Location, location, location. The Na-K pump of skeletal muscle is regulated differently at neuromuscular junctions.
Abstract
The Na,K-ATPase is essential for the contractile function of skeletal muscle, which expresses the α1 and α2 subunit isoforms of Na,K-ATPase. The α2 isozyme is predominant in adult skeletal muscles and makes a greater contribution in working compared with noncontracting muscles. Hindlimb suspension (HS) is a widely used model of muscle disuse that leads to progressive atrophy of postural skeletal muscles. This study examines the consequences of acute (6–12 h) HS on the functioning of the Na,K-ATPase α1 and α2 isozymes in rat soleus (disused) and diaphragm (contracting) muscles. Acute disuse dynamically and isoform-specifically regulates the electrogenic activity, protein, and mRNA content of Na,K-ATPase α2 isozyme in rat soleus muscle. Earlier disuse-induced remodeling events also include phospholemman phosphorylation as well as its increased abundance and association with α2 Na,K-ATPase. The loss of α2 Na,K-ATPase activity results in reduced electrogenic pump transport and depolarized resting membrane potential. The decreased α2 Na,K-ATPase activity is caused by a decrease in enzyme activity rather than by altered protein and mRNA content, localization in the sarcolemma, or functional interaction with the nicotinic acetylcholine receptors. The loss of extrajunctional α2 Na,K-ATPase activity depends strongly on muscle use, and even the increased protein and mRNA content as well as enhanced α2 Na,K-ATPase abundance at this membrane region after 12 h of HS cannot counteract this sustained inhibition. In contrast, additional factors may regulate the subset of junctional α2 Na,K-ATPase pool that is able to recover during HS. Notably, acute, low-intensity muscle workload restores functioning of both α2 Na,K-ATPase pools. These results demonstrate that the α2 Na,K-ATPase in rat skeletal muscle is dynamically and acutely regulated by muscle use and provide the first evidence that the junctional and extrajunctional pools of the α2 Na,K-ATPase are regulated differently.
INTRODUCTION
Investigations into the early molecular events that precede muscle atrophy are important for understanding the pathways involved in this disorder (Baldwin et al., 2013). Although the Na,K-ATPase is critically important for excitability, electrogenesis, and contractility of skeletal muscle (Sejersted and Sjøgaard, 2000; Clausen, 2013), its possible role in disuse-induced muscle atrophy is not known. The Na,K-ATPase is a P-type ATPase that catalyzes the active transport of K+ into and Na+ out of the cell, thereby maintaining the steep Na+ and K+ gradients that underlie the resting membrane potential (RMP) and electrical excitability. The Na,K-ATPase in skeletal muscle is composed of α-catalytic and β-glycoprotein subunits as well as a muscle-specific auxiliary FXYD1 subunit (phospholemman [PLM]), which modulates enzyme activity (Garty and Karlish, 2006; Geering, 2008). Four isoforms of the α-subunit are known to exist in tissues of vertebrates. It is generally accepted that the ubiquitous α1 isoform plays the main housekeeping role, whereas the other isoforms are expressed in a cell- and tissue-specific manner and possess additional regulatory functions that are still poorly understood (Blanco and Mercer, 1998; Geering, 2008; Krivoĭ, 2012).
The largest pool of Na,K-ATPase in a vertebrate’s body is contained in the skeletal muscles where the α1 and α2 isoforms of α subunit are expressed (Orlowski and Lingrel, 1988). The α2 isoform is expressed in high abundance in adult skeletal muscle compared with the α1 isoform and comprises up to 87% of the total α subunit (Orlowski and Lingrel, 1988; He et al., 2001). However, its functional role and mechanisms of regulation remain incompletely understood (He et al., 2001; Radzyukevich et al., 2004, 2009, 2013; Krivoi et al., 2006; Heiny et al., 2010; Krivoĭ, 2012). Studies of the specific role of the α2 Na,K-ATPase isozyme in skeletal muscle excitation, contraction, and fatigue have shown that this isozyme is specifically regulated by muscle use and enables working muscles to maintain contraction and resist fatigue, revealing its vital role in movement (Radzyukevich et al., 2004, 2013; Heiny et al., 2010; DiFranco et al., 2015). Studies of the role of the cardiac glycoside-binding site on the Na,K-ATPase α2 isoform in skeletal muscle show that this site, using circulating endogenous digitalis-like ligands, plays a unique role in the dynamic regulation of active transport and adaptations to exercise (Radzyukevich et al., 2009).
PLM is one of the most abundant phosphoproteins in skeletal and cardiac muscle. It is a member of the FXYD family of small, single membrane–spanning proteins that act as tissue-specific regulators of the Na,K-ATPase. Phosphorylation of PLM by PKA and PKC alters the enzyme’s substrate affinity or turnover in a cell- and Na,K-ATPase isoform–specific manner (Geering, 2008; Bossuyt et al., 2009; Pavlovic et al., 2013). In cardiac myocytes and skeletal muscle, PLM associates with both Na,K-ATPase α1 and α2 isoforms (Crambert et al., 2002; Reis et al., 2005; Rasmussen et al., 2008; Bossuyt et al., 2009; Chibalin et al., 2012).
Data obtained from different cells and tissues indicate that the Na,K-ATPase α2 isozyme is the more regulated subunit compared with α1. Regulation of the α2 Na,K-ATPase is determined by its functional and molecular environment, by localization in specific cellular microdomains, and by its less stable integration into the lipid membrane compared with other Na,K-ATPase α isoforms (Song et al., 2006; Kapri-Pardes et al., 2011).
Skeletal muscle activity strongly regulates the content of Na,K-ATPase, and increased muscle activity differently regulates the α1 and α2 isoforms (Yuan et al., 2007; Kristensen et al., 2008; Murphy et al., 2008; Juel, 2009; Nordsborg et al., 2010; Clausen, 2013). These effects may involve exercise-induced regulation of PLM (Rasmussen et al., 2008; Juel, 2009); however, the mechanisms of this regulation remain to be elucidated. In addition, whether skeletal muscle disuse induces isoform-specific changes in Na,K-ATPase functioning has not been investigated in detail.
It is known that hindlimb suspension (HS), widely used as an animal model of disuse, leads to progressive atrophy of postural skeletal muscles (Morey-Holton et al., 2005; Shenkman and Nemirovskaya, 2008; Baldwin et al., 2013). After 3–7 d and more of HS, the rat soleus muscle undergoes several dramatic remodeling events, including a slow to fast shift in myosin heavy chain expression, changes in ion channel expression, calcium deregulation, cytoskeleton damage, etc. (Shenkman and Nemirovskaya, 2008; Baldwin et al., 2013; Pierno et al., 2013; Ogneva et al., 2014), and a decrease of the RMP (Desaphy et al., 2001; Pierno et al., 2002; Krivoĭ et al., 2008; Tyapkina et al., 2009). The decreased RMP was shown to result from lowered electrogenic Na,K-ATPase activity (Krivoĭ et al., 2008; Tyapkina et al., 2009); specifically, α2 isozyme electrogenic activity decreases after 3 d of HS (Krivoĭ et al., 2008). Short-term (24–72 h) HS alters the Na,K-ATPase of rat soleus muscle in an isoform-specific manner and indicates that α2 Na,K-ATPase alterations precede disuse-induced muscle atrophy (Kravtsova et al., 2015). The effect of acute (hours) HS on the Na,K-ATPase isoforms is not known.
This study examines the consequences of acute HS on expression and function of the Na,K-ATPase α1 and α2 isozymes in rat soleus muscle. We subjected rats to HS for 6–12 h and analyzed its effect on the following parameters: the RMP of soleus fibers at different regions of the sarcolemma; the electrogenic transport activity of the α1 and α2 Na,K-ATPase, their protein and mRNA content; the extracellular level of acetylcholine (ACh); the plasma membrane localization of the nicotinic ACh receptors (nAChRs) and the α2 Na,K-ATPase and their functional interactions; and the phosphorylation status, abundance, and association of PLM with α2 Na,K-ATPase. In addition, we compared the characteristics of disused soleus muscle with the nonimpaired contracting diaphragm muscle of the same animals.
MATERIALS AND METHODS
Animals
Experiments were performed on male Wistar rats (180–230 g). Animals were housed in a temperature- and humidity-controlled room with food and water ad libitum. All procedures involving rats were performed in accordance with the recommendations for the Guide for the Care and Use of Laboratory Animals (http://www.nap.edu/books/0309053773/html/index.html). The protocol was approved by the Ethics Committee of St. Petersburg State University and the National Ministry of Health of the Russian Federation. The animals were subjected to HS individually in custom cages for 6 or 12 h, as described previously (Morey-Holton et al., 2005); control animals were not suspended. Soleus and diaphragm muscles were removed from euthanized animals. Freshly isolated muscles were used immediately for electrophysiological experiments, confocal microscopy imaging, or ACh assay. Some soleus muscles were quickly frozen in liquid nitrogen for later biochemical assays.
Membrane potential recording
The freshly isolated muscle with nerve stump was placed in a chamber and continuously perfused with a physiological solution containing (mM) 137 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 24 NaHCO3, 1 NaH2PO4, and 11 glucose, pH 7.4. The solution was continuously bubbled with 95% O2 and 5% CO2 and maintained at 28°C. The RMPs of muscle fibers were recorded from junctional and extrajunctional membrane regions using intracellular microelectrodes, as described previously (Krivoi et al., 2006; Heiny et al., 2010). RMPs were recorded from 20–35 different fibers within each muscle, over a total recording time of ∼5–10 min. The entire protocol was repeated in muscles from different animals.
In some experiments, a second set of RMP measurements were made before and immediately after the soleus muscle was stimulated to produce evoked contractions at 2 Hz for 5 min. Stimulation was achieved by delivering 0.l-ms isolated voltage pulses (Isostim A320; WPI) via two fine silver chloride wires and a suction electrode applied to nerve stump.
Measurement of Na,K-ATPase electrogenic activity in intact muscle
Na,K-ATPase transport activity was determined in intact muscle fibers by measuring the ouabain-sensitive change in RMP. This change is generated by electrogenic Na,K-ATPase transport and is a sensitive, real-time assay of Na,K-ATPase activity in intact skeletal muscle cells. The method is based on >100-fold difference in affinities of the rodent α1 and α2 Na,K-ATPase isoforms for ouabain. In rat skeletal muscle, 1 µM ouabain inhibits the α2 isoform without effect on the α1 isoform, whereas 500 µM ouabain completely inhibits both isoforms (Krivoi et al., 2003; Heiny et al., 2010; Chibalin et al., 2012). The electrogenic contribution of the α2 isozyme was computed as the difference in mean RMP before and after 30-min incubation in 1 µM ouabain. The electrogenic contribution of the α1 isozyme was estimated as the difference between the RMP in 1 µM ouabain and after 30-min incubation with 500 µM ouabain.
Western blot and coimmunoprecipitation assays
Frozen isolated soleus muscles were homogenized in lysis buffer (mM: 10 Tris-HCl, 250 sucrose, 1 EDTA, and 1 EGTA, 2% Triton X-100, pH 7.4, and 1 tablet protease inhibitor [EMD Millipore] per 10 ml), homogenate was centrifuged at 10,000 g, and the supernatant was collected. The protein concentration was determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Protein lysates were subsequently diluted into Laemmle buffer and heated at 56°C for 20 min.
A semiquantification of the α isoforms of Na,K-ATPase expression was performed as described previously (Matchkov et al., 2012). In brief, three 10% Tris-glycine gels were loaded with 10 µg of protein and electrotransferred after the separation onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in PBS-T (mM: 137 NaCl, 2.7 KCl, 8.2 Na2HPO4, and 1.8 KH2PO4 and 0.5% vol/vol Tween 20 at pH 7.4). The membranes were incubated overnight at 5°C with primary antibodies in PBS-T (either α1 isoform [1:2,000; Santa Cruz Biotechnology, Inc.] or α2 isoform [1:2,000; EMD Millipore] or pan-actin [1:1,000; Cell Signaling Technology]). After washing, the membranes were incubated for 1 h with horseradish peroxidase (HRP)–conjugated secondary antibody (1:4,000; Dako). The bound antibody was detected by an enhanced chemiluminescence kit (ECL; GE Healthcare). Detected protein was quantified as a ratio to pan-actin intensity using ImageJ software (National Institutes of Health).
To analyze signaling pathways, equal amounts of protein from the same samples (15 µg) were separated on precast Criterion SDS-PAGE gels of various percentages (Bio-Rad Laboratories) and transferred to PVDF membranes (Immobilion; EMD Millipore). Equal loading of protein on gels was ensured using Ponceau staining. Membranes were blocked in 7.5% milk in TBS-T for 1 h and incubated overnight with a primary antibody at 4°C. Then, the membranes were washed and incubated with the appropriate HRP-conjugated secondary antibody (Bio-Rad Laboratories) in 5% milk for 1 h at room temperature. Proteins were visualized using enhanced chemiluminescence Western blotting detection reagents were from GE Healthcare. Optical density of the bands was quantified using the Quantity One imaging system (Bio-Rad Laboratories). Antibodies against PLM were from Proteintech; phospho-PLM Ser63 and phospho-PLM Ser68 antibodies were from Abnova.
Coimmunoprecipitation assays were performed as described previously (Heiny et al., 2010). In brief, muscles were solubilized with lysis buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 20 mM Tris, pH 8.0, 1% Triton X-100, 10% [vol/vol] glycerol, 10 mM NaF, 0.5 mM Na3VO4, 5 µg/ml leupeptin, 0.2 mM phenylmethylsulfonyl fluoride, 5 µg/ml aprotinin, and 1 µM microcystin). Immunoprecipitation was performed in 1 ml of lysis buffer containing 500 µg of protein using a primary antibody to the α2 isoform of the Na,K-ATPase antibody (EMD Millipore) followed by affinity purification using protein A–agarose beads (Invitrogen). After incubation with protein A–agarose beads for 1 h at room temperature, the immuno complex was washed in lysis buffer followed by PBS. The protein samples were probed by Western blot with primary antibodies against Na,K-ATPase α2 subunit (clone McB2; provided by K. Sweadner, Massachusetts Central Hospital, Boston, MA) and PLM (Proteintech). The proteins were visualized by ECL and quantified by densitometry.
Quantitative polymerase chain reaction (PCR)
Isolated soleus muscle segments were disrupted in TissueLyser (QIAGEN). RNA isolation was performed with the Mini kit (QIAGEN) in a QIAcube robotic workstation for automated purification of RNA (QIAGEN). PCR was performed to assess the expression of specific RNAs. The reaction was executed with reverse transcription III (Invitrogen) and SUPERase (Ambion) for deactivation of RNase and DNase. The standard primer sets for quantitative PCR analyses for α1 and α2 isoforms of the Na,K-ATPase were obtained from Applied Biosystems. Quantitative PCR was performed on an MX3000P (Agilent Technologies) using TaqMan probe (FAM) technology. Gene expression was normalized to GAPDH and transferrin receptor (Ct value) levels and presented by a ΔCt value. Comparison of gene expression was derived by subtracting control ΔCt (an averaged ΔCt for the muscles which were not exposed to HS) from sample ΔCt, producing ΔΔCt. Relative gene expression was calculated as 1/(2ΔΔCt), thereby standardizing to control muscle.
Confocal microscopy and membrane localization
Ouabain at 1 µM selectively inhibits the rodent α2 Na,K-ATPase without effect on the α1 isoform, as shown previously (Krivoi et al., 2003; Chibalin et al., 2012). For selective imaging of the Na,K-ATPase α2 isoform, a freshly isolated soleus muscle was incubated for 15 min with physiological saline containing fluorescent-labeled specific ligands of the Na,K-ATPase (BODIPY-conjugated ouabain, 1 µM) and the nAChR (rhodamine-conjugated α-bungarotoxin, 1 µM). Superficial regions of the muscle were imaged with a 40×, 1.3 NA objective using a TCS SP5 confocal system (Leica) configured for concurrent viewing of rhodamine and BODIPY fluorescence, as was described previously (Heiny et al., 2010; Radzyukevich et al., 2013). Fluorescence was estimated as mean fluorescence intensity in arbitrary units (a.u.) at different sarcolemma regions using Image Pro software (Media Cybernetics). Analysis of junctional fluorescence was performed in the region defined by α-bungarotoxin staining. Extrajunctional fluorescence was calculated for an area (∼200 µm2) of muscle membrane outside of the α-bungarotoxin–positive region.
ACh assay
Fluorescence images were acquired using a CX41 microscope (Olympus) equipped with FluoLED fluorescence illuminators (Fraen) and charge-coupled device camera DP72 (Olympus). Analysis was performed using Image Pro software (Media Cybernetics).
The level of ACh released in neuromuscular preparations with native acetylcholinesterase (AChE) was estimated optically using an Amplex Red Acetylcholine Assay kit (Molecular Probes; Tsai et al., 2007; Petrov et al., 2011). A freshly isolated soleus or diaphragm muscle was incubated in a dish (“stop-flow superfusion”) in physiological saline (0.4 ml) containing choline oxidase (EC 1.1.3.17, 1 U/ml), HRP (EC 1.11.1.7, 2 U/ml), and 400 µM Amplex Red at room temperature. The solution was constantly mixed during incubation. This assay is based on the optical detection of choline released from the hydrolysis of ACh by endogenous AChE. Choline oxidase decomposes choline to betaine and H2O2, and the latter in the presence of HRP reacts with Amplex Red reagent to produce a stable fluorescent compound, resorufin, which is optically monitored. The level of residual nonhydrolysed ACh (comprising the fraction that escapes from native AChE action) was estimated in the same conditions but with the further addition of exogenous AChE (E.C. 3.1.1.7., 1 U/ml). Resorufin fluorescence was measured after incubation of the solution at room temperature for 45 min using excitation at 535 nm and emission detection at 590 nm and used to estimate the amount of ACh (calculated in a.u. of fluorescence/gram of muscle tissue). The ACh concentration was estimated by comparing resorufin fluorescence to a stand curve of resorufin fluorescence versus ACh concentration. For each measurement, we made a correction for background fluorescence caused by the release of endogenous H2O2 from reactions other than choline oxidation. Samples of 10 µl were taken for analysis.
Materials
Ouabain was obtained from Sigma-Aldrich. Rhodamine-conjugated α-bungarotoxin was from Biotium, Inc., and BODIPY-conjugated ouabain was from Invitrogen. Nicotine ((−)nicotine hydrogen tartrate) and other chemicals were from Sigma-Aldrich.
Statistics
Data are given as the mean ± SEM. Statistical significance of the difference between means was evaluated using a Student’s t test (ORIGIN 6.1 software) and one-way ANOVA (ORIGIN Pro 8 software).
RESULTS
Acute HS dynamically alters the RMP of rat muscles
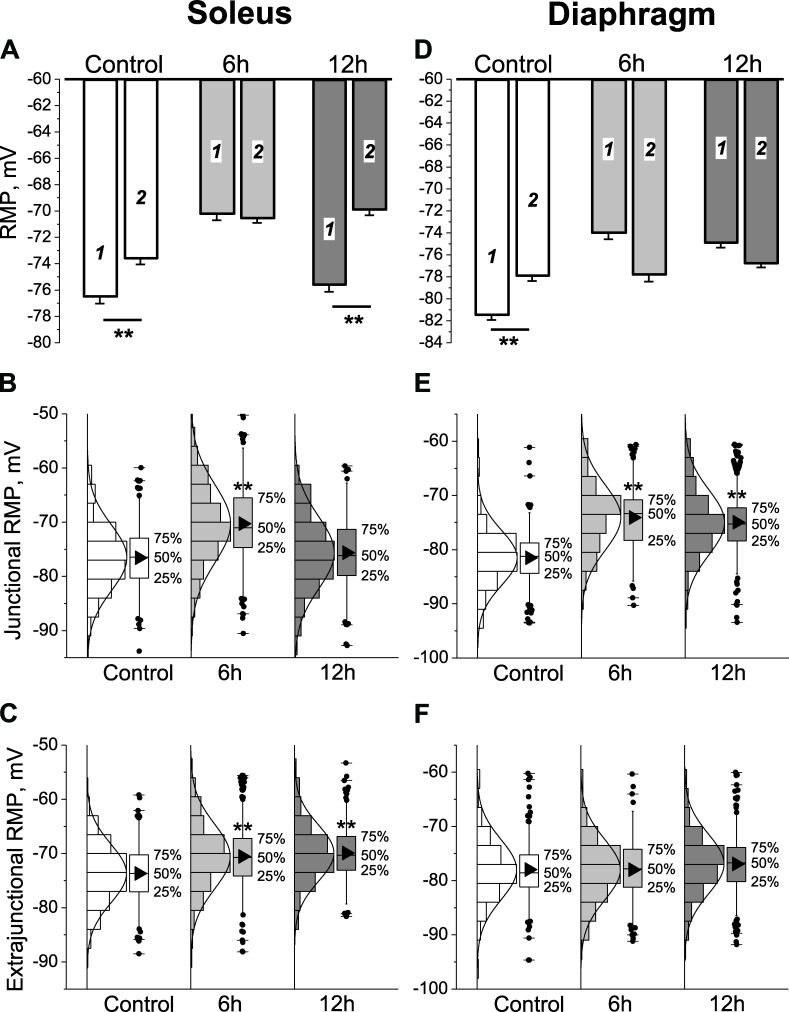
In control soleus muscles, the mean RMPs of junctional (end-plate) and extrajunctional membrane regions were −76.6 ± 0.4 mV and −73.7 ± 0.4 mV, respectively (significant difference of −2.9 ± 0.6 mV, P < 0.01). A similar local hyperpolarization of junctional membrane was observed in control diaphragm muscles (Fig. 1, A and D). The more negative RMP of junctional compared with extrajunctional membrane regions of the same muscle is consistent with previous studies (Nikolsky et al., 1994; Heiny et al., 2010) and is attributed to enhanced electrogenic activity of the Na,K-ATPase α2 isozyme at the neuromuscular junction (NMJ) of rodents (Heiny et al., 2010). Nanomolar concentrations of ACh resulting from nonquantal release stimulate the enzyme through an interaction between the nAChR and the Na,K-ATPase α2 isozyme (Krivoi et al., 2006; Heiny et al., 2010; Chibalin et al., 2012). A stable, reciprocal modulation between these proteins was further confirmed in an insect nervous system (Bao et al., 2015). Presumably, the nAChR in a desensitized conformation with high apparent affinity for agonist interacts with the Na,K-ATPase to stimulate active transport, and this effect does not require ion flow through open nAChRs (Heiny et al., 2010). The junctional membrane hyperpolarization might also involve ATP-sensitive K+ channels opening in response to local Na,K-ATPase activation and ATP depletion within a diffusion-restricted submembrane space (Kabakov, 1998; Zhu et al., 2014). In accordance with the length constant of a skeletal muscle cell, the hyperpolarization disappeared within ∼2 mm from visually identified terminal branches of the nerve.
Figure 1.
HS alters the RMP of rat soleus and diaphragm muscles. (A and D) Mean RMP of junctional (1) and extrajunctional (2) membrane regions of soleus and diaphragm fibers in control group and after 6 and 12 h of HS. **, P < 0.01, mean RMP differences between junctional and extrajunctional membrane regions. In A: control (10 muscles), 191 fibers (junctional) and 211 fibers (extrajunctional); 6 h HS (14 muscles), 288 fibers (junctional) and 303 fibers (extrajunctional); 12 h HS (10 muscles), 176 fibers (junctional) and 165 fibers (extrajunctional). In D: control (6 muscles), 154 fibers (junctional) and 184 fibers (extrajunctional); 6 h HS (5 muscles), 144 fibers (junctional) and 158 fibers (extrajunctional); 12 h HS (8 muscles), 261 fibers (junctional) and 260 fibers (extrajunctional). (B, C, E, and F) Mean RMP values were obtained from a Gaussian fit to the distribution of RMPs (solid curves) for soleus (B and C) and diaphragm (E and F) muscles in the junctional (B and E) and extrajunctional (C and F) membrane regions. Box and whisker plots are shown for each distribution, indicating median, percentiles, outliers, and mean (black triangles). **, P < 0.01, differences in mean RMPs compared with corresponding control. Error bars indicate SEM.
After 6 h of HS, the mean RMPs of both junctional and extrajunctional membranes of soleus fibers depolarized to approximately −70 mV and were similar at all regions. The mean RMP in the junctional region was −70.3 ± 0.4 mV, representing a depolarization of 6.3 ± 0.6 mV (P < 0.01) compared with control (Fig. 1 A). After 12 h of HS, the mean RMP of the junctional but not extrajunctional membrane regions returned to control values (−75.6 ± 0.5 mV). In this case, the junctional membrane was again hyperpolarized by −5.7 ± 0.7 mV compared with extrajunctional regions as in control (Fig. 1 A).
In diaphragm muscles, which continuously contract during HS, the junctional membrane depolarized to a similar extent as the disused soleus muscles after 6 h of HS. However, conversely to soleus muscles, there was no recovery of mean junctional RMP after 12 h of HS. Additionally, in contrast to soleus, the mean RMP of extrajunctional membrane regions of diaphragm muscles did not change after 6 or 12 h of HS (Fig. 1, A and D).
The RMPs of all soleus and diaphragm muscle groups showed a Gaussian distribution (Fig. 1, B, C, E, and F). Therefore, the changes produced by HS represent a simple shift of the mean RMP without change in normal distribution.
HS alters the RMP by modulating the electrogenic transport activity of the α2 Na,K-ATPase isozyme
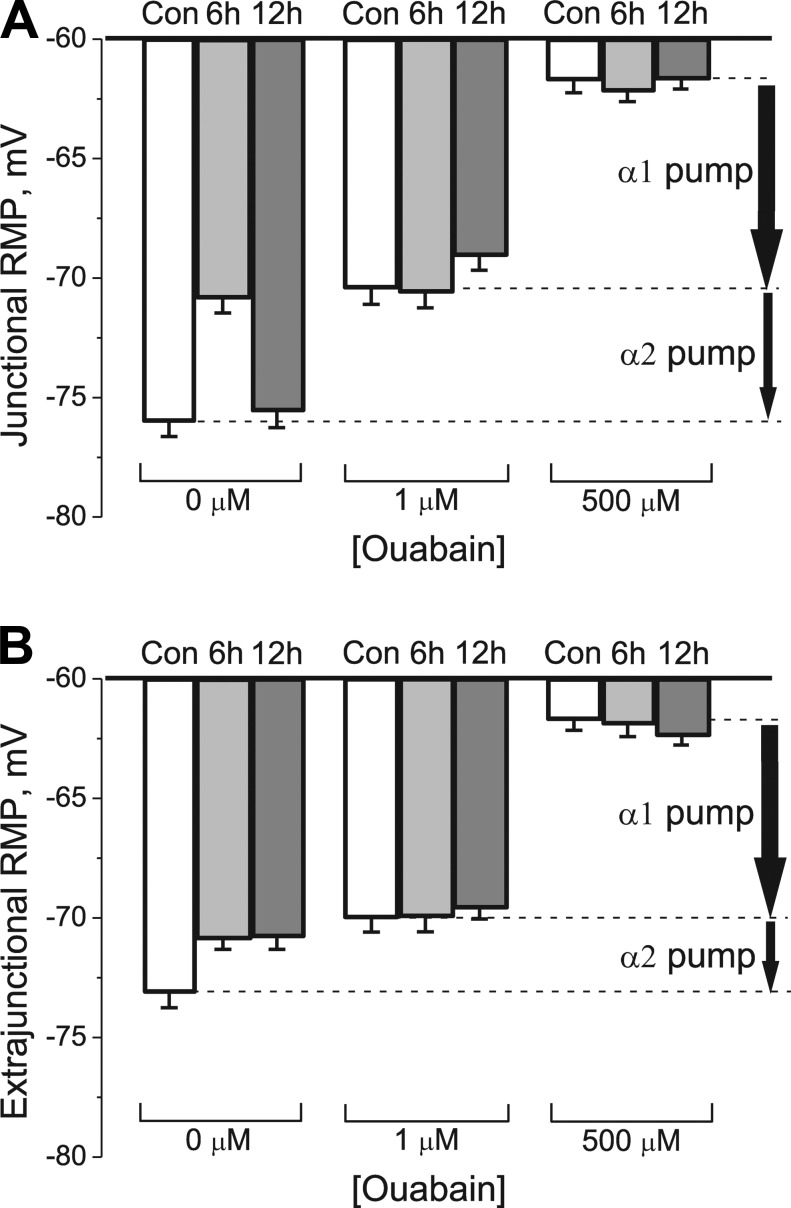
The transport activity of the Na,K-ATPase α1 and α2 isozymes was determined by measuring the ouabain-sensitive change in RMP (see Materials and methods). Fig. 2 shows the mean RMPs measured before and after exposure to 1 and 500 µM ouabain. In the junctional region of control soleus muscles, total electrogenic activity by the Na,K-ATPase contributes −14.3 ± 0.8 mV to the RMP. Of this, the α2 isozyme generates −5.5 ± 0.8 mV and the α1 isozyme generates −8.8 ± 0.8 mV. Therefore, the α2 and α1 isozymes contribute 38% and 62%, respectively, of basal Na+/K+ transport at the junctional membrane of rat soleus muscle (Figs. 2 A and 3 A).
Figure 2.
Measurement of Na,K-ATPase electrogenic activity contributed by each α isozyme in control muscles and after HS. (A and B) Changes in mean RMP induced by ouabain in rat soleus muscle fibers in control (Con) and after 6 and 12 h of HS in junctional (A) and extrajunctional (B) membrane regions. RMPs were recorded before (zero ouabain concentration) and after 30-min incubation in 1 µM ouabain and after 30-min incubation with 500 µM ouabain. Vertical arrows indicate electrogenic contributions generated by α2 and α1 Na,K-ATPase isozymes in control muscles. The RMP reported at each data point represents the mean of measurements from a total of 98–140 fibers obtained from six muscles for each group of rats. Error bars indicate SEM.
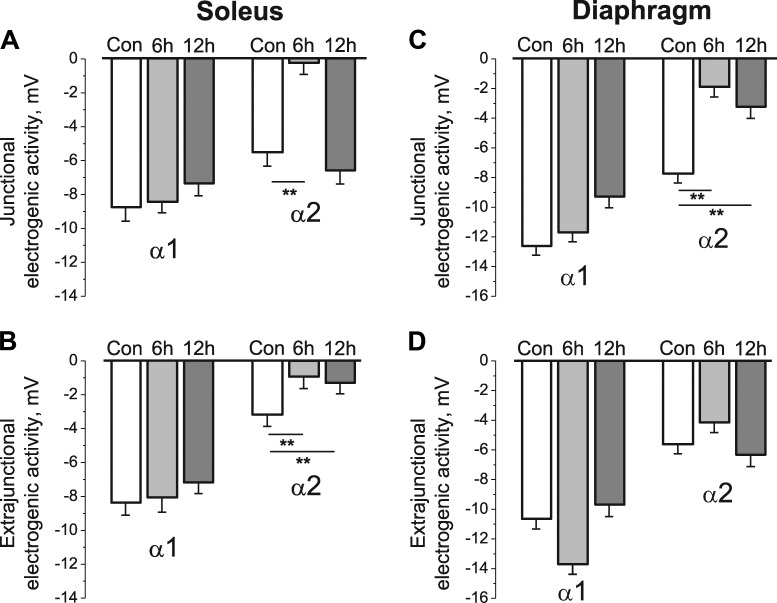
Figure 3.
HS specifically alters the electrogenic transport activity of the α2 Na,K-ATPase isozyme. (A–D) Na,K-ATPase α1 and α2 electrogenic activity measured in the junctional (A and C) and extrajunctional (B and D) membrane regions of rat soleus (A and B) and diaphragm (C and D) muscle fibers in control group (Con) and after 6 and 12 h of HS. (A and B) Six muscles for each group of rats (same muscles as in Fig. 2). (C and D) Six muscles (control), five muscles (6 h of HS), and four muscles (12 h of HS). Each value of electrogenic activity represents the mean of measurements from a total of 98–140 fibers (A and B) and 91–163 fibers (C and D). **, P < 0.01 compared with the corresponding control. Error bars indicate SEM.
HS alters these contributions in an isoform-specific manner. After 6 h of HS, the ouabain-sensitive electrogenic potential contributed by α2 isozyme decreased dramatically to only −0.2 ± 0.7 mV (P < 0.01), whereas the electrogenic potential contributed by α1 isozyme remained unchanged at −8.5 ± 0.6 mV (P < 0.01; Fig. 3 A). Therefore, the HS-induced membrane depolarization is almost entirely caused by a specific decrease in α2 isozyme activity. After 12 h of HS, α2 isozyme electrogenic activity at the NMJ recovered to control value without changes in α1 isozyme activity (Fig. 3 A). This suggests that restoration of the mean RMP at the junctional membrane after 12 h HS results from a specific increase in α2 isozyme activity.
In extrajunctional membrane regions of the soleus muscle, the activity of α1 isozyme did not change during 6 or 12 h of HS (Fig. 3 B). In contrast, α2 isozyme activity decreased from −3.2 ± 0.7 mV in control to −0.9 ± 0.7 mV (P < 0.01) after 6 h of HS. No recovery was observed, and after 12 h of HS the electrogenic activity of α2 isozyme was only −1.3 ± 0.6 mV (Fig. 3 B). Therefore, in extrajunctional membrane regions, HS of up to 12 h produces only depolarization without recovery of the RMP. Overall, electrogenic activity by the α1 isozyme is similar in junctional and extrajunctional membrane regions and is not changed by HS, whereas α2 isozyme activity is dynamically modulated.
The question arises: could changes in the RMP after HS be caused by a mechanism other than a change in the Na,K-ATPase electrogenic activity? For example, more prolonged HS of 3 d or more up-regulates voltage-dependent Na+ and ClC-1 chloride channels (Desaphy et al., 2001; Pierno et al., 2002) and down-regulates the ATP-sensitive K+ channels (Tricarico et al., 2010) in the rat soleus muscle. These permeability changes are expected to alter the RMP independent of any change in electrogenic pump potential. This possibility is unlikely caused by the finding that both control and HS-treated muscles establish the same RMP (approximately −62 mV) when the Na,K-ATPase electrogenic contribution is completely blocked with 500 µM ouabain (Fig. 2, A and B). Similar ouabain-induced RMP changes were observed in diaphragm muscles (not depicted). This result confirms that HS changes the RMP via the α2 electrogenic pump contribution rather than by a change in ion permeability.
In diaphragm muscles, which continuously contract during HS, the electrogenic activity of α1 isozyme did not change after 6 h of HS in both regions of sarcolemma. The electrogenic activity of α2 isozyme at the junctional membrane decreased to the same extent as in soleus muscle after 6 h of HS, whereas α2 isozyme activity in extrajunctional membrane (as well as RMP; Fig. 1 D) was not changed (Fig. 3, C and D). Again, conversely to soleus muscles, there was no recovery of junctional α2 isozyme electrogenic activity after 12 h of HS; the electrogenic activity of the extrajunctional α2 isozyme pool was not change after both 6 and 12 h of HS (Fig. 3, C and D).
Therefore, acute HS preferentially depolarizes the RMPs of skeletal muscle fibers through corresponding isoform-specific changes in the electrogenic transport activity of the α2 Na,K-ATPase, and the junctional and extrajunctional pools of α2 Na,K-ATPase are regulated differently.
Low-intensity muscle workload specifically restores the electrogenic activity of α2 Na,K-ATPase
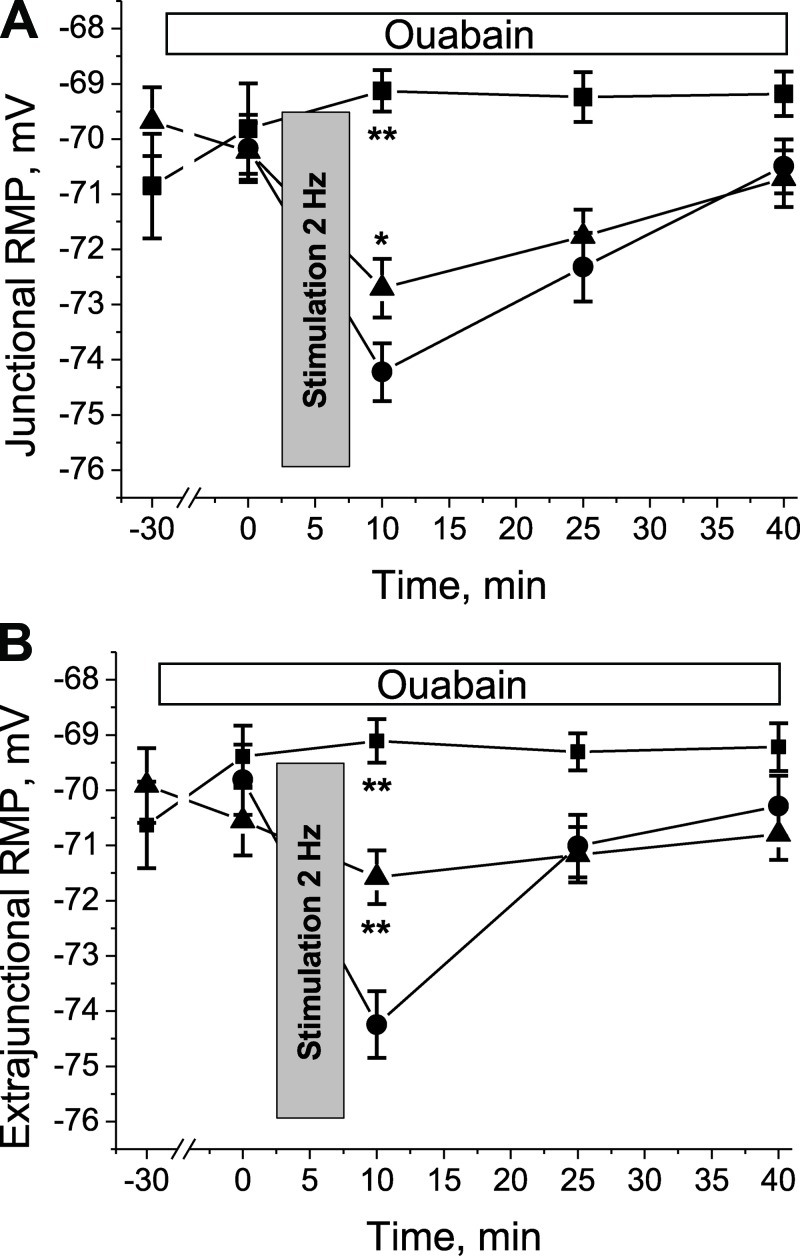
In some experiments, RMP measurements were repeated on HS-suspended soleus muscles before and immediately after the muscle was stimulated to produce evoked contractions at 2 Hz for 5 min (see Materials and methods). This protocol hyperpolarizes the RMP by stimulating the Na,K-ATPase (Hicks and McComas, 1989), and extracellular K+ accumulation might specifically stimulate the α2 Na,K-ATPase (DiFranco et al., 2015). This low-intensity workload transiently hyperpolarized both junctional and extrajunctional sarcolemma regions of soleus muscles after 6 h of HS (Fig. 4). The hyperpolarization was inhibited in a dose-dependent manner by 100 nM to 1 µM ouabain (Fig. 4), indicating that it arises from ouabain-sensitive α2 Na,K-ATPase electrogenic activity. This result provides further evidence that α2 Na,K-ATPase activity is specifically responsive to muscle use.
Figure 4.
Repetitive stimulation of rat soleus muscle after 6 h of HS specifically restores the electrogenic transport activity of the α2 Na,K-ATPase isozyme. (A and B) RMPs were measured in the junctional (A) and extrajunctional (B) membrane regions before and immediately after the soleus muscles were stimulated at 2 Hz for 5 min (see Materials and methods). The experiments were performed without ouabain (circles, six muscles) and in the presence of 100 nM (triangles, eight muscles) or 1 µM (squares, six muscles) ouabain (30-min preincubation). *, P < 0.05; **, P < 0.01 compared with corresponding value in the absence of ouabain. Error bars indicate SEM.
It should be noted that, if the excitation-related Na,K-ATPase activity causes ATP depletion in a local subsarcolemmal space (Kabakov, 1998; Zhu et al., 2014), ATP-sensitive K+ channels will open and contribute an additional component to the measured hyperpolarization. In any case, these data indicate that a low-intensity workload can specifically restore the activity of the α2 Na,K-ATPase isozyme in hindlimb-suspended muscles. This result provides further evidence that the activity of α2 Na,K-ATPase depends strongly on muscle use and that it is retained in the sarcolemma during 6 h of HS (see also Fig. 9).
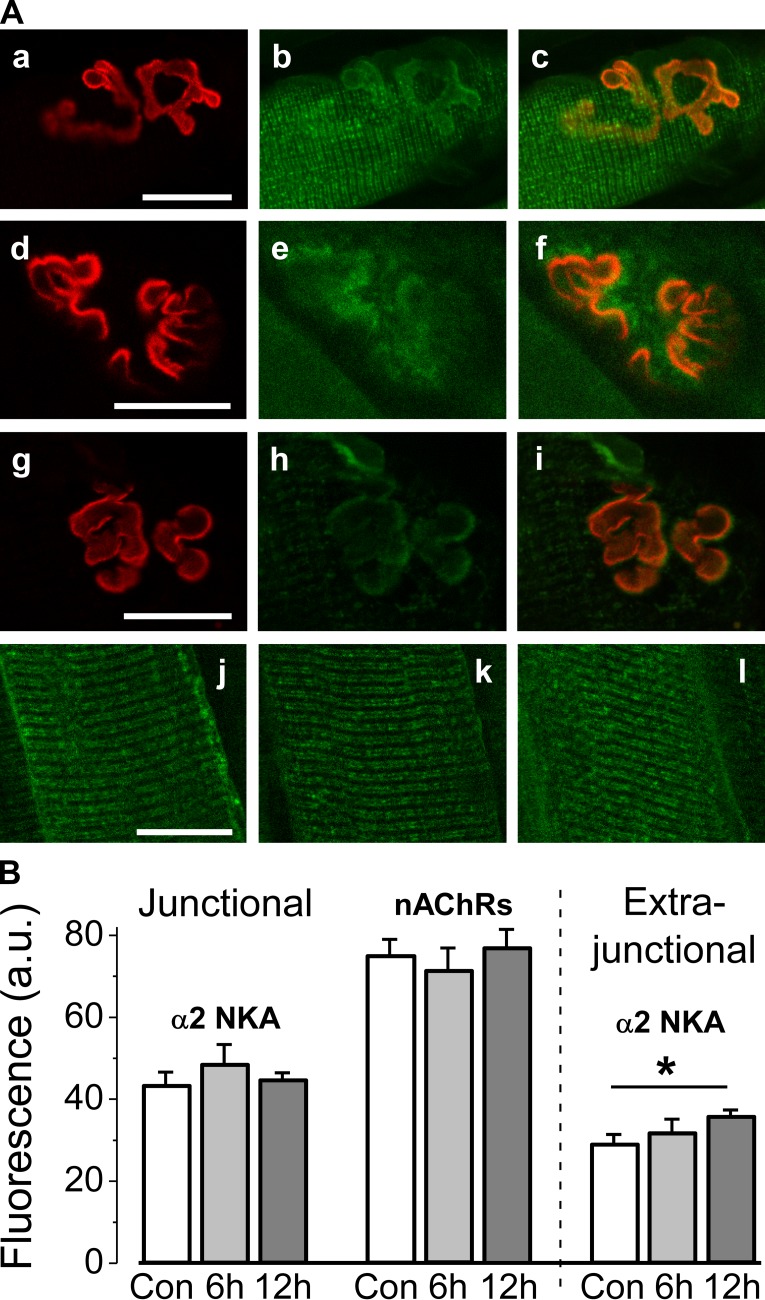
Figure 9.
Investigation of α2 Na,K-ATPase membrane localization. (A) Representative images. (a–c) Control. The Na,K-ATPase α2 isozyme and the nAChRs colocalize at the muscle end-plate. A control rat soleus muscle was dual labeled with rhodamine-conjugated α-bungarotoxin (red channel, a) and BODIPY-conjugated ouabain (green channel, b). Overlap is shown (orange channel, c). (d–i) Same labeling after 6 and 12 h of HS, respectively. (j–l) Muscles singly labeled with 1 µM BODIPY-ouabain, showing specific α2 Na,K-ATPase localization in the transverse tubules and sarcolemma where the Na,K-ATPase α2 isozyme is known to be expressed. (j) Control. (k) After 6 h of HS. (l) After 12 h of HS. Same confocal settings were used for panels a–l. (B) Averaged fluorescence (a.u.) from eight control soleus muscles (white bars: 57 junctional and 57 extrajunctional membrane regions), eight muscles after 6 h of HS (light gray bars: 48 junctional and 48 extrajunctional membrane regions), and after 12 h of HS (gray bars: 7 muscles, 34 junctional regions; 10 muscles, 48 extrajunctional regions). Error bars indicate SEM. *, P < 0.05 compared with control. Bars: (A, a–i) 25 µm; (A, j–l) 20 µm.
HS of 12 h specifically alters α2 Na,K-ATPase protein and mRNA content in whole homogenate
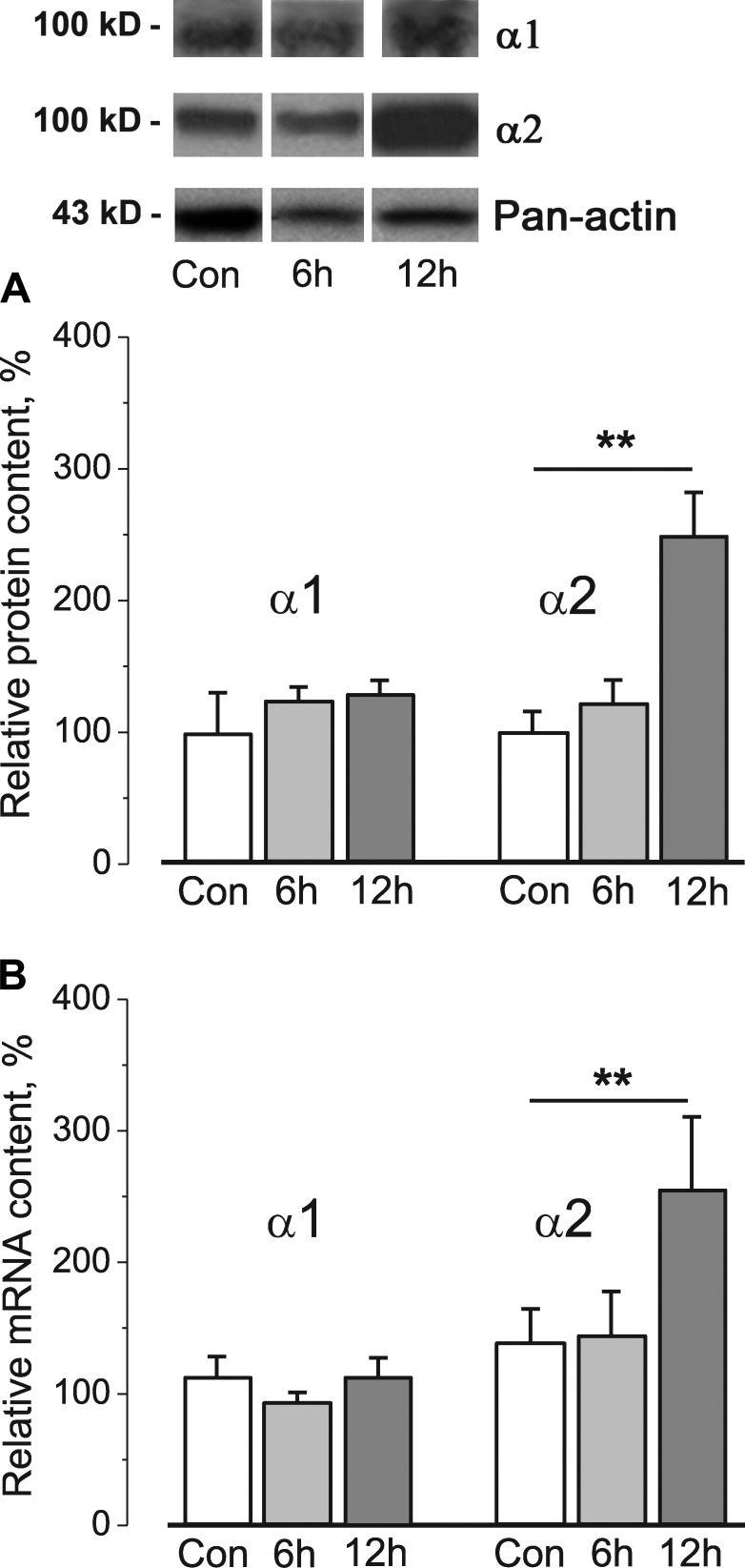
6 h of HS did not change total Na,K-ATPase α1 or α2 protein content (Fig. 5 A) or their mRNA (Fig. 5 B) measured in whole homogenates from soleus muscles. However, after 12 h of HS, both α2 protein content and mRNA increased, whereas α1 content and mRNA were unchanged (Fig. 5, A and B). Therefore, the initial decrease in activity is not explained by reduced α2 Na,K-ATPase protein content, whereas increased α2 protein content may contribute to the restoration of junctional α2 activity at 12 h of HS. However, even the increased α2 Na,K-ATPase protein and mRNA content after 12 h of HS cannot counteract the sustained inhibition of Na,K-ATPase α2 isozyme activity at extrajunctional membrane regions (Fig. 3 B). In sum, these data suggest that the decrease in α2 Na,K-ATPase activity in the soleus muscle is caused by an acute regulation of enzyme activity by muscle use, rather than by altered protein or mRNA content.
Figure 5.
12 h of HS specifically alters α2 Na,K-ATPase protein and mRNA content. (A and B) Relative Na,K-ATPase α1 and α2 protein (A) and mRNA content (B) in whole homogenates from the rat soleus muscles of control rats (Con) and after 6 and 12 h of HS. In A, the expression levels of α1 and α2 proteins were semiquantified by normalization of band intensities to the band intensity of pan-actin made from the same lysate. Top panels show representative immunoblots. In A and B, data were normalized to the mean level of expression under control conditions. Columns show mean data from 8–10 (A) and 5–16 (B) different muscle samples. **, P < 0.01 compared with control. Error bars indicate SEM.
Role of PLM in the early disuse-induced remodeling events
To investigate the molecular mechanisms that regulate Na,K-ATPase α2 activity in response to muscle disuse, we investigated the expression and phosphorylation state of proteins known to regulate Na,K-ATPase activity. PLM, a partner protein for the Na,K-ATPase, plays an important role in its regulation (for reviews see Geering [2008] and Pavlovic et al. [2013]).
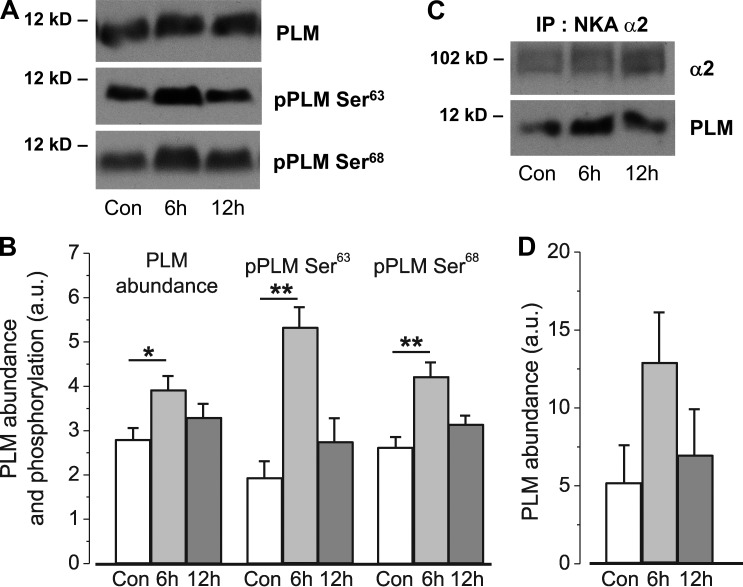
In our study, PLM abundance increased 1.4-fold (P < 0.05) after 6 h of HS. Notably, the phosphorylation of PLM on Ser63 and Ser68 also increased significantly after 6 h of HS, increasing 2.75-fold (P < 0.01) and 1.6-fold (P < 0.01), respectively. These effects were transient, and after 12 h of HS, PLM abundance and phosphorylation tended to return toward control levels (Fig. 6, A and B). The association of PLM with the α2 Na,K-ATPase measured as coimmunoprecipitation also was strong and tended to increase after 6 h of HS (P = 0.11; Fig. 6, C and D).
Figure 6.
HS alters PLM abundance, phosphorylation, and its association with α2 Na,K-ATPase. (A and B) PLM abundance and phosphorylation at Ser63 and Ser68. (C and D) PLM coimmunoprecipitates with the Na,K-ATPase α2 subunit. Bar graphs show the mean density from 10 (A and B) and 4 (C and D) measurements of different muscle samples. Representative immunoblots are shown. *, P < 0.05; **, P < 0.01 compared with corresponding control. Error bars indicate SEM.
These data provide evidence to suggest that 6 h of HS might affect the Na,K-ATPase α2 by a phosphorylation-dependent mechanism and by increased PLM abundance and its association with α2 Na,K-ATPase. We cannot exclude the possibility that a subpopulation of the Na,K-ATPase α2 may be activated by increased phosphorylation of PLM. However, as a net effect, we observe α2 pump inhibition.
HS does not alter the functional interaction of nAChR with α2 Na,K-ATPase
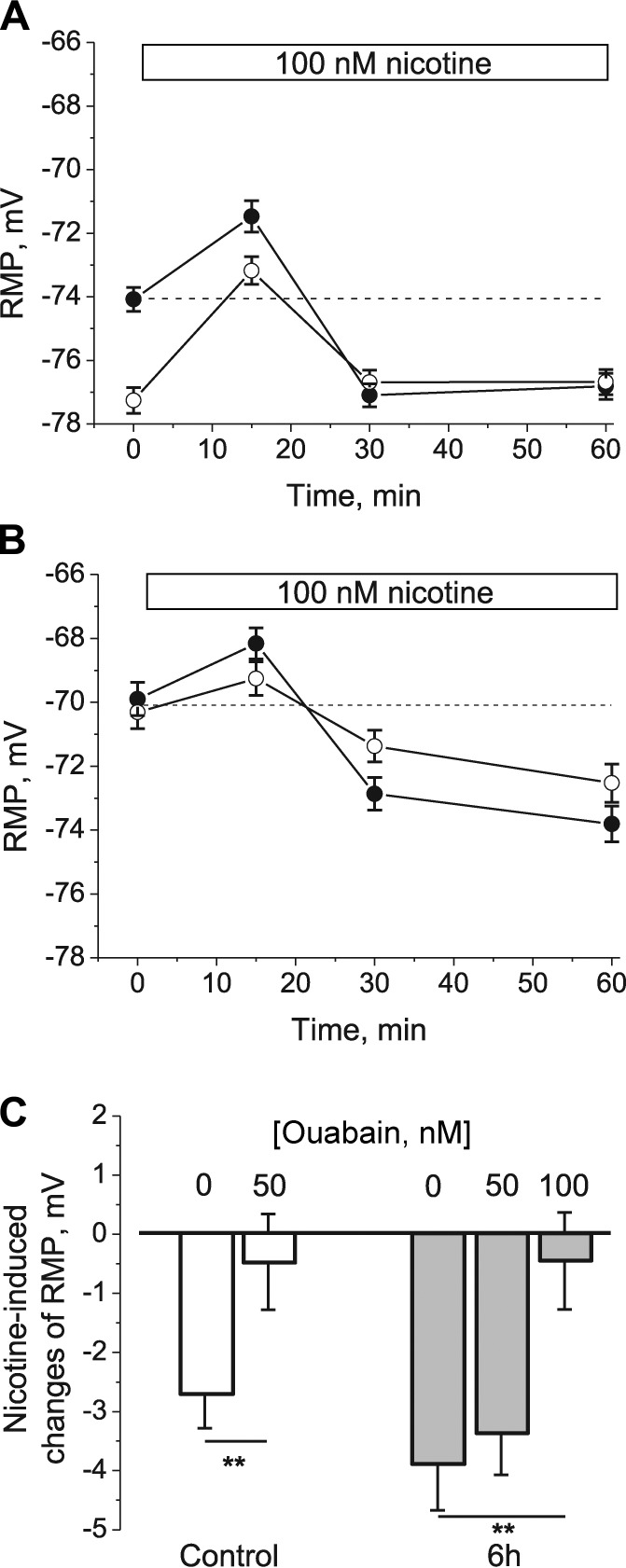
To confirm that α2 Na,K-ATPase remains in the membrane and is functional during acute HS, we tested whether the Na,K-ATPase α2 isozyme is able to be stimulated by nanomolar concentrations of nAChR agonists. This effect is based on the functional interaction between the nAChRs and the Na,K-ATPase (Krivoi et al., 2006; Heiny et al., 2010). Upon exposure of rat soleus muscles to 100 nM nicotine, a small but significant (P < 0.01) depolarization was detected in both junctional and extrajunctional membrane regions. The depolarization, expected if 100 nM nicotine initially opens a small number of nAChRs, was followed by sustained hyperpolarization (Fig. 7 A).
Figure 7.
HS does not alter the functional interaction between nAChR and α2 Na,K-ATPase. (A–C) Changes in membrane potential induced by 100 nM nicotine in rat soleus muscle fibers in control (A), after 6 h of HS (B), and in the presence of ouabain (C). In A and B, junctional membrane (open circles), extrajunctional membrane (closed circles); horizontal bars indicate the periods when nicotine was present in the perfusate. In C, muscles were preincubated with the indicated concentration of ouabain for 30 min before nicotine addition. RMPs were recorded from extrajunctional membrane regions. Changes in RMP were calculated as the difference between the potential measured before and after 60 min of nicotine action. **, P < 0.01 compared with corresponding value in the absence (0 nM) of ouabain. Error bars indicate SEM.
After 6 h of HS, the initial RMPs of both sarcolemma regions depolarized to approximately −70 mV; however, Na,K-ATPase activity in both regions was able to be stimulated further by nicotine, indicating the continued presence of Na,K-ATPase in both junctional and extrajunctional membrane (Fig. 7 B). The nicotine-induced hyperpolarization was inhibited by 50 or 100 nM ouabain (Fig. 7 C), consistent with previous data attributing this hyperpolarization to specific stimulation of ouabain-sensitive Na,K-ATPase α2 isozyme activity (Krivoi et al., 2003; Heiny et al., 2010). These data indicate that the Na,K-ATPase α2 isozyme, even after being inhibited by 6 h of HS, remains capable of dynamically increasing its transport activity in response to nanomolar nAChR agonist.
HS does not alter the regulation of α2 Na,K-ATPase electrogenic transport by extracellular ACh
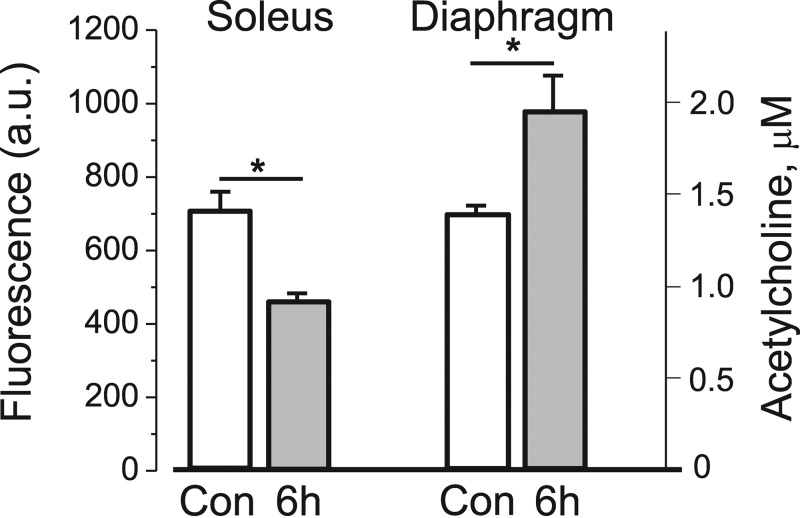
It is possible that HS might lower the level of endogenous nonquantal ACh release and thereby selectively decrease α2 activity through the nAChR/α2Na,K-ATPase interaction (Krivoi et al., 2006; Heiny et al., 2010). To test this possibility, we measured the level of extracellular ACh released from resting soleus muscles and nerve endings using optical detection of choline released from the hydrolysis of ACh by endogenous AChE. These experiments as well as experiments with the addition of exogenous AChE (not depicted) demonstrate that choline fluorescence decreases by at most 25–35% after 6 h of HS (Fig. 8). This result indicates that 6 h of HS induces a small decrease in extracellular ACh levels in soleus muscles. In contrast, choline fluorescence in diaphragm muscles increases by the same small amount after 6 h of HS (Fig. 8). These opposite changes in ACh levels suggest that the significant decrease in α2 Na,K-ATPase transport activity (Fig. 3) induced in both soleus and diaphragm muscles after 6 h HS is not explained by changes in extracellular ACh levels.
Figure 8.
Extracellular ACh levels in control and after 6 h of HS. Choline fluorescence (a.u.) after 15-min incubation of soleus and diaphragm muscles from control rats (white bars, four muscles) and rats after 6 h of HS (gray bars, four muscles). *, P < 0.05 compared with the corresponding control. The fluorescence was compared with the ACh standard curve to estimate the amount of ACh (µM) accumulated in 0.4 ml of bathing solution normalized per gram of muscle tissue (right y axis). Error bars indicate SEM.
Investigation of α2 Na,K-ATPase localization in the sarcolemma
It is possible that HS may alter the localization of α2 Na,K-ATPase in the sarcolemma and thereby alter its electrogenic contribution in different membrane regions. We investigated this possibility by imaging the NMJ of intact soleus muscles dual labeled with fluorescent, specific ligands of the Na,K-ATPase (BODIPY-conjugated ouabain, 1 µM) and the nAChR (rhodamine-conjugated α-bungarotoxin). In control muscles, the fluorescent ouabain signal overlaps with the α-bungarotoxin signal at the end-plate regions, confirming that the α2 Na,K-ATPase and the nAChR colocalize at the postsynaptic NMJ (Fig. 9 A, a–c), as reported previously (Heiny et al., 2010). 6 and 12 h of HS did not affect the colocalization between these proteins (Fig. 9 A, d–f and g–i) and did not alter the fluorescence signal from either α2 Na,K-ATPase or nAChRs (Fig. 9 B). These observations confirm that α2 Na,K-ATPase distribution at the NMJ does not change after both 6 and 12 h of HS, times when electrogenic activity by the α2 pump is completely lost and then recovered, respectively (Fig. 3 A). The stable colocalization of the nAChRs with the α2 Na,K-ATPase after 6 h of HS is also expected from the finding that the functional interaction between these proteins remains (see above, Fig. 7).
In addition to the NMJ, the α2 Na,K-ATPase isozyme is also present on extrajunctional sarcolemma and transverse tubule membranes (Williams et al., 2001). Consistent with this expectation, the fluorescent ouabain signal is also detected on extrajunctional sarcolemma in the transverse tubules, evident as double rows of label with a repeat pattern of two per sarcomere. This pattern is expected from the dual transverse-tubule openings at the A-I junctions of mammalian muscle (Fig. 9 A, j), consistent with previous studies (Heiny et al., 2010; Radzyukevich et al., 2013). As with the junctional membrane, 6 h of HS did not alter the extrajunctional localization (Fig. 9 A, k) or fluorescence signal (Fig. 9 B) from the Na,K-ATPase α2 isozyme. However, after 12 h of HS, the fluorescence from the α2 Na,K-ATPase increased by 22% (P < 0.05; Fig. 9 B), suggesting the increased abundance in the extrajunctional sarcolemma. This finding corresponds well with enhanced α2 Na,K-ATPase protein and mRNA content after 12 h of HS (Fig. 5). Nevertheless, no recovery of extrajunctional α2 pump activity was observed at this time point (Fig. 3 B). Collectively, these data suggest that α2 Na,K-ATPase is retained in the soleus muscle sarcolemma during 6–12 h of HS, and disuse-induced changes of α2 pump activity cannot be explained by altered membrane localization.
DISCUSSION
The novelty of this study is that acute disuse dramatically and specifically alters the activity, mRNA, and protein content of the Na,K-ATPase α2 isoform, and this represents an early event in the progression to muscle atrophy. The decreased α2 Na,K-ATPase activity depolarizes the RMP by reducing the negative potential contributed by electrogenic pump transport, and this may occur in part via a PLM-dependent regulatory mechanism. Intriguingly, junctional and extrajunctional pools of the α2 Na,K-ATPase are regulated differently.
The Na,K-ATPase is obligatory for excitability, electrogenesis, and the contractility of skeletal muscles (Sejersted and Sjøgaard, 2000; Clausen, 2013). The skeletal muscle pool of Na,K-ATPase demonstrates a high level of plasticity in response to a variety of physiological conditions. Its transport activity is modulated over a wide dynamic range by several acute interventions. These have been reviewed extensively and include membrane excitation, muscle contraction, circulating hormones, neurotransmitters, and endogenous inhibitors. In addition, longer-term interventions such as exercise training or chronic hormonal status can modulate the protein content of Na,K-ATPase (Clausen, 2013). The wide regulatory range of Na,K-ATPase activity and content allows skeletal muscles to match Na+/K+ transport capacity to the varying demands of resting and contracting muscle and to adapt to chronic changes in levels of inactivity, activity, or training.
Although adaptations of Na,K-ATPase content and activity in response to increased physiological loading are well documented (Yuan et al., 2007; Kristensen et al., 2008; Murphy et al., 2008; Juel, 2009; Nordsborg et al., 2010; Benziane et al., 2012; Clausen, 2013), the effects of physical inactivity induced by functional unloading and other conditions (Leivseth et al., 1992; Krivoĭ et al., 2008; Boon et al., 2012; Kravtsova et al., 2015; Perry et al., 2015) are relatively few in numbers. The molecular mechanisms underlying the changes have not been investigated.
Adult skeletal muscle coexpresses α1 and α2 isoforms of the Na,K-ATPase catalytic and transport α subunit (Orlowski and Lingrel, 1988). Most prior studies of the regulation of Na,K-ATPase in skeletal muscle by acute interventions have measured total Na,K-ATPase activity or content contributed by the combined α1 and α2 isoform pools. However, the α1 and α2 isoforms in skeletal muscle have distinct distributions with respect to content and membrane localization, suggesting that they play distinct physiological roles. The α1 isoform comprises up to 40% of total Na,K-ATPase α subunit and is expressed only on the outer plasma membrane. The α2 isozyme comprises 60–80% of total Na,K-ATPase content (Orlowski and Lingrel, 1988; He et al., 2001), and the majority of α2 isozyme is expressed in the interior transverse tubule membranes, with smaller pools localized to the postsynaptic NMJ and surface caveolae (Williams et al., 2001; Heiny et al., 2010). In resting, noncontracting skeletal muscles, the α2 isozyme operates far below its maximum transport capacity. Although the α1 isozyme provides up to 75% of the Na+/K+ transport needed to maintain basal ion gradients and the resting potential, the α2 Na,K-ATPase contributes only 25% of basal Na+/K+ transport (Krivoi et al., 2003; Chibalin et al., 2012). Because of the large size of the α2 Na,K-ATPase pool, even small changes in specific α2 isozyme activity are expected to produce large changes in total Na+/K+ transport capacity.
Several recent studies suggest that the α2 Na,K-ATPase pool provides a reserve transport capacity that can be activated in response to acute changes in muscle use, to meet the increased demands of contracting muscles for active Na+/K+ transport. The activity of the α2 pool at the NMJ increases rapidly during periods of nerve activity, through an interaction between the ACh-bound nAChR and the Na,K-ATPase α2 isozyme (Krivoi et al., 2003, 2006; Heiny et al., 2010). The resulting hyperpolarization primes the muscle junctional membrane to be more responsive to nerve input during periods of increased muscle use. The extrajunctional pool of Na,K-ATPase α2 is also stimulated by this mechanism, to a lesser extent.
The extrajunctional pool of α2 isozyme, presented in the surface caveolae and transverse tubules, plays an important role in clearing the excitation-related increases in extracellular K+ and intracellular Na+ that accompany action potential excitation. Studies of mice with a targeted KO of α2 isozyme in the skeletal muscles indicate that stimulation of α2 isozyme activity during muscle use is absolutely required for maintaining contractility and preventing fatigue in working muscles (Radzyukevich et al., 2013; DiFranco et al., 2015).
This study examined whether the Na,K-ATPase α1 and α2 isoforms in skeletal muscle are differentially regulated by acute decreases in muscle use. Acute muscle disuse induced by 6 h of HS dramatically reduced the Na,K-ATPase α2 transport activity of resting soleus muscles. Decreased α2 Na,K-ATPase activity is caused by a decrease in enzyme activity rather than by altered protein and mRNA content or localization in the sarcolemma. In addition, HS does not alter α2 Na,K-ATPase colocalization and functional interaction with the nAChRs.
Longer periods of HS up to 12 h selectively up-regulate α2 isoform protein and mRNA content. It was shown that decreased Na,K-ATPase activity in skeletal muscle at fatigue is reciprocally correlated with increased α2 mRNA expression, suggesting a possible signal transduction role for depressed Na,K-ATPase activity on α isoform gene expression (Petersen et al., 2005). The increased α2 mRNA expression may be a compensatory response to preserve muscle function. However, even the increased α2 Na,K-ATPase protein content and enhanced membrane abundance after 12 h of HS cannot counteract the sustained inhibition of α2 isozyme activity at extrajunctional membrane regions.
Notably, acute HS did not alter the activity and content of the α1 Na,K-ATPase isoform. This finding is consistent with the emerging consensus that the α1 isoform in muscle, as in other tissues, plays the major role in maintaining basal ion gradients, osmotic balance, and the resting potential, and it further supports the idea that the Na,K-ATPase α2 isoform in skeletal muscle is the more regulated isoform that can respond to changes in muscle use.
HS is expected to induce systemic changes in the animal, in addition to muscle-specific changes in the inactive hindlimb muscles. Some studies indicate that animals show an initial, transient stress response to HS (Thomason and Booth, 1990). By comparing α2 Na,K-ATPase regulation in the inactive soleus muscle with the continuously active diaphragm muscle from the same animal, we were able to study the role of muscle inactivity per se on α2 Na,K-ATPase regulation. The decrease in α2 Na,K-ATPase activity on extrajunctional membranes, where the majority of α2 pump is localized, occurred only in the inactive soleus muscle. An interesting result was that the α2 activity in the junctional membranes decreased for both diaphragm and soleus muscles during 6 h of HS. However, conversely to soleus, there was no recovery of junctional α2 isozyme activity in diaphragm muscle after 12 h of HS. This finding indicates that the junctional and extrajunctional pools of α2 isozyme are regulated differently. The extrajunctional pool of the α2 isozyme is regulated by disuse, whereas the small subset of α2 Na,K-ATPase at the junctional membrane is regulated by disuse as well as by additional systemic factors (possibly circulating stress factors) related to HS. Importantly, application of a low-intensity workload is able to restore the electrogenic transport activity of both junctional and extrajunctional pools of α2 Na,K-ATPase, providing further evidence that regulation of Na,K-ATPase α2 activity depends strongly on muscle use. The molecular mechanisms that differentially regulate the α2 Na,K-ATPase in different membrane compartments remain to be elucidated.
The molecular mechanisms by which disuse down-regulates the activity of α2 Na,K-ATPase are not known. This study investigated whether PLM plays a role in this regulation. Other studies have shown that PLM acts as a brake on the Na,K-ATPase, whereas phosphorylation removes this brake and increases pump activity by increasing the affinity of intracellular Na+ sites (reviewed in Geering [2008] and Pavlovic et al. [2013]). In skeletal muscle, at least 30% of α1 and α2 Na,K-ATPase isoforms are associated with PLM (Rasmussen et al., 2008). The increased PLM abundance and increased association with the α2 Na,K-ATPase after 6 h of HS suggest that inhibition by PLM may underlie the acute, total inhibition of α2 Na,K-ATPase electrogenic activity. In contrast, we detected an increase in PLM phosphorylation, which is expected to stimulate the Na,K-ATPase. PLM phosphorylation can be induced by multiple signaling pathways and receptors, including stress-related β-adrenergic receptors acting through PKA. Short-term HS may alter PLM regulation of Na,K-ATPase by more than one mechanism, with the dominant effect toward the reduced activity.
Other factors related to muscle disuse may be also involved in the inhibition of α2 Na,K-ATPase functional activity during acute HS. Reduced EMG and neurogram activity after HS (Ohira et al., 2002; De-Doncker et al., 2005) is expected to reduce PKC activity. In fact, PKC activity is known to decrease after short HS (Pierno et al., 2007), and this in turn could alter the phosphorylation state of the Na,K-ATPase α or PLM subunits (Walaas et al., 1994; Mahmmoud and Cornelius, 2002). Additional studies of the regulation of Na,K-ATPase activity, in particular the less-studied α2 isoform, will be needed to interpret these results.
Physiological implications
Other studies also suggest that the α2 Na,K-ATPase plays a special role in adaptations to skeletal muscle disuse. Chronic disuse resulting from spinal cord injury (Boon et al., 2012) or knee injury (Perry et al., 2015) significantly decreases the α2 Na,K-ATPase content in skeletal muscles. The disuse-induced decrease in Na,K-ATPase α2 electrogenic activity and steady membrane depolarization is expected to decrease muscle excitability and contractility. Maintaining the necessary level of skeletal muscle RMP is essential for many physiological processes, including ion homeostasis and normal action potential generation. The disuse-induced steady depolarization of junctional membrane is expected to inactivate Na+ channels and lower the safety factor for neuromuscular transmission by increasing the threshold for generation of the muscle action potentials (Wood and Slater, 2001). Depolarization of extrajunctional membrane is expected to decrease excitability and impair action potential generation and voltage-dependent excitation-contraction coupling. Also, loss in Na,K-ATPase α2 activity should result in an additional excitation-related K+ load in the T-tubules in working muscles (DiFranco et al., 2015). These effects are in a direction to exaggerate muscle weakness under conditions in which the safety factor for excitation is already impaired. Disuse-induced changes in the Na,K-ATPase are also expected to alter the function of Na+-dependent transporters and other processes that depend on the Na+ gradient established by the Na,K-ATPase. Our data provide the first evidence that such remodeling during HS starts early and that the α2 Na,K-ATPase is specifically altered. Considering the unique role of the α2 Na,K-ATPase isozyme in excitation, contraction, and adaptation to exercise (Radzyukevich et al., 2004, 2009, 2013; Krivoĭ, 2012), disuse-induced α2 Na,K-ATPase alterations might be an important player in adaptations of skeletal muscle to altered use.
Acknowledgments
We are very grateful to St. Petersburg State University Research Center for Molecular and Cell Technologies and personally to Nikolai A. Kostin for assistance with confocal microscopy experiments.
This study was supported for I.I. Krivoi by Russian Foundation for Basic Research (RFBR) grant #13-04-00973 and St. Petersburg State University research grants #1.50.1621.2013 and #1.38.231.2014; for V.V. Matchkov by the Danish Research Council grant #09-063499 and the Novo Nordisk Foundation grant #NNF14OC0012731; for A.V. Chibalin by Swedish Research Council grant #K2013-55X-14191-12-3, the Strategic Research Program in Diabetes at Karolinska Institutet, and the Novo Nordisk Foundation grant #10559; for A.M. Petrov by RFBR grant #14-04-00094; for A.L. Zefirov by RFBR grant #14-04-01232; and for J.A. Heiny by National Institutes of Health grant #1 R01 AR063710.
The authors declare no competing financial interests.
Author contributions: V.V. Kravtsova, A.M. Petrov, A.N. Vasiliev, E.V. Bouzinova, and B. Benziane performed all experiments, data collection, and analysis. V.V. Matchkov, A.V. Chibalin, and A.L. Zefirov were involved in the interpretation of data. I.I. Krivoi and J.A. Heiny designed and wrote the manuscript. The project was directed by I.I. Krivoi. All authors approved the final version of the manuscript for publication.
Eduardo Rios served as editor.
Footnotes
Abbreviations used in this paper:
- ACh
- acetylcholine
- AChE
- acetylcholinesterase
- a.u.
- arbitrary units
- HRP
- horseradish peroxidase
- HS
- hindlimb suspension
- nAChR
- nicotinic ACh receptor
- NMJ
- neuromuscular junction
- PCR
- polymerase chain reaction
- PLM
- phospholemman
- RMP
- resting membrane potential
References
- Baldwin K.M., Haddad F., Pandorf C.E., Roy R.R., and Edgerton V.R.. 2013. Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front. Physiol. 4:284 10.3389/fphys.2013.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Sun H., Xiao Y., Zhang Y., Wang X., Xu X., Liu Z., Fang J., and Li Z.. 2015. Functional interaction of nicotinic acetylcholine receptors and Na+/K+ ATPase from Locusta migratoria manilensis (Meyen). Sci. Rep. 5:8849 10.1038/srep08849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benziane B., Björnholm M., Pirkmajer S., Austin R.L., Kotova O., Viollet B., Zierath J.R., and Chibalin A.V.. 2012. Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells. J. Biol. Chem. 287:23451–23463. 10.1074/jbc.M111.331926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G., and Mercer R.W.. 1998. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275:F633–F650. [DOI] [PubMed] [Google Scholar]
- Boon H., Kostovski E., Pirkmajer S., Song M., Lubarski I., Iversen P.O., Hjeltnes N., Widegren U., and Chibalin A.V.. 2012. Influence of chronic and acute spinal cord injury on skeletal muscle Na+-K+-ATPase and phospholemman expression in humans. Am. J. Physiol. Endocrinol. Metab. 302:E864–E871. 10.1152/ajpendo.00625.2011 [DOI] [PubMed] [Google Scholar]
- Bossuyt J., Despa S., Han F., Hou Z., Robia S.L., Lingrel J.B., and Bers D.M.. 2009. Isoform specificity of the Na/K-ATPase association and regulation by phospholemman. J. Biol. Chem. 284:26749–26757. 10.1074/jbc.M109.047357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalin A.V., Heiny J.A., Benziane B., Prokofiev A.V., Vasiliev A.V., Kravtsova V.V., and Krivoi I.I.. 2012. Chronic nicotine modifies skeletal muscle Na,K-ATPase activity through its interaction with the nicotinic acetylcholine receptor and phospholemman. PLoS One. 7:e33719 10.1371/journal.pone.0033719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. 2013. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: functional significance. J. Gen. Physiol. 142:327–345. 10.1085/jgp.201310980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crambert G., Fuzesi M., Garty H., Karlish S., and Geering K.. 2002. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl. Acad. Sci. USA. 99:11476–11481. 10.1073/pnas.182267299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Doncker L., Kasri M., Picquet F., and Falempin M.. 2005. Physiologically adaptive changes of the L5 afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J. Exp. Biol. 208:4585–4592. 10.1242/jeb.01931 [DOI] [PubMed] [Google Scholar]
- Desaphy J.-F., Pierno S., Léoty C., George A.L. Jr., De Luca A., and Camerino D.C.. 2001. Skeletal muscle disuse induces fibre type-dependent enhancement of Na+ channel expression. Brain. 124:1100–1113. 10.1093/brain/124.6.1100 [DOI] [PubMed] [Google Scholar]
- DiFranco M., Hakimjavadi H., Lingrel J.B., and Heiny J.A.. 2015. Na,K-ATPase α2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K+. J. Gen. Physiol. 146:281–294. 10.1085/jgp.201511407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., and Karlish S.J.. 2006. Role of FXYD proteins in ion transport. Annu. Rev. Physiol. 68:431–459. 10.1146/annurev.physiol.68.040104.131852 [DOI] [PubMed] [Google Scholar]
- Geering K. 2008. Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 17:526–532. 10.1097/MNH.0b013e3283036cbf [DOI] [PubMed] [Google Scholar]
- He S., Shelly D.A., Moseley A.E., James P.F., James J.H., Paul R.J., and Lingrel J.B.. 2001. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281:R917–R925. [DOI] [PubMed] [Google Scholar]
- Heiny J.A., Kravtsova V.V., Mandel F., Radzyukevich T.L., Benziane B., Prokofiev A.V., Pedersen S.E., Chibalin A.V., and Krivoi I.I.. 2010. The nicotinic acetylcholine receptor and the Na,K-ATPase α2 isoform interact to regulate membrane electrogenesis in skeletal muscle. J. Biol. Chem. 285:28614–28626. 10.1074/jbc.M110.150961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks A., and McComas A.J.. 1989. Increased sodium pump activity following repetitive stimulation of rat soleus muscles. J. Physiol. 414:337–349. 10.1113/jphysiol.1989.sp017691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C. 2009. Na+-K+-ATPase in rat skeletal muscle: muscle fiber-specific differences in exercise-induced changes in ion affinity and maximal activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296:R125–R132. 10.1152/ajpregu.90760.2008 [DOI] [PubMed] [Google Scholar]
- Kabakov A.Y. 1998. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys. J. 75:2858–2867. 10.1016/S0006-3495(98)77728-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapri-Pardes E., Katz A., Haviv H., Mahmmoud Y., Ilan M., Khalfin-Penigel I., Carmeli S., Yarden O., and Karlish S.J.D.. 2011. Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J. Biol. Chem. 286:42888–42899. 10.1074/jbc.M111.293852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsova V.V., Matchkov V.V., Bouzinova E.V., Vasiliev A.N., Razgovorova I.A., Heiny J.A., and Krivoi I.I.. 2015. Isoform-specific Na,K-ATPase alterations precede disuse-induced atrophy of rat soleus muscle. BioMed Res. Int. 2015:720172 10.1155/2015/720172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M., Rasmussen M.K., and Juel C.. 2008. Na+-K+ pump location and translocation during muscle contraction in rat skeletal muscle. Pflugers Arch. 456:979–989. 10.1007/s00424-008-0449-x [DOI] [PubMed] [Google Scholar]
- Krivoĭ I.I. 2012. [Regulatory function of the Na,K-ATPase alpha2 isoform]. Biofizika. 57:771–788. [PubMed] [Google Scholar]
- Krivoi I., Vasiliev A., Kravtsova V., Dobretsov M., and Mandel F.. 2003. Porcine kidney extract contains factor(s) that inhibit the ouabain-sensitive isoform of Na,K-ATPase (α2) in rat skeletal muscle: a convenient electrophysiological assay. Ann. N. Y. Acad. Sci. 986:639–641. 10.1111/j.1749-6632.2003.tb07272.x [DOI] [PubMed] [Google Scholar]
- Krivoi I.I., Drabkina T.M., Kravtsova V.V., Vasiliev A.N., Eaton M.J., Skatchkov S.N., and Mandel F.. 2006. On the functional interaction between nicotinic acetylcholine receptor and Na+,K+-ATPase. Pflugers Arch. 452:756–765. 10.1007/s00424-006-0081-6 [DOI] [PubMed] [Google Scholar]
- Krivoĭ I.I., Kravtsova V.V., Altaeva E.G., Kubasov I.V., Prokofiev A.V., Drabkina T.M., Nikolsky E.E., and Shenkman B.S.. 2008. [Decrease in the electrogenic contribution of Na,K-ATPase and resting membrane potential as a possible mechanism of calcium ion accumulation in filaments of the rat musculus soleus subjected to the short-term gravity unloading]. Biofizika. 53:1051–1057. [PubMed] [Google Scholar]
- Leivseth G., Clausen T., Everts M.E., and Bjordal E.. 1992. Effects of reduced joint mobility and training on Na,K-ATPase and Ca-ATPase in skeletal muscle. Muscle Nerve. 15:843–849. 10.1002/mus.880150714 [DOI] [PubMed] [Google Scholar]
- Mahmmoud Y.A., and Cornelius F.. 2002. Protein kinase C phosphorylation of purified Na,K-ATPase: C-terminal phosphorylation sites at the α- and γ-subunits close to the inner face of the plasma membrane. Biophys. J. 82:1907–1919. 10.1016/S0006-3495(02)75540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchkov V.V., Moeller-Nielsen N., Dam V.S., Nourian Z., Briggs Boedtkjer D.M., and Aalkjaer C.. 2012. The α2 isoform of the Na,K-pump is important for intercellular communication, agonist-induced contraction, and EDHF-like response in rat mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 303:H36–H46. 10.1152/ajpheart.00673.2011 [DOI] [PubMed] [Google Scholar]
- Morey-Holton E., Globus R.K., Kaplansky A., and Durnova G.. 2005. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv. Space Biol. Med. 10:7–40. 10.1016/S1569-2574(05)10002-1 [DOI] [PubMed] [Google Scholar]
- Murphy K.T., Nielsen O.B., and Clausen T.. 2008. Analysis of exercise-induced Na+-K+ exchange in rat skeletal muscle in vivo. Exp. Physiol. 93:1249–1262. 10.1113/expphysiol.2008.042457 [DOI] [PubMed] [Google Scholar]
- Nikolsky E.E., Zemková H., Voronin V.A., and Vyskocil F.. 1994. Role of non-quantal acetylcholine release in surplus polarization of mouse diaphragm fibres at the endplate zone. J. Physiol. 477:497–502. 10.1113/jphysiol.1994.sp020210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg N.B., Kusuhara K., Hellsten Y., Lyngby S., Lundby C., Madsen K., and Pilegaard H.. 2010. Contraction-induced changes in skeletal muscle Na+,K+ pump mRNA expression — importance of exercise intensity and Ca2+-mediated signalling. Acta Physiol. (Oxf.). 198:487–498. 10.1111/j.1748-1716.2009.02057.x [DOI] [PubMed] [Google Scholar]
- Ogneva I.V., Biryukov N.S., Leinsoo T.A., and Larina I.M.. 2014. Possible role of non-muscle alpha-actinins in muscle cell mechanosensitivity. PLoS One. 9:e96395 10.1371/journal.pone.0096395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira M., Hanada H., Kawano F., Ishihara A., Nonaka I., and Ohira Y.. 2002. Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn. J. Physiol. 52:235–245. 10.2170/jjphysiol.52.235 [DOI] [PubMed] [Google Scholar]
- Orlowski J., and Lingrel J.B.. 1988. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic α isoform and β subunit mRNAs. J. Biol. Chem. 263:10436–10442. [PubMed] [Google Scholar]
- Pavlovic D., Fuller W., and Shattock M.J.. 2013. Novel regulation of cardiac Na pump via phospholemman. J. Mol. Cell. Cardiol. 61:83–93. (published erratum appears in J. Mol. Cell. Cardiol. 2014. 69:75) 10.1016/j.yjmcc.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Perry B.D., Levinger P., Morris H.G., Petersen A.C., Garnham A.P., Levinger I., and McKenna M.J.. 2015. The effects of knee injury on skeletal muscle function, Na+, K+-ATPase content, and isoform abundance. Physiol. Rep. 3:e12294 10.14814/phy2.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Murphy K.T., Snow R.J., Leppik J.A., Aughey R.J., Garnham A.P., Cameron-Smith D., and McKenna M.J.. 2005. Depressed Na+-K+-ATPase activity in skeletal muscle at fatigue is correlated with increased Na+-K+-ATPase mRNA expression following intense exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289:R266–R274. 10.1152/ajpregu.00378.2004 [DOI] [PubMed] [Google Scholar]
- Petrov A.M., Naumenko N.V., Uzinskaya K.V., Giniatullin A.R., Urazaev A.K., and Zefirov A.L.. 2011. Increased non-quantal release of acetylcholine after inhibition of endocytosis by methyl-β-cyclodextrin: the role of vesicular acetylcholine transporter. Neuroscience. 186:1–12. 10.1016/j.neuroscience.2011.04.051 [DOI] [PubMed] [Google Scholar]
- Pierno S., Desaphy J.-F., Liantonio A., De Bellis M., Bianco G., De Luca A., Frigeri A., Nicchia G.P., Svelto M., Léoty C., et al. . 2002. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain. 125:1510–1521. 10.1093/brain/awf162 [DOI] [PubMed] [Google Scholar]
- Pierno S., Desaphy J.-F., Liantonio A., De Luca A., Zarrilli A., Mastrofrancesco L., Procino G., Valenti G., and Conte Camerino D.. 2007. Disuse of rat muscle in vivo reduces protein kinase C activity controlling the sarcolemma chloride conductance. J. Physiol. 584:983–995. 10.1113/jphysiol.2007.141358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno S., Camerino G.M., Cannone M., Liantonio A., De Bellis M., Digennaro C., Gramegna G., De Luca A., Germinario E., Danieli-Betto D., et al. . 2013. Paracrine effects of IGF-1 overexpression on the functional decline due to skeletal muscle disuse: Molecular and functional evaluation in hindlimb unloaded MLC/mIgf-1 transgenic mice. PLoS One. 8:e65167 10.1371/journal.pone.0065167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzyukevich T.L., Moseley A.E., Shelly D.A., Redden G.A., Behbehani M.M., Lingrel J.B., Paul R.J., and Heiny J.A.. 2004. The Na+-K+-ATPase α2-subunit isoform modulates contractility in the perinatal mouse diaphragm. Am. J. Physiol. Cell Physiol. 287:C1300–C1310. 10.1152/ajpcell.00231.2004 [DOI] [PubMed] [Google Scholar]
- Radzyukevich T.L., Lingrel J.B., and Heiny J.A.. 2009. The cardiac glycoside binding site on the Na,K-ATPase α2 isoform plays a role in the dynamic regulation of active transport in skeletal muscle. Proc. Natl. Acad. Sci. USA. 106:2565–2570. 10.1073/pnas.0804150106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzyukevich T.L., Neumann J.C., Rindler T.N., Oshiro N., Goldhamer D.J., Lingrel J.B., and Heiny J.A.. 2013. Tissue-specific role of the Na,K-ATPase α2 isozyme in skeletal muscle. J. Biol. Chem. 288:1226–1237. 10.1074/jbc.M112.424663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M.K., Kristensen M., and Juel C.. 2008. Exercise-induced regulation of phospholemman (FXYD1) in rat skeletal muscle: implications for Na+/K+-ATPase activity. Acta Physiol. (Oxf.). 194:67–79. 10.1111/j.1748-1716.2008.01857.x [DOI] [PubMed] [Google Scholar]
- Reis J., Zhang L., Cala S., Jew K.N., Mace L.C., Chung L., Moore R.L., and Ng Y.-C.. 2005. Expression of phospholemman and its association with Na+-K+-ATPase in skeletal muscle: effects of aging and exercise training. J. Appl. Physiol. 99:1508–1515. 10.1152/japplphysiol.00375.2005 [DOI] [PubMed] [Google Scholar]
- Sejersted O.M., and Sjøgaard G.. 2000. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 80:1411–1481. [DOI] [PubMed] [Google Scholar]
- Shenkman B.S., and Nemirovskaya T.L.. 2008. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J. Muscle Res. Cell Motil. 29:221–230. 10.1007/s10974-008-9164-7 [DOI] [PubMed] [Google Scholar]
- Song H., Lee M.Y., Kinsey S.P., Weber D.J., and Blaustein M.P.. 2006. An N-terminal sequence targets and tethers Na+ pump α2 subunits to specialized plasma membrane microdomains. J. Biol. Chem. 281:12929–12940. 10.1074/jbc.M507450200 [DOI] [PubMed] [Google Scholar]
- Thomason D.B., and Booth F.W.. 1990. Atrophy of the soleus muscle by hindlimb unweighting. J. Appl. Physiol. 68:1–12. [DOI] [PubMed] [Google Scholar]
- Tricarico D., Mele A., Camerino G.M., Bottinelli R., Brocca L., Frigeri A., Svelto M., George A.L. Jr., and Camerino D.C.. 2010. The KATP channel is a molecular sensor of atrophy in skeletal muscle. J. Physiol. 588:773–784. 10.1113/jphysiol.2009.185835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.J., Tsai Y.C., and Shen C.K.. 2007. G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J. Exp. Med. 204:1273–1280. 10.1084/jem.20062481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyapkina O., Volkov E., Nurullin L., Shenkman B., Kozlovskaya I., Nikolsky E., and Vyskocil F.. 2009. Resting membrane potential and Na+,K+-ATPase of rat fast and slow muscles during modeling of hypogravity. Physiol. Res. 58:599–603. [DOI] [PubMed] [Google Scholar]
- Walaas S.I., Czernik A.J., Olstad O.K., Sletten K., and Walaas O.. 1994. Protein kinase C and cyclic AMP-dependent protein kinase phosphorylate phospholemman, an insulin and adrenaline-regulated membrane phosphoprotein, at specific sites in the carboxy terminal domain. Biochem. J. 304:635–640. 10.1042/bj3040635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.W., Resneck W.G., Kaysser T., Ursitti J.A., Birkenmeier C.S., Barker J.E., and Bloch R.J.. 2001. Na,K-ATPase in skeletal muscle: two populations of β-spectrin control localization in the sarcolemma but not partitioning between the sarcolemma and the transverse tubules. J. Cell Sci. 114:751–762. [DOI] [PubMed] [Google Scholar]
- Wood S.J., and Slater C.R.. 2001. Safety factor at the neuromuscular junction. Prog. Neurobiol. 64:393–429. 10.1016/S0301-0082(00)00055-1 [DOI] [PubMed] [Google Scholar]
- Yuan X., Lin Z., Luo S., Ji G., Yuan C., and Wu Y.. 2007. Effects of different magnitudes of cyclic stretch on Na+-K+-ATPase in skeletal muscle cells in vitro. J. Cell. Physiol. 212:509–518. 10.1002/jcp.21047 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Sierra A., Burnett C.M., Chen B., Subbotina E., Koganti S.R., Gao Z., Wu Y., Anderson M.E., Song L.S., et al. . 2014. Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. J. Gen. Physiol. 143:119–134. 10.1085/jgp.201311063 [DOI] [PMC free article] [PubMed] [Google Scholar]