru I, Tasa G, Must A, Toover E, Lang A, …Shlik J. Association testing of panic disorder candidate genes using CCK-4 challenge in healthy volunteers. Neuroscience Letters. 2008;446(2–3):88–92. doi: 10.1016/j.neulet.2008.09.052. [DOI] [PubMed] [Google Scholar]
- Maurage P, de Timary P, Billieux J, Collignon M, Heeren A. Attentional alterations in alcohol dependence are underpinned by specific executive control deficits. Alcoholism, Clinical and Experimental Research. 2014;38(7):2105–2112. doi: 10.1111/acer.12444. [DOI] [PubMed] [Google Scholar]
- Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, …Stamatoyannopoulos JA. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337(6099):1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka K, Yoshida Y, Matsushita S, Higuchi S, Maruyama K, Niino N, …Funakoshi A. Association of cholecystokinin-A receptor gene polymorphism with alcohol dependence in a Japanese population. Alcohol and Alcoholism. 2004;39(1):25–28. doi: 10.1093/alcalc/agh002. [DOI] [PubMed] [Google Scholar]
- Neuner I, Stocker T, Kellermann T, Ermer V, Wegener HP, Eickhoff SB, …Shah NJ. Electrophysiology meets fMRI: neural correlates of the startle reflex assessed by simultaneous EMG-fMRI data acquisition. Human Brain Mapping. 2010;31(11):1675–1685. doi: 10.1002/hbm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Harada S. Polymorphisms of the CCK, CCKAR and CCKBR genes: an association with alcoholism study. Journal of Studies on Alcohol. 2001;62(4):413–421. doi: 10.15288/jsa.2001.62.413. [DOI] [PubMed] [Google Scholar]
- Okubo T, Harada S, Higuchi S, Matsushita S. Investigation of quantitative trait loci in the CCKAR gene with susceptibility to alcoholism. Alcoholism, Clinical and Experimental Research. 2002;26(8 Suppl):2S–5S. doi: 10.1097/01.ALC.0000026826.96191.FB. [DOI] [PubMed] [Google Scholar]
- Owens DC, Johnstone EC, Miller P, Macmillan JF, Crow TJ. Duration of untreated illness and outcome in schizophrenia: test of predictions in relation to relapse risk. British Journal of Psychiatry. 2010;196(4):296–301. doi: 10.1192/Bjp.Bp.109.067694. [DOI] [PubMed] [Google Scholar]
- Parker SC, Hansen L, Abaan HO, Tullius TD, Margulies EH. Local DNA topography correlates with functional noncoding regions of the human genome. Science. 2009;324(5925):389–392. doi: 10.1126/science.1169050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, …Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam LE, Roth WT. Effects of stimulus repetition, duration, and rise time on startle blink and automatically elicited P300. Psychophysiology. 1990;27(3):275–297. doi: 10.1111/j.1469-8986.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Sanjuan J, Toirac I, Gonzalez JC, Leal C, Molto MD, Najera C, De Frutos R. A possible association between the CCK-AR gene and persistent auditory hallucinations in schizophrenia. European Psychiatry. 2004;19(6):349–353. doi: 10.1016/j.eurpsy.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Schall U, Catts SV, Karayanidis F, Ward PB. Auditory event-related potential indices of fronto-temporal information processing in schizophrenia syndromes: valid outcome prediction of clozapine therapy in a three-year follow-up. International Journal of Neuropsychopharmacology. 1999;2(2):83–93. doi: 10.1017/S1461145799001418. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Kelsoe JR, Braff DL, Wilhelmsen KC. Some possible genetic parallels across alcoholism, bipolar disorder and schizophrenia. Journal of Studies on Alcohol. 2003;64(2):157–159. doi: 10.15288/jsa.2003.64.157. [DOI] [PubMed] [Google Scholar]
- Shlik J, Zhou Y, Koszycki D, Vaccarino FJ, Bradwejn J. Effects of CCK-4 infusion on the acoustic eye-blink startle and psychophysiological measures in healthy volunteers. J Psychopharmacol. 1999;13(4):385–390. doi: 10.1177/026988119901300409. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Archives of General Psychiatry. 1994;51(2):139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Braff DL, Geyer MA. The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol. 1992;6(2):176–190. doi: 10.1177/026988119200600210. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Neurophysiology and neuropharmacology of short lead interval startle modification. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge, UK: Cambridge University Press; 1999. pp. 114–133. [Google Scholar]
- Swerdlow NR, Light GA, Sprock J, Calkins ME, Green MF, Greenwood TA, …Braff DL. Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophrenia Research. 2014;152(2–3):503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T, Terayama H, Waga C, Okayama T, Ikeda K, Fukunishi I, Iwahashi K. Cholecystokinin (CCK) and the CCKA receptor gene polymorphism, and smoking behavior. Psychiatry Research. 2005;133(2–3):123–128. doi: 10.1016/j.psychres.2003.06.004. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. Journal of Clinical and Experimental Neuropsychology. 2009;31(6):706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanakoski J, Virkkunen M, Naukkarinen H, Goldman D. No association of CCK and CCK(B) receptor polymorphisms with alcohol dependence. Psychiatry Research. 2001;102(1):1–7. doi: 10.1016/s0165-1781(01)00246-3. [DOI] [PubMed] [Google Scholar]
- Wilson J, Markie D, Fitches A. Cholecystokinin system genes: associations with panic and other psychiatric disorders. Journal of Affective Disorders. 2012;136(3):902–908. doi: 10.1016/j.jad.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Yen CF, Cheng CP, Huang CF, Ko CH, Yen JY, Chang YP, Chen CS. Relationship between psychosocial adjustment and executive function in patients with bipolar disorder and schizophrenia in remission: the mediating and moderating effects of insight. Bipolar Disorders. 2009;11(2):190–197. doi: 10.1111/j.1399-5618.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, DelBello MP, Getz GE, Shear PK, Strakowski SM. Anterior cingulate subregion volumes and executive function in bipolar disorder (vol 8, pg 281, 2006) Bipolar Disorders. 2006;8(5):522–522. doi: 10.1111/j.1399-5618.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Zorko M, Marusic A, Cebasek-Travnik Z, Bucik V. The frontal lobe hypothesis: impairment of executive cognitive functions in chronic alcohol in-patients. Psychiatr Danub. 2004;16(1–2):21–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.