Abstract
Heart rate variability (HRV) reflects the healthiness of autonomic nervous system, which is associated with exercise capacity. We therefore investigated whether HRV could predict the exercise capacity in the adults with cardiac syndrome X (CSX). A total of 238 subjects (57±12 years, 67.8% men), who were diagnosed as CSX by the positive exercise stress test and nearly normal coronary angiogram were enrolled. Power spectrum from the 24-hour recording of heart rate was analyzed in frequency domain using total power (TP) and spectral components of the very low frequency (VLF), low frequency (LF) and high frequency (HF) ranges. Among the study population, 129 subjects with impaired exercise capacity during the treadmill test had significantly lower HRV indices than those with preserved exercise capacity (≥90% of the age predicted maximal heart rate). After accounting for age, sex, and baseline SBP and heart rate, VLF (odds ratio per 1SD and 95% CI: 2.02, 1.19–3.42), LF (1.67, 1.10–2.55), and TP (1.82, 1.17–2.83) remained significantly associated with preserved exercise capacity. In addition, increased HRV indices were also associated with increased exercise duration, rate-pressure product, and heart rate recovery, independent of age, body mass index, and baseline SBP and heart rate. In subgroup analysis, HRV indices demonstrated similar predictive values related to exercise capacity across various subpopulations, especially in the young. In patients with CSX, HRV was independently associated with exercise capacity, especially in young subjects. The healthiness of autonomic nervous system may have a role in modulating the exercise capacity in patients with CSX.
Introduction
Cardiac syndrome X (CSX), a clinical condition characterized by exertional angina, exercise induced myocardial ischemia, and normal coronary angiogram [1], is associated with coronary microvascular insufficiency [2]. It has been suggested that automonic dysfunction may contribute to the increased vasomotion of the pre-arteriolar coronary vessels in CSX [3], as demonstrated in studies employing myocardial 123I-metaiodobenzylguanidine scintigraphy [4] or assessing baroreflex sensitivity [5] and heart rate variability (HRV) [5, 6]. In addition, the parasympathetic withdrawal quantitated by HRV is associated with reduced coronary flow reserve, antedating episodes of dynamic myocardial ischemia [6]. CSX patients may have a good long-term survival. However, the attendant morbidity is not negligible [7], since the symptomatic patients may confront significant limitation of physical activities due to chest pain.
Spectral analysis of HRV has been used widely as a non-invasive technique for examining sympathetic and parasympathetic nervous outflows to the heart, which have been associated with the presence and the prognosis of cardiac disorders, including coronary artery disease (CAD), fatal arrhythmia, and heart failure [8–10]. It has been shown that regular physical activity or exercise training may reverse the autonomic neural remodeling in subjects with CAD, myocardial infarction, or heart failure [11–13] with resultant improvement in the clinical outcomes [14]. However, HRV indices were not always related to physical activity or exercise tolerability in patient [15] or in the general population [16]. It remains unknown whether the cardiac autonomic function modulates exercise capacity in CSX patients. In the present study, we therefore investigated the association between HRV and exercise capacity in the patients with CSX.
Methods
Study population
The study population was drawn from an intramural registry of Taipei Veterans General Hospital (TARGET registry), conducted to enroll patients referred for non-invasive studies due to the clinical impression of CAD. In addition to treadmill exercise test, ambulatory ECG monitoring, echocardiography, the medical history, the findings of physical examinations, and biochemical examinations were also prospective logged in a web-based electronic medical recording system. Estimated glomerular filtration rate (eGFR) was calculated with the published formula for Chinese [17]. Patients with stable angina, who have received both exercise treadmill test (CASE T2100, General Electric Company, USA) and 24-hour ambulatory ECG monitoring (Medilog FD4, Oxford Instruments, UK) before coronary angiogram, were eligible for this study.
From 2009 to 2013, a total of 238 subjects who have sinus rhythm, myocardial ischemia documented by the exercise stress test, and consequent normal or nearly normal coronary angiogram on the basis of the visual inspection by two experienced cardiologists were enrolled to the present analysis. Patients with significant valvular heart disease, heart failure, cardiomyopathy, atrial arrhythmia, sick sinus syndrome, pacing rhythm, pulmonary disease, stroke and other neurological disorders, or musculoskeletal pain has been excluded. The investigation conformed to the principles outlined in the Declaration of Helsinki, and the institutional review board of Taipei Veterans General Hospital approved the study. The participants provided their written informed consent to participate in this study, and the institutional review board approved this consent procedure.
Exercise stress test
Participants were refrained from smoking or drinking beverages containing caffeine or alcohol for 24 hours before exercise stress test and 24-hour ambulatory ECG monitoring. All patients would undergo symptom-limited exercise testing using a Bruce protocol. Twelve-lead ECG was obtained throughout the test, and exercise blood pressures were measured at baseline, during the last minute of each 3-minute stage, at the moment of maximum effort, and at 1 minute after the test with the arm relaxed at the side without holding on to the side bar of the treadmill, using an incorporated automatic BP monitor. (CASE T2100, General Electric Company, USA) A physician and two experienced technicians conducted the exercise test, and patients were encouraged continuing exercise if there was no severe electrocardiographic ischemia or unstable hemodynamics. The maximal systolic blood pressure (SBP) was defined as the highest value achieved during the test. Total exercise duration was documented and the rate-pressure product was the product of maximal achieved SBP and heart rate during exercise. Heart rate recovery is defined as the difference in heart rate between peak exercise and 1 minute later [18]. Patients who achieved more than 90% of the age predicted maximal heart rate were defined to have preserved exercise capacity, while the others had impaired exercise capacity.
Measures of Heart Rate Variability
A 24-hour ECG signal provided by the use of 3-channel digital recorders (Medilog FD4, Oxford Instruments, UK) was stored for off-line analyzing. The digitized ECG signals were processed and analyzed using open source HRV algorithms [19]. Briefly, the time series of R-R intervals was filtered to remove ectopic beats (such as supraventricular or ventricular ectopic beats), missing or noisy segments by linear interpolation from the surrounding signal. Only subjects with over 18 hours of good quality of normal-to-normal interbeat intervals were entered the subsequent HRV analyses. For each R-R time series, the tachogram was re-sampled at 4 Hz (http://ecg.mit.edu/dbag/tach-1.htm) to generate a uniformly spaced time series.
The computational algorithms for spectral HRV indices employed in this study are available publicly at www.physionet.org. Spectral HRV measures [20] were calculated using Fast Fourier transform with Welch window and include high-frequency power (HF; 0.15–0.40 Hz), low-frequency power (LF; 0.04–0.15 Hz), and very-low-frequency power (VLF; 0.003–0.04 Hz). The spectral HRV indices were calculated for each non-overlapping 5 minutes window of R-R time series and the average of VLF, LF, and HF spectral power was obtained to represent the overall HRV measures for a subject. A natural logarithmic transformation was used to normalize the distribution of the spectral HRV measures. LF power is suggested to be modulated by both sympathetic and parasympathetic activities, whereas HF power is primarily modulated by parasympathetic activity [21, 22]. The LF/HF ratio was computed as a measure of the sympathovagal balance toward sympathetic activity [20, 23]. The physiological mechanism underlying VLF power is disputed but has been suggested to be partially mediated by the renin-angiotensin-aldosterone system [20, 24, 25].
Statistical analysis
Means, standard deviations, and percentages were used to describe the characteristics of participants in the study. Student’s t-test and Chi-square test were used for comparisons between subjects with and without achieving more than 90% of the age predicted maximal heart rate. Uni- and multi-variate linear regression analyses were performed to examine the associations of HRV indices with exercise capacity, in terms of exercise duration and achieved METs. The association of each HRV index with exercise capacity was separately evaluated by multi-variate logistic regression analyses with adjustments for age, gender, baseline SBP and heart rate, and use of β-blockers and renin-angiotensin system blockers. Forward stepwise multiple logistic regression analyses were used to compare the predictive values of HRV indices after accounting for age, gender, baseline SBP and heart rate. Subgroup analysis was performed stratified by age of 60 year-old, gender, and presence of co-morbidities. A p value < 0.05 was considered statistically significant, and all statistical analyses were carried out using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Among the total of 238 patents (aged 57.0 ± 12.4 years, 67.8% men), 109 subjects (45.8% of total population) achieved more than 90% of the maximal age predicted heart rate during exercise stress test. Comparing to those without such achievement, subjects with good exercise capacity were characterized by younger age and less hypertension. (Table 1) Gender distribution, body mass index, diabetic prevalence, renal function, lipid profiles, and fasting blood sugar were similar between these 2 groups. In addition, patients with good exercise capacity were less likely to take non-dihydropiridine calcium channel blockers (CCBs) and renin-angiotensin system blockade. Otherwise, the prescriptions of β-blockers, α-blockers, and Statin between 2 groups were the same.
Table 1. Baseline characteristics of the study population.
| Impaired exercise capacity, n = 129 | Preserved exercise capacity, n = 109 | p-value | |
|---|---|---|---|
| Age, years | 59.97 ± 11.18 | 53.55 ± 12.95 | <0.001 |
| Male gender, n (%) | 82(63.6) | 80(73.4) | 0.139 |
| BMI, kg/m2 | 25.40 ± 3.92 | 24.77 ± 2.96 | 0.191 |
| Co-morbidities | |||
| Hypertension, n (%) | 67 (51.9) | 34 (31.2) | 0.002 |
| Diabetes, n (%) | 28 (21.7) | 20 (18.3) | 0.631 |
| Biochemistry profiles | |||
| eGFR, ml/min/1.73 m2 | 84.67 ± 30.44 | 88.48 ± 26.17 | 0.328 |
| Cholesterol, mg/dL | 180.42 ± 30.56 | 176.69 ± 32.48 | 0.388 |
| HDL-cholesterol, mg/dL | 52.31 ± 16.05 | 49.67 ± 13.37 | 0.217 |
| LDL-cholesterol, mg/dL | 111.69 ± 30.55 | 109.60 ± 28.38 | 0.627 |
| Triglyceride, mg/dL | 111.29 ± 74.97 | 118.97 ± 76.51 | 0.455 |
| Fasting Glucose, mg/dL | 102.22 ± 22.90 | 99.42 ± 24.42 | 0.381 |
| Prescribed medications | |||
| Dihydropyridine CCB, n (%) | 40 (31) | 10 (9.2) | <0.001 |
| β-blockers, n (%) | 38 (29.5) | 21 (19.3) | 0.096 |
| α-blockers, n (%) | 4 (3.1) | 1 (0.9) | 0.474 |
| RAS blockers, n (%) | 45 (34.9) | 23 (21.1) | 0.028 |
| Statin n (%) | 34 (26.4) | 23 (21.1) | 0.427 |
BMI = body mass index; CCB = calcium channel blocker; eGFR = estimated glomerular filtration rate; HDL = high density lipoprotein; LDL = low density lipoprotein; METs = metabolic equivalents; RAS = renin-angiotensin system.
No doubt patients with good exercise capacity experienced longer exercise test duration, and they achieved higher METs and Bruce treadmill test stage. (Table 2) The baseline SBP and pulse pressure (PP) were lower and heart rate was higher in patients with good exercise capacity. But the diastolic blood pressure and mean arterial blood pressure were similar. Regarding HRV indices, VLF power, LF power, and total power (TP), but not LF/HF were higher in the patients with good exercise capacity. However, there was a borderline significantly difference in HF power between the two groups.
Table 2. Characteristics of the exercise stress testing and heart rate variability in patients with impaired and preserved exercise capacity.
| Impaired exercise capacity, n = 129 | Preserved exercise capacity, n = 109 | p-value | |
|---|---|---|---|
| Exercise stress test | |||
| Total exercise duration, s | 475.15 ± 165.28 | 535.32 ± 150.77 | 0.004 |
| Achieved METs | 10.62 ± 2.64 | 11.53 ± 2.35 | 0.006 |
| Achieved Bruce Treadmill stage | 3.21 ± 0.96 | 3.53 ± 0.85 | 0.007 |
| Baseline blood pressure and heart rate | |||
| SBP, mmHg | 127.05 ± 19.49 | 121.86 ± 18.07 | 0.036 |
| DBP, mmHg | 76.47 ± 13.11 | 76.81 ± 11.45 | 0.836 |
| MAP, mmHg | 93.33 ± 13.83 | 91.83 ± 12.46 | 0.382 |
| PP, mmHg | 50.57 ± 14.97 | 45.06 ± 13.61 | 0.003 |
| Heart rate, beats/min | 73.40 ± 13.45 | 82.93 ± 16.32 | <0.001 |
| Maximal achieved blood pressure and heart rate | |||
| SBP, mmHg | 154.86 ± 30.79 | 149.57 ± 28.53 | 0.173 |
| DBP, mmHg | 74.06 ± 15.32 | 75.10 ± 14.56 | 0.594 |
| MAP, mmHg | 100.99 ± 17.78 | 99.92 ± 15.90 | 0.619 |
| PP, mmHg | 80.80 ± 28.55 | 74.47 ± 26.84 | 0.081 |
| Heart rate, beats/min | 140.12 ± 17.69 | 181.16 ± 71.17 | <0.001 |
| Increase of blood pressure and heart rate | |||
| SBP change, mmHg | 30.95 ± 30.50 | 36.92 ± 22.62 | 0.093 |
| Heart rate change, beats/min | 62.32 ± 20.88 | 97.79 ± 70.15 | <0.001 |
| Rate-pressure product, bpm*mmHg | 21804.88 ± 5476.91 | 25990.32 ± 4719.74 | <0.001 |
| Heart rate variability | |||
| Mean heart rate, beats/min | 77.73 ± 13.52 | 83.37 ± 14.70 | 0.002 |
| *VLF, ms2 | 3498.19 ± 2.80 | 4817.45 ± 1.81 | 0.004 |
| *LF, ms2 | 871.31 ± 3.00 | 1261.43 ± 2.29 | 0.005 |
| *HF, ms2 | 595.86 ± 3.67 | 804.32 ± 2.97 | 0.052 |
| *TP, ms2 | 5324.10 ± 2.53 | 7186.80 ± 1.88 | 0.004 |
| LF/HF | 1.82 ± 1.26 | 1.90 ± 1.21 | 0.612 |
*Geometric mean and standard deviation.
Rate-pressure product = maximal heart rate * maximal SBP.
BMI = body mass index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HF = high frequency component of the heart rate variability power spectrum; LF = low frequency component of heart rate variability power spectrum; MAP = mean blood pressure; METs = metabolic equivalents; RAS = renin-angiotensin system; SBP = systolic blood pressure; TP = total power of the heart rate variability power spectrum; VLF = very low frequency of the heart rate variability power spectrum.
Predictors of achieving 90% predicted heart rate
Age, heart rate, SBP, PP as well as HRV indices, including VLF power, LF power and TP were all predictors of achieving 90% maximal predicted heart rate. (Table 3) After accounting for age, gender, baseline SBP and heart rate in multivariate logistic regression analysis, VLF power, LF power, and TP, but not HF power significantly predicted the achievement of 90% predicted heart rate. (Table 4) With further adjustments for the use of β-blockers and renin-angiotensin system blockade, VLF, LF power and TP power remained associated with exercise capacity.
Table 3. Determinants of achieving more than 90% of the age predicted maximal heart rate during exercise stress test: univariate logistic regression analysis.
| Variable | Odd ratio (95% CI) | P value |
|---|---|---|
| Age, 1SD = 12.40 year | 0.573 (0.431–0.762) | <0.001 |
| Baseline heart rate, 1SD = 14.38 bpm | 1.512 (1.150–1.987) | 0.003 |
| Baseline SBP, 1SD = 18.99 mmHg | 0.756 (0.580–0.984) | 0.037 |
| Baseline PP, 1SD = 14.60 mmHg | 0.673 (0.513–0.883) | 0.004 |
| Heart rate variability indices | ||
| lnVLF, 1SD = 0.87 ms2 | 1.722 (1.192–2.486) | 0.004 |
| lnLF, 1SD = 1.00 ms2 | 1.499 (1.125–1.996) | 0.006 |
| lnHF, 1SD = 1.22 ms2 | 1.295 (0.996–1.683) | 0.053 |
| lnTP, 1SD = 0.82 ms2 | 1.554 (1.144–2.110) | 0.005 |
| LF/HF, 1SD = 1.23 | 1.068 (0.828–1.379) | 0.611 |
CI = confidence interval; HF = high frequency component of heart rate variability power spectrum; LF = very low frequency component of heart rate variability power spectrum; PP = pulse pressure; SBP = systolic blood pressure; SD = standard deviation; TP = total power of heart rate variability power spectrum; VLF = very low frequency component of heart rate variability power spectrum.
Table 4. Determinants of achieving more than 90% of the age predicted maximal heart rate during exercise stress test: multi-variate logistic regression analysis*.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| lnVLF, 1SD 0.87 ms2 | 2.016 (1.189–3.420) | 0.009 | 1.989 (1.154–3.427) | 0.013 |
| lnLF, 1SD 1.00 ms2 | 1.673 (1.096–2.552) | 0.017 | 1.639 (1.072–2.506) | 0.022 |
| lnHF, 1SD 1.22 ms2 | 1.419 (1.000–2.015) | 0.050 | 1.420 (0.996–2.025) | 0.053 |
| lnTP, 1SD 0.82 ms2 | 1.820 (1.170–2.830) | 0.008 | 1.792 (1.143–2.809) | 0.011 |
*each index of heart rate variability was evaluated separately
Model 1: accounting for age, sex, baseline systolic blood pressure and heart rate
Model 2: Model 1 + use of β-blockers and renin-angiotensin system blockers
CI = confidence interval; HF = high frequency component of heart rate variability power spectrum; LF = very low frequency component of heart rate variability power spectrum; OR = odds ratio; TP = total power; VLF = very low frequency component of heart rate variability power spectrum.
Determinants of exercise duration
While age, body mass index, and baseline SBP, PP and HR significantly correlated with total exercise duration, rate-pressure product during treadmill test, and heart rate recovery, VLF, LF and TP were also related to those measures. After accounting for age, body mass index, and baseline SBP and HR, VLF, LF and TP remained associated with total exercise duration and rate-pressure product. (Table 5) In addition, VLF, LF, HF and TP were associated with heart rate recovery in multivariate linear regression analysis. (Table 5)
Table 5. Correlates of exercise duration, achieved metabolic equivalent, and rate- pressure product with heart rate variability*†.
| Exercise duration | Rate-Pressure product | Heart rate recovery | ||||
|---|---|---|---|---|---|---|
| Standardized coefficient | P value | Standardized coefficient | P value | Standardized coefficient | P value | |
| lnVLF, ms2 | 0.252 | <0.001 | 0.261 | <0.001 | 0.246 | 0.001 |
| lnLF, ms2 | 0.151 | 0.035 | 0.122 | 0.049 | 0.244 | 0.002 |
| lnHF, ms2 | 0.032 | 0.649 | 0.057 | 0.361 | 0.201 | 0.008 |
| lnTP, ms2 | 0.193 | 0.006 | 0.186 | 0.003 | 0.252 | 0.001 |
| LF/HF | 0.153 | 0.029 | 0.015 | 0.809 | -0.075 | 0.322 |
*accounting for age, body mass index, baseline systolic blood pressure and heart rate.
†accounting for age, body mass index, baseline systolic blood pressure and heart rate.
HF = high frequency component of heart rate variability power spectrum; LF = very low frequency component of heart rate variability power spectrum; TP = total power of heart rate variability power spectrum; VLF = very low frequency component of heart rate variability power spectrum.
Subgroup analysis in predicting the achievement of 90% predicted heart rate
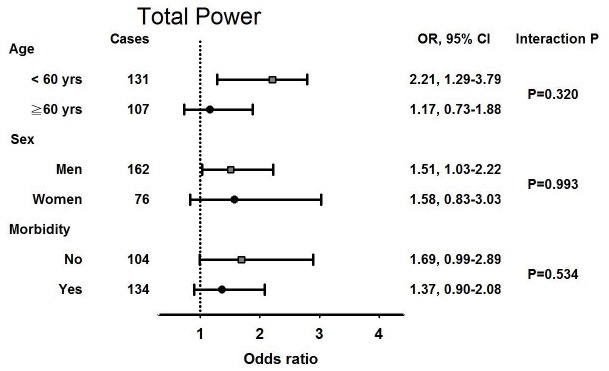
In subgroup analysis, high total power HRV was significantly associated with the achievement of 90% predicted heart rate in in younger rather than older subjects, and in male rather than female subjects, after accounting for age. (Fig 1) However, there was no substantial interaction across the subgroups, regarding age, gender, and co-morbidities. In addition, VLF, LF and HF also demonstrated similar clinical correlates with exercise capacity in subgroup analyses. (S1–S3 Figs)
Fig 1. Odds ratio (OR) and 95% confidence interval (CI) per increment of 1 standard deviation of total power heart rate variability power spectrum for preserved exercise capacity in the younger (< 60 years) and the older (≥ 60 years) subjects; women and men; subjects with and without hypertension or diabetes, after accounting for age.
Discussion
The present study demonstrated that exercise intolerance indeed prevailed in patients with CSX, while 54.2% of the study population had ischemia-limited impaired exercise capacity. In addition, subjects with impaired exercise tolerance were characterized by higher SBP and PP, and lower HRV indices. These HRV indices were not only related to exercise duration and rate-pressure product, but also independent predictors of ischemia associated exercise intolerance in CSX patients. Furthermore, the relations of HRV to exercise tolerability consistently existed in various subpopulations of the younger and older subjects, men and women, and subjects with and without co-morbidities.
Heart rate variability and exercise capacity
It has been known that autonomic activity may affect the endurance of exercise, because parasympathetic withdraw and a sympathetic augmentation [26] during dynamic training may increase heart rate, reduce the muscle blood flow and thus oxygen supply. Accordingly, the cardiac autonomic activity could correlate with the exercise tolerability, as assessed by the anaerobic threshold or the peak oxygen uptake [27]. In the community-based Cardiovascular Health Study of 985 adults, physical activity of walking distance or walking pace was both cross-sectionally and longitudinally associated with higher HRV [28]. Over 5 years, those who increased their walking pace or walking distance had more favorable HRV indices in comparison with the others. Not only in general populations but also in athletes and patients with existed cardiovascular disease, exercise training program may propose favorable impacts on cardiac autonomic activity with increasing HRV [29, 30].
Heart rate variability and cardiac syndrome X
An autonomic imbalance has been observed in subjects with CSX, involving the dynamic variations in the vasomotor tone of coronary microcirculations and thereafter precipitating the transient ischemic episodes [6, 31]. While some studies demonstrated that patents with CSX had lower HRV comparing to healthy controls [5, 32], Gulli et al. further suggested parasympathetic impairment rather than sympathetic over-excitation in the pathogenesis of CSX [33]. Moreover, Lee et al. indicated that the vagal withdraw was involved in the pathogenesis of dynamic myocardial ischemia, because HF power and root mean square of the successive differences (RMSSD) were significantly reduced before ischemic episodes [6]. Accordingly, cardiac autonomic activity may be associated with ischemia-limited exercise in patients with CSX.
In the present study, we demonstrated an agreeing result that patients with CSX who achieved 90% maximal predicted heart rate during treadmill exercise test had higher HRV comparing to those who did not. The association was independent of age, sex, baseline blood pressure and heart rate, and uses of antihypertensive agents. HRV was also independently correlated with exercise duration and rate pressure product, indicating that cardiac autonomic activity was essential to the cardiopulmonary function in patients with CSX [34].
Heart rate variability and exercise capacity in the elderly with cardiac syndrome X
Measures of HRV decrease linearly with age, indicating an age-related reduction in the responsiveness of autonomic activity [35]. Levy et al. reported that older subjects might have less parasympathetic withdrawal than the young subjects during peak exercise [36]. In the present study, we showed that all HRV indices were more predictive in predicting ischemia-limited exercise intolerance in patients younger than 60 years (Fig 1 and S1–S3 Figs). In contrast, the correlations between HRV indices and exercise capacity were similar in men and women, and in patients with and without hypertension or diabetes.
Study limitation
There are several limitations in the present observational study. First, good exercise capacity and exercise training are usually related to a lower resting heart rate, which is not observed in this study. But subjects with poor exercise capacity in this study tend to take more beta-blockers, which would confound the mean and resting heart rate. Although we have done multiple logistic regression analyses, accounting for the confounders and medications, omitted variable bias remained. Second, we have excluded subjects with documented pulmonary disease. However, some patients might have limited exercise capacity due to abnormal pulmonary function, which was not examined in the present study. In addition, the participants may not be compliant to the study protocol; even we did have experienced technicians encouraging them to achieve their predicted peak heart rate. Third, the exercise capacity is determined not only cardiopulmonary function, but also non-cardiac factors, including mental stress. Although the coefficients of the associations between HRV and exercise duration, rate-pressure product or heart rate recovery in multiple linear models were relatively small, it still suggested a consistent relation of HRV and exercise. Finally, a causal relationship between autonomic activity and exercise intolerance in patients with CSX could not be established in the present cross-sectional study. A longitudinal study may be warranted.
Conclusions
In patients with CSX, a reduction in cardiac autonomic activity is associated with impaired exercise capacity. Since anaerobic exercise training may improve autonomic activity, patients with CSX may be encouraged to exercise regularly to augment the favorable autonomic function and subsequently improve their quality of life.
Supporting Information
(TIF)
(TIF)
(TIF)
Acknowledgments
The authors would like to thank numerous research assistants at Taipei Veterans General Hospital, Taiwan for their assistance with data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by Taipei Veterans General Hospital (V103B-017), and Ministry of Health and Welfare, Taiwan (MOHW-104-TDU-B-211-113003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kemp HG Jr. (1973) Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol; 32:375–6. [DOI] [PubMed] [Google Scholar]
- 2.Cannon RO 3rd, Bonow RO, Bacharach SL, Green MV, Rosing DR, et al. (1985) Left ventricular dysfunction in patients with angina pectoris, normal epicardial coronary arteries, and abnormal vasodilator reserve. Circulation; 71:218–26. [DOI] [PubMed] [Google Scholar]
- 3.Epstein SE and Cannon RO 3rd (1986) Site of increased resistance to coronary flow in patients with angina pectoris and normal epicardial coronary arteries. J Am Coll Cardiol; 8:459–61. [DOI] [PubMed] [Google Scholar]
- 4.Lanza GA, Giordano A, Pristipino C, Calcagni ML, Meduri G, et al. (2000) Relationship between myocardial 123I-metaiodobenzylguanidine scintigraphic uptake and heart rate variability in patients with syndrome X. Ital Heart J; 1:221–5. [PubMed] [Google Scholar]
- 5.Adamopoulos S, Rosano GM, Ponikowski P, Cerquetani E, Piepoli M, et al. (1998) Impaired baroreflex sensitivity and sympathovagal balance in syndrome X. Am J Cardiol; 82:862–8. [DOI] [PubMed] [Google Scholar]
- 6.Lee WL, Chen JW, Lin SJ, Hsu NW, Chang MS, et al. (1998) Parasympathetic withdrawal antedates dynamic myocardial ischemia in patients with syndrome X. Int J Cardiol; 66:253–60. [DOI] [PubMed] [Google Scholar]
- 7.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, et al. (1995) Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol; 25:807–14. [DOI] [PubMed] [Google Scholar]
- 8.Wennerblom B, Lurje L, Tygesen H, Vahisalo R and Hjalmarson A (2000) Patients with uncomplicated coronary artery disease have reduced heart rate variability mainly affecting vagal tone. Heart; 83:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipovic M, Jeger R, Probst C, Girard T, Pfisterer M, et al. (2003) Heart rate variability and cardiac troponin I are incremental and independent predictors of one-year all-cause mortality after major noncardiac surgery in patients at risk of coronary artery disease. J Am Coll Cardiol; 42:1767–76. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Jiang Z, Li C and Shu M (2014) Prediction of heart rate variability on cardiac sudden death in heart failure patients: A systematic review. Int J Cardiol; 174:857–60. 10.1016/j.ijcard.2014.04.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiilavuori K, Toivonen L, Naveri H and Leinonen H (1995) Reversal of autonomic derangements by physical training in chronic heart failure assessed by heart rate variability. Eur Heart J; 16:490–5. [DOI] [PubMed] [Google Scholar]
- 12.Malfatto G, Facchini M, Sala L, Branzi G, Bragato R, et al. (1998) Effects of cardiac rehabilitation and beta-blocker therapy on heart rate variability after first acute myocardial infarction. Am J Cardiol; 81:834–40. [DOI] [PubMed] [Google Scholar]
- 13.Munk PS, Butt N and Larsen AI (2010) High-intensity interval exercise training improves heart rate variability in patients following percutaneous coronary intervention for angina pectoris. Int J Cardiol; 145:312–4. 10.1016/j.ijcard.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 14.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, et al. (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA; 301:1439–50. 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piotrowicz E, Baranowski R, Piotrowska M, Zielinski T and Piotrowicz R (2009) Variable effects of physical training of heart rate variability, heart rate recovery, and heart rate turbulence in chronic heart failure. Pacing Clin Electrophysiol; 32 Suppl 1:S113–5. 10.1111/j.1540-8159.2008.02266.x [DOI] [PubMed] [Google Scholar]
- 16.Bosquet L, Gamelin FX and Berthoin S (2007) Is aerobic endurance a determinant of cardiac autonomic regulation? Eur J Appl Physiol; 100:363–9. [DOI] [PubMed] [Google Scholar]
- 17.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, et al. (2006) Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol; 17:2937–44. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD and Lauer MS (2001) Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation; 104:1911–6. [PubMed] [Google Scholar]
- 19.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, et al. (2000) PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation; 101: E215–20. [DOI] [PubMed] [Google Scholar]
- 20.Task-Force (1996) Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology: Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation; 93:1043–65. [PubMed] [Google Scholar]
- 21.Katona PG and Jih F (1975) Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol; 39:801–5. [DOI] [PubMed] [Google Scholar]
- 22.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, et al. (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol; 248:H151–3. [DOI] [PubMed] [Google Scholar]
- 23.Malliani A, Lombardi F and Pagani M (1994) Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J; 71:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor JA, Carr DL, Myers CW and Eckberg DL (1998) Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation; 98:547–55. [DOI] [PubMed] [Google Scholar]
- 25.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, et al. (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science; 213:220–2. [DOI] [PubMed] [Google Scholar]
- 26.Iellamo F (2001) Neural mechanisms of cardiovascular regulation during exercise. Auton Neurosci; 90:66–75. [DOI] [PubMed] [Google Scholar]
- 27.Silvilairat S, Wongsathikun J, Sittiwangkul R, Pongprot Y and Chattipakorn N (2011) Heart rate variability and exercise capacity of patients with repaired tetralogy of Fallot. Pediatr Cardiol; 32:1158–63. 10.1007/s00246-011-0040-7 [DOI] [PubMed] [Google Scholar]
- 28.Soares-Miranda L, Sattelmair J, Chaves P, Duncan GE, Siscovick DS, et al. (2014) Physical activity and heart rate variability in older adults: the Cardiovascular Health Study. Circulation; 129:2100–10. 10.1161/CIRCULATIONAHA.113.005361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murad K, Brubaker PH, Fitzgerald DM, Morgan TM, Goff DC Jr., et al. (2012) Exercise training improves heart rate variability in older patients with heart failure: a randomized, controlled, single-blinded trial. Congest Heart Fail; 18:192–197. 10.1111/j.1751-7133.2011.00282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahle A, Nordlander R and Bergfeldt L (1999) Aerobic group training improves exercise capacity and heart rate variability in elderly patients with a recent coronary event. A randomized controlled study. Eur Heart J; 20:1638–46. [DOI] [PubMed] [Google Scholar]
- 31.Meeder JG, Blanksma PK, Crijns HJ, Anthonio RL, Pruim J, et al. (1995) Mechanisms of angina pectoris in syndrome X assessed by myocardial perfusion dynamics and heart rate variability. Eur Heart J; 16:1571–7. [DOI] [PubMed] [Google Scholar]
- 32.Rosano GM, Ponikowski P, Adamopoulos S, Collins P, Poole-Wilson PA, et al. (1994) Abnormal autonomic control of the cardiovascular system in syndrome X. Am J Cardiol; 73:1174–9. [DOI] [PubMed] [Google Scholar]
- 33.Gulli G, Cemin R, Pancera P, Menegatti G, Vassanelli C, et al. (2001) Evidence of parasympathetic impairment in some patients with cardiac syndrome X. Cardiovasc Res; 52:208–16. [DOI] [PubMed] [Google Scholar]
- 34.(1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation; 93:1043–65. [PubMed] [Google Scholar]
- 35.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, et al. (1999) Effect of aging on gender differences in neural control of heart rate. Am J Physiol; 277:H2233–9. [DOI] [PubMed] [Google Scholar]
- 36.Levy WC, Cerqueira MD, Harp GD, Johannessen KA, Abrass IB, et al. (1998) Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol; 82:1236–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.