Abstract
Tyrosine hydroxylase (TH) is a rate-limiting enzyme broadly expressed in noradrenergic and dopaminergic neurons in the central nervous system [57,70]. TH is also expressed by peripheral sympathetic neurons [98] as well as by enteric neurons within the gut [81,84]. Over 30 years ago, TH was unexpectedly discovered in developing and adult rodent cranial and dorsal root ganglion (DRG) neurons. Today, TH-expressing DRG neurons are being re-discovered as a relevant subpopulation. This review addresses the emerging importance of TH-expressing DRG neurons in sensation and pain mechanisms, focusing specifically on: 1) their nature as C-low threshold mechanoreceptors (C-LTMRs); 2) their involvement in nociception/pain; and 3) their catecholaminergic phenotype.
TH-expressing DRG neurons – a unique subpopulation
The presence of TH in peripheral sensory neurons was initially described in the field of neuronal development in the 1980s and 90s in rat [33,35,36,40,41,82,83] and avian [55,102] embryonic cranial and spinal ganglia. This was later confirmed in mouse [3,20,32] and also by means of TH gene promoter-driven expression of the Escherichia coli reporter gene lacZ [42,92], supporting the concept of dynamic developmental changes in neurotransmitter phenotype. However, it was soon discovered that many sensory neurons maintain TH protein and transcript synthesis during adulthood (Figure 1A). Moreover, today we know that they comprise one of seven key neuronal lineages in mouse DRGs [54,95]. Interestingly, whereas neuronal precursors are identified for most of these lineages, ancestors of TH-expressing DRG neurons remain unknown [54].
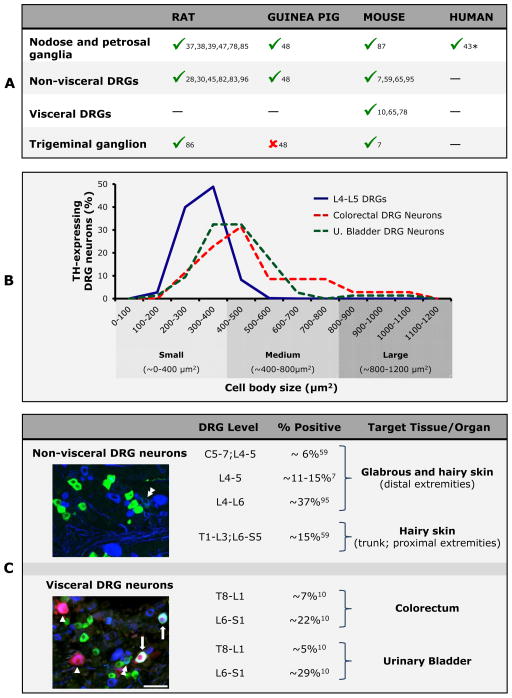
Figure 1. Morphological features of TH-expressing DRG neurons (quantitative and qualitative data in B, C is based on mouse).
(A) Adult TH-expressing DRG neurons have been detected in a number of species, including rat, guinea pig and mouse (—, not determined; * in humans, only embryonic expression has so far been studied [43]). (B) TH-expressing mouse DRG neurons are primarily small and medium-sized. Interestingly, differences can be observed when comparing visceral and non-visceral neurons, the former being represented by both small and medium-sized neurons, while the latter most often are small in size [data summarized from [7,10] and pooled to show differences between subpopulations. Percentage refers to the distribution among neuronal soma sizes of the total number of neurons measured for each target tissue/organ (921 non-visceral; 35 colonic and 74 urinary bladder)]. (C) On average, 15% of mouse DRG neurons express TH, although variations between DRGs innervating visceral versus non-visceral tissues or glabrous versus hairy skin are observed. Micrographs show examples of TH- and CGRP-expressing DRG neurons innervating non-visceral or visceral tissues, and the patterns of coexpression between the two markers. (Upper micrograph; L4-5 DRG neurons) TH-expressing DRG neurons (in green) innervating non-visceral tissues very seldom coexpress CGRP (in blue), and only rarely the two markers can be detected in the same neuron (double arrowhead). (Lower micrograph; S1 DRG neurons; in red, neurons innervating the urinary bladder) TH-expressing DRG neurons innervating the urinary bladder often coexpress CGRP (arrows). Additional bladder-projecting DRG neurons expressing CGRP but lacking TH are observed (arrowheads). Finally, several TH- (in green) or CGRP-only (in red) neurons are detected in the S1 DRG, and again a rare TH/CGRP coexpressing neurons (double arrowhead) Scale bar: 50 μm, applies for both micrographs.
Various immunohistochemical [7,10,41,45,48,59,82,83,96], in situ hybridization [7] and more recent large-scale single-cell RNA sequencing [95] studies documented that most adult rodent TH-expressing DRG neurons are small (Figure 1B). Only in mice is TH also detected in medium-sized adult visceral DRG neurons [10], or transiently in large DRG neurons during prenatal life [64]. The number of TH-expressing DRG neurons varies between studies, depending on species and DRG level (Figure 1C). On average, ~15% of all adult DRG neurons express TH, although TH mRNA is reportedly expressed in ~37% of L4–L6 mouse DRG neurons [95]. Whether TH transcripts are translated into protein in all neurons remains to be established.
The classical characterization of small and medium-sized DRG neurons as “peptidergic” (calcitonin gene-related peptide (CGRP)-expressing) or “non-peptidergic” (binding isolectin B4, IB4) [13,56,66] applies only partially to TH-expressing neurons (Figure 2A) [54,95], and appears to depend on the targeted tissue/organ. In fact, non-visceral TH-expressing DRG neurons are non-peptidergic and do not bind IB4 [7,10,32,54,59,82,95]. Even galanin, upregulated in small DRG neurons after peripheral nerve injury [15,90,97], remains absent in TH-expressing neurons under such conditions in mouse [7]. In contrast, a large percentage of TH-expressing DRG neurons targeting the colorectum or the urinary bladder of mouse are peptidergic [10].
Figure 2. Neurochemical features of TH-expressing DRG neurons.
(A) Neurochemical expression in DRG somata of unidentified L4-5 DRG neurons (not identified to tissue of innervation), and those innervating hairy skin or viscera (
 , present;
, present;
 , absent; —, not determined). TH-expressing DRG neurons exhibit distinctive neurochemical features, some of which appear to be dependent on target (e.g., compare CGRP expression between neurons innervating hairy skin and viscera). Although VGLUT2 expression remains to be confirmed in somata of TH-expressing DRG neurons projecting to hairy skin and viscera (δ), the presence of the transporter in nerve terminals in close apposition to hair follicles [8], and the fact that virtually all visceral DRG neurons express VGLUT2 [11,12], suggest that TH-expressing DRG neurons synthesize both VGLUT2 and VGLUT3. (B) The presence of enzymes associated with catecholamine synthesis and metabolism, including TH and associated molecules (NET-1), supports the existence of dopaminergic (1) or noradrenergic (2) phenotypes for TH-expressing DRG neurons (shading denotes enzymes and catecholamines present or secreted from DRG somata). Abbreviations: Cav3.2, T-type calcium channel Cav.3.2; Gfrα2, GDNF family receptor alpha-2; MRGPRs, mas related G-protein coupled receptors; NaV1.8/9, voltage gated sodium channel 1.8/9; NFH, neurofilament high; NtsR1, neurotensin receptor type 1; P2X3, P2X purinoceptor 3; Piezo2, Piezo-type mechanosensitive ion channel component 2; RET, rearranged in transformation proto-oncogene; SP, substance P; TAFA4, chemokine-like protein TAFA4; TrkA, Tropomyosin receptor kinase A; TRPA1, Transient receptor potential cation channel, member A1; VGLUT, vesicular glutamate transporter type; Y2R, neuropeptide tyrosine receptor, type 2.
, absent; —, not determined). TH-expressing DRG neurons exhibit distinctive neurochemical features, some of which appear to be dependent on target (e.g., compare CGRP expression between neurons innervating hairy skin and viscera). Although VGLUT2 expression remains to be confirmed in somata of TH-expressing DRG neurons projecting to hairy skin and viscera (δ), the presence of the transporter in nerve terminals in close apposition to hair follicles [8], and the fact that virtually all visceral DRG neurons express VGLUT2 [11,12], suggest that TH-expressing DRG neurons synthesize both VGLUT2 and VGLUT3. (B) The presence of enzymes associated with catecholamine synthesis and metabolism, including TH and associated molecules (NET-1), supports the existence of dopaminergic (1) or noradrenergic (2) phenotypes for TH-expressing DRG neurons (shading denotes enzymes and catecholamines present or secreted from DRG somata). Abbreviations: Cav3.2, T-type calcium channel Cav.3.2; Gfrα2, GDNF family receptor alpha-2; MRGPRs, mas related G-protein coupled receptors; NaV1.8/9, voltage gated sodium channel 1.8/9; NFH, neurofilament high; NtsR1, neurotensin receptor type 1; P2X3, P2X purinoceptor 3; Piezo2, Piezo-type mechanosensitive ion channel component 2; RET, rearranged in transformation proto-oncogene; SP, substance P; TAFA4, chemokine-like protein TAFA4; TrkA, Tropomyosin receptor kinase A; TRPA1, Transient receptor potential cation channel, member A1; VGLUT, vesicular glutamate transporter type; Y2R, neuropeptide tyrosine receptor, type 2.
Expression of a number of other neurochemical markers in TH-expressing DRG neurons has been analyzed (Figure 2A). The expression of transient receptor potential channel, subfamily V, member 1 (TRPV1) remains, however, controversial, since absence [54,56,95], minor [22] or abundant presence [27,53] in TH-expressing DRG neurons in mouse [27] has been reported.
Altogether, mammalian sensory ganglia contain a select, lineage-specific, subpopulation of permanently or transiently expressing TH neurons with unique neurochemical coding, possibly influenced by the target tissue/organ (see [54,56,59]).
Effects of nerve injury
TH expression in primary afferent neurons is affected by peripheral nerve injury. This was first observed in nodose and petrosal ganglia after axotomy of the vagus nerve or its branches as a reduction in the percentage of TH-expressing neurons [29,41,104], accompanied by a decrease in TH catalytic activity [39]. In mouse DRGs, axotomy of the sciatic [7] or pelvic nerves [65], or spinal nerve ligation [101] results in downregulated expression of TH mRNA [7] and/or protein [7,65], or of TH promoter-driven expression of enhanced green fluorescent protein [101]. In rat, sciatic nerve chronic constriction injury (CCI) leads to reduced TH mRNA content in L4-5 DRGs [30].
Changes in TH expression after injury could derive from alterations in peripheral trophic support, as suggested by TH downregulation in nodose ganglion neurons after colchicine-induced inhibition of vagal trophic factor axoplasmic transport [103]. In contrast, dorsal rhizotomy, a “central nerve injury” model, fails to alter TH expression in DRG neurons [39]. However, exactly which neurotrophic factors influence TH-expressing adult DRG neurons appears controversial. In fact, while their lack of TrkA and expression of RET and Gfrα2 (Figure 2A) [49,59,95] suggest a role for neurturin and glial cell line-derived neurotrophic factor (GDNF) [75], Gfrα2 or RET deletion in mice does not affect the development of non-peptidergic DRG neurons [49,62] or their expression of TH [49]. In contrast, TH-expressing neurons in developing mouse nodose and petrosal ganglia are reported to rely on peripherally available brain-derived neurotrophic factor, GDNF or both for their survival [18] while remaining nerve growth factor-independent [20].
In conclusion, the expression of TH is typically downregulated by peripheral nerve injury. If other insults such as tissue inflammation also result in changes in the expression of TH in sensory neurons, and what trophic factor/s may be involved, it remains to be established.
Roles in normal sensation
Li and colleagues [59] reported that all C-LTMRs innervating hairy skin as longitudinal lanceolate nerve endings expressed TH. Moreover, they noted that together with Aβ- and Aδ-LTMRs, C-LTMRs comprise functionally distinct mechanosensory end organs, with their central projections integrated within discrete dorsal horn LTMR columns [59]. C-LTMRs normally convey innocuous mechanical (hair deflection and light touch) and cooling sensations [56,59,105]. This has been confirmed in studies analyzing the role of TAFA4, a chemokine-like protein [16], or the T-type calcium channel Cav3.2 [22], both highly co-expressed with TH in C-LTMRs [16,22]. In addition, C-LTMRs appear to be involved in processing gentle and affective touch (e.g., nurturing), in a manner common to humans and rodents [61,72]. Thus, TH-expressing C-LTMRs are likely participants of “emotional touch” (see [105]).
Roles in nociception/pain
Recent studies using transgenic mice revealed a role of TH-expressing DRG neurons in non-visceral pain mechanisms. The vesicular glutamate transporter 2 (VGLUT2) is essential for the processing of acute pain and heat hyperalgesia [89]. When VGLUT2 is selectively deleted in TH-expressing mouse DRG neurons [53], responses to noxious mechanical stimulation are unaffected whereas responses to noxious heat (potentially suggesting coexpression with TRPV1) and inflammation are decreased. Deletion of TAFA4, selectively coexisting with TH in C-LTMRs, results in enhanced and prolonged mechanical hypersensitivity after either inflammation or CCI, suggesting that TAFA4 modulates the excitability of these neurons [16]. Finally, deletion of Cav3.2, a key regulator of sensory neuron excitability expressed both in Aδ-LTMRs and TH-expressing C-LTMRS, reduces spared nerve injury-induced mechanical and cold allodynia and attenuates tissue inflammation-induced hypersensitivity [22].
Less is known of the role of TH-expressing DRG neurons in visceral pain. However, pharmacological blockade of Cav3.2 in rats prevents the occurrence of colonic hypersensitivity induced by intracolonic sodium butyrate [21]. This effect appears to take place in peptidergic, IB4-negative, Cav3.2-expressing colorectal DRG neurons [63], although it remains to be established if they also express TH, as mentioned above for mice [22]. Also, patients with classic or non-ulcerative interstitial cystitis show increases in the number of TH-IR nerve fibers innervating the urinary bladder, likely due to outgrowth of sympathetic neuron projections [77]. However, TH-expressing DRG neurons may also participate and thus, along with sympathetic input, modulate urinary bladder sensitivity.
Potential catecholaminergic phenotype and pain modulation
There are currently three hypotheses regarding the catecholaminergic phenotype of TH-expressing DRG neurons. The first, for which evidence is strongest, is that TH-expressing DRG neurons synthesize and use dopamine or L-DOPA as neurotransmitters. Thus, a number of dopamine-associated enzymes and enzymatic products were identified in rat [5,51,52,99] and mouse [27] DRG (Figure 2B). Synthesis of dopamine was also reported in chick [80] and rat [5,99] DRGs, and additional support comes from studies in cranial sensory neurons, where the dopaminergic nature of TH-expressing neurons has been confirmed [19,31,34,67]. In humans, dopamine and associated metabolites were identified in peripheral nerves [50].
Dopamine release by DRG neurons would modulate pain at two specific targets: 1) Central DRG nerve terminals, and 2) Local spinal interneurons and projection neurons. Dopamine receptors 1–5 (D1–5Rs) have been reported in rat DRG neurons using RT-PCR [100], western blot and immunohistochemistry [23]. These receptors are functional, as established by the inhibitory effects of D1- and D2Rs [58,68], or D1- and D5Rs [23], on the electrical activity of predominantly small DRG neurons [23,58,68]. Such effects could modulate both the excitability of DRG nociceptors as well as neurotransmitter release from their central terminals. In support, activation of D2Rs inhibits presynaptic N-type calcium channel currents in rat chemosensory cranial visceral afferents, resulting in inhibition of neurotransmitter release [44]. Functional D2Rs have not only been documented at pre-, but also at postsynaptic sites in the substantia gelatinosa (lamina II) of rat [94]. Importantly, stimulation of the A11 cell group, possibly the major supraspinal source of dopamine (see [46]), results in antinociception, most likely by D2R-dependent inhibition of substantia gelatinosa neurons, both in rats exposed to noxious stimuli [94] or after peripheral nerve injury [2,71]. Activation of spinal D2Rs also attenuates carrageenan-induced hyperalgesia [24,25,60,73]. Finally, an antinociceptive role has been ascribed to dopamine in human patients suffering fibromyalgia [69] or restless leg syndrome [93].
Potential synthesis and release of L-DOPA by DRG neurons could also serve in antinociception. L-DOPA, after conversion to dopamine, reduces nociceptive behavior induced by intrathecal administration of substance P in rats, via a D2R-dependent mechanism [91]. In rats with CCI, intrathecally injected L-DOPA reduces mechanical and thermal hyperalgesia, again through D2R actions [14]. Interestingly, the D2R-dependent antinociceptive effect seems to be enhanced after CCI [14] or spinal nerve ligation [2,76], possibly due to injury-induced D2R upregulation in pre- and/or post-synaptic sites in the dorsal horn [2].
The second hypothesis, based on a single study in rats with bilateral lumbar sympathectomy and adrenalectomy, suggests that TRPV1-expressing DRG neurons release noradrenaline (but not dopamine) from their peripheral terminals upon knee joint capsaicin administration (Figure 2B). The authors also detected in these rats presence of norepinephrine transporter-1 (NET-1, for reuptake of extracellular norepinephrine and dopamine [74]), dopamine β-hydroxylase (DBH) and monoamino oxidase type A (MAO-A) in DRGs. They also found that neonatal capsaicin treatment alters the expression of DBH and MAO-A [17]. Therefore, a sensory neuron source of peripheral noradrenaline release should not be discounted when discussing the effects of adrenoceptor blockers on pain states [79].
The third hypothesis is that TH is not active in primary afferent neurons, based on the reported weak expression or absence of any other cathecolaminergic trait in guinea pig [48], mouse [7] and rat [82,83,96] non-visceral DRG neurons, and in mouse visceral DRG neurons [10]. However, mouse diencephalic A11 dopaminergic neurons have been shown to lack, for example, the dopamine transporter [46]. Therefore, absence of related molecules should not necessarily rule out a specific catecholaminergic phenotype.
Summary
In conclusion, more than 30 years after being discovered, TH-expressing DRG neurons are recognized as a key subpopulation during DRG development, with emerging roles in adulthood in several species. However, future studies will be necessary to (1) provide definitive proof of the synthesis of catecholamines by TH-expressing DRG neurons. 2) If synthesis of dopamine, L-DOPA or both is established, their potential somatic release and action on dopamine receptor-expressing DRG neurons should be explored (as observed for substantia nigra neurons, which engage in somatodendritic release [1,4,6,26,86]). 3) Determine whether TH-expressing (catecholaminergic?) DRG neurons are present in humans. 4) Analyze the impact of selective deletion or pharmacological manipulation of TH in DRG neurons on visceral and non-visceral pain. 5) Analyze the implications for functionality and/or trophic factor support of the observed differences in abundance of TH-expressing DRG neurons, relative to spinal segmental level and target organ/tissue.
Finally, while data on cell lineage or molecular signature of whole DRGs is relevant for understanding the role of sensory neurons, their morphological and functional diversity is importantly reliant on the tissue innervated. A more comprehensive characterization of DRG subpopulations, including target-identified TH-expressing ones, bears the potential for an improved understanding of the involvement of each DRG neuron subpopulation in discrete types of sensory and pain mechanisms.
Acknowledgments
I wish to thank Professors G.F. Gebhart (Director of the Center for Pain Research, University of Pittsburgh, Pittsburgh, USA), Kim B. Seroogy (Department of Neurology, University of Cincinnati, Cincinnati, USA), Tomas Hökfelt (Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden) and Dr. Carly J. McCarthy (Faculty of Biomedical Sciences, Austral University, Buenos Aires, Argentina), for valuable comments on this manuscript and continued support through these years of research on TH in sensory neurons. Figures and portions of the data summarized in this review are the result of work previously funded by the Swedish Research Council (04X-2887), the Wallenberg Foundation, the Knut and Alice Wallenberg Foundation, the Wallenberg Consortium North, a Carrillo Oñativia Grant and the National Institutes of Health (NIH; grants NS035790 and DK093525). The author is currently supported by CONICET, Austral University and Fondo Nacional para la Investigación Científica y Tecnológica (FONCyT).
Footnotes
Conflict of interest statement
Pablo R. Brumovsky declares no conflicts of interest.
References
- 1.Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- 2.Aira Z, Barrenetxea T, Buesa I, Gomez-Esteban JC, Azkue JJ. Synaptic upregulation and superadditive interaction of dopamine D2- and mu-opioid receptors after peripheral nerve injury. Pain. 2014;155:2526–33. doi: 10.1016/j.pain.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Baetge G, Schneider KA, Gershon MD. Development and persistence of catecholaminergic neurons in cultured explants of fetal murine vagus nerves and bowel. Development. 1990;110:689–701. doi: 10.1242/dev.110.3.689. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist F, Shahabi HN, Nissbrandt H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003;973:81–91. doi: 10.1016/s0006-8993(03)02555-1. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand A, Weil-Fugazza J. Sympathectomy does not modify the levels of dopa or dopamine in the rat dorsal root ganglion. Brain Res. 1995;681:201–4. doi: 10.1016/0006-8993(95)00267-t. [DOI] [PubMed] [Google Scholar]
- 6.Björklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- 7.Brumovsky P, Villar MJ, Hökfelt T. Tyrosine hydroxylase is expressed in a subpopulation of small dorsal root ganglion neurons in the adult mouse. Exp Neurol. 2006;200:153–165. doi: 10.1016/j.expneurol.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Brumovsky P, Watanabe M, Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 9.Brumovsky PR. VGLUTs in Peripheral Neurons and the Spinal Cord: Time for a Review. ISRN Neurol. 2013;2013:1–28. doi: 10.1155/2013/829753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brumovsky PR, La JH, McCarthy CJ, Hökfelt T, Gebhart GF. Dorsal root ganglion neurons innervating pelvic organs in the mouse express tyrosine hydroxylase. Neuroscience. 2012;223:77–91. doi: 10.1016/j.neuroscience.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brumovsky PR, Robinson DR, La JH, Seroogy KB, Lundgren KH, Albers KM, Kiyatkin ME, Seal RP, Edwards RH, Watanabe M, Hökfelt T, Gebhart GF. Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum. J Comp Neurol. 2011;519:3346–3366. doi: 10.1002/cne.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brumovsky PR, Seal RP, Lundgren KH, Seroogy KB, Watanabe M, Gebhart GF. Expression of Vesicular Glutamate Transporters in Sensory and Autonomic Neurons Innervating the Mouse Bladder. J Urol. 2012;189:2342–2349. doi: 10.1016/j.juro.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brumovsky PR, Villar MJ, Hökfelt T. Retrograde Cellular Changes in Primary Afferent and Sympathetic Neurons after Nerve Injury. In: Schmidt R, Gebhart GF, editors. Encyclopedia of Pain. Springer; Berlin: 2013. pp. 3407–3415. [Google Scholar]
- 14.Cobacho N, De la Calle JL, Gonzalez-Escalada JR, Paino CL. Levodopa analgesia in experimental neuropathic pain. Brain Res Bull. 2010;83:304–9. doi: 10.1016/j.brainresbull.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Corness J, Shi TJ, Xu ZQ, Brulet P, Hökfelt T. Influence of leukemia inhibitory factor on galanin/GMAP and neuropeptide Y expression in mouse primary sensory neurons after axotomy. Exp Brain Res. 1996;112:79–88. doi: 10.1007/BF00227180. [DOI] [PubMed] [Google Scholar]
- 16.Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P, Alonso S, Francois A, Barrere C, Seal R, Landry M, Eschallier A, Alloui A, Bourinet E, Delmas P, Le Feuvre Y, Moqrich A. TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep. 2013;5:378–88. doi: 10.1016/j.celrep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Dina OA, Khasar SG, essandri-Haber N, Bogen O, Chen X, Green PG, Reichling DB, Messing RO, Levine JD. Neurotoxic catecholamine metabolite in nociceptors contributes to painful peripheral neuropathy. Eur J Neurosci. 2008;28:1180–1190. doi: 10.1111/j.1460-9568.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–589. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finley JC, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neuroscience. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- 20.Forgie A, Kuehnel F, Wyatt S, Davies AM. In vivo survival requirement of a subset of nodose ganglion neurons for nerve growth factor. Eur J Neurosci. 2000;12:670–676. doi: 10.1046/j.1460-9568.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- 21.Francois A, Kerckhove N, Meleine M, Alloui A, Barrere C, Gelot A, Uebele VN, Renger JJ, Eschalier A, Ardid D, Bourinet E. State-dependent properties of a new T-type calcium channel blocker enhance Ca(V)3.2 selectivity and support analgesic effects. Pain. 2013;154:283–93. doi: 10.1016/j.pain.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Francois A, Schuetter N, Laffray S, Sanguesa J, Pizzoccaro A, Dubel S, Mantilleri A, Nargeot J, Noel J, Wood JN, Moqrich A, Pongs O, Bourinet E. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. 2015;10:370–382. doi: 10.1016/j.celrep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Galbavy W, Safaie E, Rebecchi MJ, Puopolo M. Inhibition of tetrodotoxin-resistant sodium current in dorsal root ganglia neurons mediated by D1/D5 dopamine receptors. Mol Pain. 2013;9:60. doi: 10.1186/1744-8069-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Zhang Y, Wu G. Effects of dopaminergic agents on carrageenan hyperalgesia in rats. Eur J Pharmacol. 2000;406:53–58. doi: 10.1016/s0014-2999(00)00649-x. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Zhang Y, Wu G. Effects of dopaminergic agents on carrageenan hyperalgesia after intrathecal administration to rats. Eur J Pharmacol. 2001;418:73–77. doi: 10.1016/s0014-2999(01)00930-x. [DOI] [PubMed] [Google Scholar]
- 26.Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- 27.Goswami SC, Mishra SK, Maric D, Kaszas K, Gonnella GL, Clokie SJ, Kominsky HD, Gross JR, Keller JM, Mannes AJ, Hoon MA, Iadarola MJ. Molecular Signatures of Mouse TRPV1-Lineage Neurons Revealed by RNA-Seq Transcriptome Analysis. J Pain. 2014;15:1338–59. doi: 10.1016/j.jpain.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardebo JE, Suzuki N, Ekblad E, Owman C. Vasoactive intestinal polypeptide and acetylcholine coexist with neuropeptide Y, dopamine-beta-hydroxylase, tyrosine hydroxylase, substance P or calcitonin gene-related peptide in neuronal subpopulations in cranial parasympathetic ganglia of rat. Cell Tissue Res. 1992;267:291–300. doi: 10.1007/BF00302967. [DOI] [PubMed] [Google Scholar]
- 29.Helke CJ, Rabchevsky A. Axotomy alters putative neurotransmitters in visceral sensory neurons of the nodose and petrosal ganglia. Brain Res. 1991;551:44–51. doi: 10.1016/0006-8993(91)90911-e. [DOI] [PubMed] [Google Scholar]
- 30.Herradon G, Ezquerra L, Nguyen T, Wang C, Siso A, Franklin B, Dilorenzo L, Rossenfeld J, Silos-Santiago I, Alguacil LF. Noradrenergic and opioidergic alterations in neuropathy in different rat strains. Neurosci Lett. 2008;438:186–189. doi: 10.1016/j.neulet.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 31.Hertzberg T, Brosenitsch T, Katz DM. Depolarizing stimuli induce high levels of dopamine synthesis in fetal rat sensory neurons. Neuroreport. 1995;7:233–237. [PubMed] [Google Scholar]
- 32.Ichikawa H, Mo Z, Xiang M, Sugimoto T. Brn-3a deficiency increases tyrosine hydroxylase-immunoreactive neurons in the dorsal root ganglion. Brain Res. 2005;1036:192–195. doi: 10.1016/j.brainres.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa H, Rabchevsky A, Helke CJ. Presence and coexistence of putative neurotransmitters in carotid sinus baro- and chemoreceptor afferent neurons. Brain Res. 1993;611:67–74. doi: 10.1016/0006-8993(93)91778-q. [DOI] [PubMed] [Google Scholar]
- 34.Iturriaga R, Cerpa V, Zapata P, Alcayaga J. Catecholamine release from isolated sensory neurons of cat petrosal ganglia in tissue culture. Brain Res. 2003;984:104–110. doi: 10.1016/s0006-8993(03)03118-4. [DOI] [PubMed] [Google Scholar]
- 35.Jonakait GM, Markey KA, Goldstein M, Black IB. Transient expression of selected catecholaminergic traits in cranial sensory and dorsal root ganglia of the embryonic rat. Dev Biol. 1984;101:51–60. doi: 10.1016/0012-1606(84)90116-7. [DOI] [PubMed] [Google Scholar]
- 36.Katz DM. A catecholaminergic sensory neuron phenotype in cranial derivatives of the neural crest: regulation by cell aggregation and nerve growth factor. J Neurosci. 1991;11:3991–4002. doi: 10.1523/JNEUROSCI.11-12-03991.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz DM, Adler JE, Black IB. Expression and regulation of tyrosine hydroxylase in adult sensory neurons in culture: effects of elevated potassium and nerve growth factor. Brain Res. 1986;385:68–73. doi: 10.1016/0006-8993(86)91548-9. [DOI] [PubMed] [Google Scholar]
- 38.Katz DM, Adler JE, Black IB. Catecholaminergic primary sensory neurons: autonomic targets and mechanisms of transmitter regulation. Fed Proc. 1987;46:24–29. [PubMed] [Google Scholar]
- 39.Katz DM, Black IB. Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J Neurosci. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz DM, Erb MJ. Developmental regulation of tyrosine hydroxylase expression in primary sensory neurons of the rat. Dev Biol. 1990;137:233–242. doi: 10.1016/0012-1606(90)90250-m. [DOI] [PubMed] [Google Scholar]
- 41.Katz DM, Markey KA, Goldstein M, Black IB. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. Proc Natl Acad Sci USA. 1983;80:3526–3530. doi: 10.1073/pnas.80.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SJ, Lee JW, Chun HS, Joh TH, Son JH. Monitoring catecholamine differentiation in the embryonic brain and peripheral neurons using E. coli lacZ as a reporter gene. Mol Cells. 1997;7:394–8. [PubMed] [Google Scholar]
- 43.Kiyokawa H, Katori Y, Cho KH, Murakami G, Kawase T, Cho BH. Reconsideration of the autonomic cranial ganglia: an immunohistochemical study of mid-term human fetuses. Anat Rec (Hoboken) 2012;295:141–9. doi: 10.1002/ar.21516. [DOI] [PubMed] [Google Scholar]
- 44.Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol. 2009;101:2270–8. doi: 10.1152/jn.91304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi S, Mwaka ES, Baba H, Kokubo Y, Yayama T, Kubota M, Nakajima H, Meir A. Microvascular system of the lumbar dorsal root ganglia in rats. Part II: neurogenic control of intraganglionic blood flow. J Neurosurg Spine. 2010;12:203–209. doi: 10.3171/2009.8.SPINE08895. [DOI] [PubMed] [Google Scholar]
- 46.Koblinger K, Fuzesi T, Ejdrygiewicz J, Krajacic A, Bains JS, Whelan PJ. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS One. 2014;9:e109636. doi: 10.1371/journal.pone.0109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kummer W, Bachmann S, Neuhuber WL, Hanze J, Lang RE. Tyrosine-hydroxylase-containing vagal afferent neurons in the rat nodose ganglion are independent from neuropeptide-Y-containing populations and project to esophagus and stomach. Cell Tissue Res. 1993;271:135–144. doi: 10.1007/BF00297551. [DOI] [PubMed] [Google Scholar]
- 48.Kummer W, Gibbins IL, Stefan P, Kapoor V. Catecholamines and catecholamine-synthesizing enzymes in guinea-pig sensory ganglia. Cell Tissue Res. 1990;261:595–606. doi: 10.1007/BF00313540. [DOI] [PubMed] [Google Scholar]
- 49.Kupari J, Airaksinen MS. Different requirements for GFRalpha2-signaling in three populations of cutaneous sensory neurons. PLoS One. 2014;9:e104764. doi: 10.1371/journal.pone.0104764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lackovic Z, Kleinman J, Karoum F, Neff NH. Dopamine and its metabolites in human peripheral nerves: is there a widely distributed system of peripheral dopaminergic nerves? Life Sci. 1981;29:917–922. doi: 10.1016/0024-3205(81)90393-3. [DOI] [PubMed] [Google Scholar]
- 51.Lackovic Z, Neff NH. Evidence for the existence of peripheral dopaminergic neurons. Brain Res. 1980;193:289–292. doi: 10.1016/0006-8993(80)90969-5. [DOI] [PubMed] [Google Scholar]
- 52.Lackovic Z, Neff NH. Minireview. Evidence that dopamine is a neurotransmitter in peripheral tissues. Life Sci. 1983;32:1665–1674. doi: 10.1016/0024-3205(83)90827-5. [DOI] [PubMed] [Google Scholar]
- 53.Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallen-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35:373–381. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Le Douarin NM, Xue ZG, Smith J. In vivo and in vitro studies on the segregation of autonomic and sensory cell lineages. J Physiol (Paris) 1985;80:255–261. [PubMed] [Google Scholar]
- 56.Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat. 2014;8:21. doi: 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- 58.Li GH, Guan BC, Li ZW. Effects of dopamine, SKF-38393 and R(−)-NPA on ATP-activated currents in rat DRG neurons. Can J Physiol Pharmacol. 2005;83:267–277. doi: 10.1139/y05-010. [DOI] [PubMed] [Google Scholar]
- 59.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu QS, Qiao JT, Dafny N. D2 dopamine receptor involvement in spinal dopamine-produced antinociception. Life Sci. 1992;51:1485–1492. doi: 10.1016/0024-3205(92)90558-7. [DOI] [PubMed] [Google Scholar]
- 61.Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 62.Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–54. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 63.Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrere C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, Eschalier A, Bourinet E, Ardid D. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A. 2011;108:11268–73. doi: 10.1073/pnas.1100869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsushita N, Kobayashi K, Miyazaki J. Fate of transient catecholaminergic cell types revealed by site-specific recombination in transgenic mice. J Neurosci Res. 2004;78:7–15. doi: 10.1002/jnr.20229. [DOI] [PubMed] [Google Scholar]
- 65.McCarthy CJ, Tomasella E, Malet M, Seroogy KB, Hokfelt T, Villar MJ, Gebhart GF, Brumovsky PR. Axotomy of tributaries of the pelvic and pudendal nerves induces changes in the neurochemistry of mouse dorsal root ganglion neurons and the spinal cord. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMahon SB, Priestley JV. Nociceptor Plasticity. In: Hunt SP, Koltzenburg M, editors. The Neurobiology of Pain. Oxford Univ. Press; Oxford: 2005. pp. 35–64. [Google Scholar]
- 67.Misu Y, Goshima Y, Miyamae T. Is DOPA a neurotransmitter? Trends Pharmacol Sci. 2002;23:262–8. doi: 10.1016/s0165-6147(02)02013-8. [DOI] [PubMed] [Google Scholar]
- 68.Molokanova EA, Tamarova ZA. The effects of dopamine and serotonin on rat dorsal root ganglion neurons: an intracellular study. Neuroscience. 1995;65:859–67. doi: 10.1016/0306-4522(94)00488-q. [DOI] [PubMed] [Google Scholar]
- 69.Nagakura Y, Oe T, Aoki T, Matsuoka N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain. 2009;146:26–33. doi: 10.1016/j.pain.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 70.Nagatsu T, Levitt M, Udenfriend S. Tyronsine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 71.Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–56. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 72.Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- 73.Paalzow GH. L-dopa induces opposing effects on pain in intact rats: (−)-sulpiride, SCH 23390 or alpha-methyl-DL-p-tyrosine methylester hydrochloride reveals profound hyperalgesia in large antinociceptive doses. J Pharmacol Exp Ther. 1992;263:470–479. [PubMed] [Google Scholar]
- 74.Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- 75.Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–91. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Park HJ, Joo HS, Kim YH, Kwon OK, Lee J, Kim ES, Moon DE. Anti-allodynic effects of levodopa in neuropathic rats. Yonsei Med J. 2013;54:330–5. doi: 10.3349/ymj.2013.54.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peeker R, Aldenborg F, Dahlstrom A, Johansson SL, Li JY, Fall M. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J Urol. 2000;163:1112–1115. [PubMed] [Google Scholar]
- 78.Peeters PJ, Aerssens J, de Hoogt R, Stanisz A, Gohlmann HW, Hillsley K, Meulemans A, Grundy D, Stead RH, Coulie B. Molecular profiling of murine sensory neurons in the nodose and dorsal root ganglia labeled from the peritoneal cavity. Physiol Genomics. 2006;24:252–63. doi: 10.1152/physiolgenomics.00169.2005. [DOI] [PubMed] [Google Scholar]
- 79.Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 80.Philippe E, Zhou C, Audet G, Geffard M, Gaulin F. Expression of dopamine by chick primary sensory neurons and their related targets. Brain Res Bull. 1993;30:227–230. doi: 10.1016/0361-9230(93)90248-a. [DOI] [PubMed] [Google Scholar]
- 81.Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Price J, Mudge AW. A subpopulation of rat dorsal root ganglion neurones is catecholaminergic. Nature. 1983;301:241–243. doi: 10.1038/301241a0. [DOI] [PubMed] [Google Scholar]
- 84.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 85.Regalia J, Cai F, Helke C. Streptozotocin-induced diabetes and the neurochemistry of vagal afferent neurons. Brain Res. 2002;938:7–14. doi: 10.1016/s0006-8993(02)02456-3. [DOI] [PubMed] [Google Scholar]
- 86.Reynolds AJ, Kaasinen SK, Hendry IA. Retrograde axonal transport of dopamine beta hydroxylase antibodies by neurons in the trigeminal ganglion. Neurochem Res. 2005;30:703–12. doi: 10.1007/s11064-005-6864-x. [DOI] [PubMed] [Google Scholar]
- 87.Sang Q, Young HM. The origin and development of the vagal and spinal innervation of the external muscle of the mouse esophagus. Brain Res. 1998;809:253–68. doi: 10.1016/s0006-8993(98)00893-2. [DOI] [PubMed] [Google Scholar]
- 88.Sarre S, Yuan H, Jonkers N, Van HA, Ebinger G, Michotte Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J Neurochem. 2004;90:29–39. doi: 10.1111/j.1471-4159.2004.02471.x. [DOI] [PubMed] [Google Scholar]
- 89.Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci US A. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi TJ, Cui JG, Meyerson BA, Linderoth B, Hökfelt T. Regulation of galanin and neuropeptide Y in dorsal root ganglia and dorsal horn in rat mononeuropathic models: possible relation to tactile hypersensitivity. Neuroscience. 1999;93:741–757. doi: 10.1016/s0306-4522(99)00105-0. [DOI] [PubMed] [Google Scholar]
- 91.Shimizu T, Iwata S, Morioka H, Masuyama T, Fukuda T, Nomoto M. Antinociceptive mechanism of L-DOPA. Pain. 2004;110:246–9. doi: 10.1016/j.pain.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 92.Son JH, Min N, Joh TH. Early ontogeny of catecholaminergic cell lineage in brain and peripheral neurons monitored by tyrosine hydroxylase-lacZ transgene. Brain Res Mol Brain Res. 1996;36:300–8. doi: 10.1016/0169-328x(95)00255-q. [DOI] [PubMed] [Google Scholar]
- 93.Stiasny-Kolster K, Pfau DB, Oertel WH, Treede RD, Magerl W. Hyperalgesia and functional sensory loss in restless legs syndrome. Pain. 2013;154:1457–63. doi: 10.1016/j.pain.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 94.Taniguchi W, Nakatsuka T, Miyazaki N, Yamada H, Takeda D, Fujita T, Kumamoto E, Yoshida M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain. 2011;152:95–105. doi: 10.1016/j.pain.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 95.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 96.Vega JA, Amenta F, Hernandez LC, del Valle ME. Presence of catecholamine-related enzymes in a subpopulation of primary sensory neurons in dorsal root ganglia of the rat. Cell Mol Biol. 1991;37:519–530. [PubMed] [Google Scholar]
- 97.Villar MJ, Cortés R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Hökfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- 98.von Euler US. Adrenergic neurotransmitter functions. Science. 1971;173:202–206. doi: 10.1126/science.173.3993.202. [DOI] [PubMed] [Google Scholar]
- 99.Weil-Fugazza J, Onteniente B, Audet G, Philippe E. Dopamine as trace amine in the dorsal root ganglia. Neurochem Res. 1993;18:965–969. doi: 10.1007/BF00966754. [DOI] [PubMed] [Google Scholar]
- 100.Xie GX, Jones K, Peroutka SJ, Palmer PP. Detection of mRNAs and alternatively spliced transcripts of dopamine receptors in rat peripheral sensory and sympathetic ganglia. Brain Res. 1998;785:129–135. doi: 10.1016/s0006-8993(97)01394-2. [DOI] [PubMed] [Google Scholar]
- 101.Xie W, Strong JA, Mao J, Zhang JM. Highly localized interactions between sensory neurons and sprouting sympathetic fibers observed in a transgenic tyrosine hydroxylase reporter mouse. Mol Pain. 2011;7:53. doi: 10.1186/1744-8069-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xue ZG, Smith J, Le Douarin NM. Differentiation of catecholaminergic cells in cultures of embryonic avian sensory ganglia. Proc Natl Acad Sci USA. 1985;82:8800–8804. doi: 10.1073/pnas.82.24.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhuo H, Lewin AC, Phillips ET, Sinclair CM, Helke CJ. Inhibition of axoplasmic transport in the rat vagus nerve alters the numbers of neuropeptide and tyrosine hydroxylase messenger RNA-containing and immunoreactive visceral afferent neurons of the nodose ganglion. Neuroscience. 1995;66:175–87. doi: 10.1016/0306-4522(94)00561-i. [DOI] [PubMed] [Google Scholar]
- 104.Zhuo H, Sinclair C, Helke CJ. Plasticity of tyrosine hydroxylase and vasoactive intestinal peptide messenger RNAs in visceral afferent neurons of the nodose ganglion upon axotomy-induced deafferentation. Neuroscience. 1994;63:617–26. doi: 10.1016/0306-4522(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 105.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346:950–4. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]