Abstract
Dysregulated fear, or the persistence of high levels of fear in low-threat contexts, is an early risk factor for the development of anxiety symptoms. Previous work has suggested both propensities for over-control and under-control of fearfulness as risk factors for anxiety problems, each of which may be relevant to observations of dysregulated fear. Given difficulty disentangling over-control and under-control through traditional behavioral measures, we used delta–beta coupling to begin to understand the degree to which dysregulated fear may reflect propensities for over- or under-control. We found that toddlers who showed high levels of dysregulated fear evidenced greater delta–beta coupling at frontal and central electrode sites as preschoolers relative to children who were low in dysregulated fear. Importantly, these differences were not observed when comparisons were made based on fear levels in high threat contexts. Results suggest dysregulated fear may involve tendencies toward over-control at the neural level.
Keywords: Delta–beta coupling, Anxiety risk, Dysregulated fear
1. Introduction
Extreme fearfulness in childhood is associated with a sevenfold increase in risk for being diagnosed with an anxiety disorder (Clauss and Blackford, 2012). Despite this large effect, rates of stability in fearfulness (Kagan et al., 1988, Pfeifer et al., 2002) and its associations with subsequent disorder (Biederman et al., 2001, Hirshfeld et al., 1992) are highly variable, making it difficult to understand how and for whom early fear leads to disorder. Recent work suggests that risk may be greatest for children who are highly fearful in low-threat contexts (Buss, 2011, Buss et al., 2013). This work describes dysregulated patterns of fear (Cole et al., 1994) in which observed fear is unmatched to contextual incentives (Buss, 2011). It remains unclear, however, whether dysregulated fear is associated with the under-engagement or over-engagement of regulatory resources expected to mitigate fearful behaviors. In fact, both tendencies for under-control (Murray and Kochanska, 2002) and over-control (Eisenberg et al., 2001) have been suggested as factors of risk for anxiety problems. This has resulted in ambiguity about the processes that should be targeted for programs of intervention and treatment. In the current study, we explore a behavioral neuroscience approach to understand whether dysregulated fear is associated with trait-level propensities for the over- or under-engagement of regulatory processing at the neural level.
Dysregulated fear reflects a lack of modulation of fear across contexts (Buss, 2011). Greater dysregulated fear is associated with longer, more intense expressions of fear in both high-threat and low-threat contexts. High levels of fearfulness in low threat contexts, in particular, distinguish dysregulated fear from traditional fear-based risk. Importantly, dysregulated fear predicts anxiety risk even when traditionally-assessed fear is statistically controlled (Buss, 2011, Buss et al., 2013), suggesting that a focus on high levels of fear in low-threat contexts may be critical for identifying early risk for disorder.
Still, the processes that underlie dysregulated fear are unclear. Consistent with differing theoretical perspectives on emotion regulation, greater dysregulated fear may reflect two types of disruptions in emotion processing. From a functionalist perspective, dysregulated fear may reflect a lack of coordination of response systems (Campos et al., 1994, Levenson, 1999) such that fear functions to mount behavioral responses (e.g., autonomic arousal, withdrawal) to one's environment even if such a response is unnecessary. From this orientation, it is sensible that dysregulated fear is linked to a propensity for over-control or an underlying readiness to respond to threat even when threat is not apparent. This is consistent with previous behavioral and neuroscience reports linking propensities for over-control to greater fear-based risk for anxiety problems (Brooker et al., 2011, Brooker and Buss, 2014).
Alternatively, dysregulated fear may reflect a disruption in the down-regulation of negative emotion, or an inability to alter the time course and/or intensity of the fear response (Thompson, 1990). From this orientation, dysregulated fear might reflect under-control, or a lack of availability of regulatory resources and ultimately an inability to abate the fear response. This perspective is also consistent with past work showing that lower levels of self-control predict greater risk for behavior problems (Eisenberg et al., 2001). At first glance, these two perspectives may appear to be separated only by nuance. However, their distinction is critical at the intervention and treatment levels. If dysregulated fear is associated with propensities for under-control, then interventions focused on enhancing processes of self-control and regulation may be most effective for preventing disorder. If, however, dysregulated fear is associated with over-control, then these same interventions risk affirming, or even enhancing, the very tendencies that put children at risk. Thus, the aim of the current work was to examine the association of dysregulated fear with trait-level propensities for under- or over-control in children.
The majority of work characterizing dysregulated fear has been done via investigations of observed behavior. However, trait-level propensities for regulation, including under- versus over-control, are notoriously difficult to disentangle at the behavioral level (Cole et al., 2004, Gross and Thompson, 2007). This difficulty has led to an increased focus on physiological measures in studies of emotion and development. At least one study has shown positive associations between dysregulated fear and physiological measures in infants, including baseline autonomic activity and diurnal cortisol levels (Buss et al., 2004). Baseline measures provide unique information about the dispositional regulatory style of the individual, including a dispositional readiness to respond to challenges in one's environment (Coan et al., 2006, Davidson, 2002, Gunnar, 1992). In this way, baseline measures may be most appropriate for questions about trait-level tendencies for over- or under-control.
Despite this utility of baseline assessments, autonomic arousal and diurnal cortisol reflect fairly ubiquitous processes through which it would be difficult to separate individual tendencies for under- or over-control. A more optimal measure would allow for some degree of distinction between emotion-based behavioral responses that are often linked to subcortical neural activity, and more cognitive processes of regulation, often linked to cortical neural activity. From this approach, relative increases in subcortical activity without parallel increases in cortical activity would reflect under-control, as motivational and emotion processes become heightened in absence of downregulation. Similarly, simultaneous increases in cortical and subcortical activity would reflect increasing propensities for over-control, as both excitatory and regulatory mechanisms become active. Clearly, the invasiveness of recording neural activity from deep-brain structures associated with emotional arousal makes such procedures inappropriate for research with children. Similarly, the sensitivity of Functional Magnetic Resonance Imaging (fMRI) to movement artifact, coupled with its temporal imprecision, make it suboptimal for examinations of real-time emotion processing in very young children (Byars et al., 2002). However, parallel changes in cortical and subcortical activity have been linked to oscillations within specific frequency bands of the electroencephalogram (EEG) in both children and adults.
Power in the delta frequency band of the EEG is the predominant frequency in early life as neural activity develops into its adult-like form (Bell, 1998, Stern et al., 2001). Delta oscillations are visible in primitive animal brains (González et al., 1999) and, in humans, have been linked to generators in subcortical areas and linked to motivational, reward, and emotional processes (Knyazev, 2007, Uhlhaas and Singer, 2006). In contrast, power in the beta frequency band of the EEG is associated with alertness, with greater beta power visible during periods of cognitive processing (Ray and Cole, 1985). Although their neural bases are not entirely clear, fast-wave oscillations such as beta are believed to reflect intra-cortical connections that are important for attention and higher cognitive functions (Engel et al., 2001, Ray and Cole, 1985) which exert an inhibitory influence on subcortical systems (Robinson, 1999).
While the spatial resolution of EEG limits the degree to which real-time oscillations can be linked to specific neural structures, relations between slow (e.g., delta) and fast (e.g., beta) wave activity are believed to reflect functional interactions between cortical and subcortical circuitry (Knyazev and Slobodskaya, 2003, Knyazev, 2007). Indeed, physiological studies have suggested that the stimulation of brainstem and limbic areas of the brain result in increased slow-wave activity (Gray, 1982, Guyton, 1976) while fast-wave beta oscillations are associated with increased activity in cortico-cortical circuits (Knyazev and Slobodskaya, 2003). Greater positive associations between delta and beta power are believed reflect functional coherence between cortical (i.e., cerebral cortex) and subcortical (i.e., limbic) structures. Thus, it has been proposed that delta–beta coupling may reflect, in real time, efforts by cognitively-oriented, cortical systems to regulate reactivity in emotionally-oriented, subcortical systems (Knyazev, 2007, Knyazev et al., 2006), providing a proxy for emotion-regulation processes. Although these types of interpretations remain tentative, this theory is consistent with evidence that greater delta–beta coupling has been associated with greater anxiety in adults (Knyazev, 2011, Miskovic et al., 2010), greater trait-level inhibition (Putman, 2011, Van Peer et al., 2008), and heritable levels of risk for anxiety problems in children (Miskovic et al., 2011a). Furthermore, delta–beta coupling is reduced in concert with the remediation of symptoms following treatment for Social Anxiety Disorder (Miskovic et al., 2011b).
Cumulatively, this body of work suggests that delta–beta coupling provides an avenue by which questions about links between under-vs. over-control and dysregulated fear during childhood may be explored. Specifically, propensities for over-control, which would increasingly engage cortical regulatory networks as subcortical reactivity increases, should be visible as greater baseline delta–beta coupling. In contrast, propensities for under-control, which would engage cognitive resources for regulation to a lesser degree as subcortical activity increases, would result in smaller associations between cortical and subcortical function.
Thus, two competing hypotheses may be derived. If dysregulated fear is linked to trait-level propensities for over-control, then differences in baseline delta–beta coupling should be visible in children who tend to show high versus low levels of fear in low-threat contexts. Specifically, high levels of fear in a low-threat context should be associated with greater baseline coupling relative to low levels of fear in a high-threat context. In contrast, if dysregulated fear is linked to trait-level propensities for under-control, then differences in baseline coupling should be visible in children who show high versus low levels of fear in high-threat contexts. Specifically, high levels of fear in a high-threat context should be associated with greater delta–beta coupling relative to low levels of fear in a high-threat context. Moreover, if delta–beta coupling is simply reflective of general tendencies to respond with fear, then baseline delta–beta coupling should be associated with high levels of fear, relative to low levels of fear, in both high- and low-threat contexts. We tested each of these possibilities in the current study. Critically, because we view both dysregulated fear and delta–beta coupling as trait-level qualities, we tested their association using a longitudinal study design, eliminating state-level confounds that might artificially increase the degree to which the two measures appear to be related.
2. Methods
2.1. Participants
Participants for the current study were a subset of families participating in a larger study of emotional development in children. Sixty-six families who had visited the laboratory at child age 2 years (M = 2.04, SD = 0.04) were invited to participate in a follow-up psychophysiological assessment on which the current study is based. Inclusion criteria for participation in the follow-up assessment required that children be 4.5 years of age (M = 4.59; SD = 0.13), without any known developmental delays or neurological impairments, and free of psychostimulant medications. One invited family withdrew from the project, 7 families did not respond to invitations to participate, 3 families had moved away from the area, 13 families declined participation, and 1 family failed to show for their laboratory visit. Thus, 41 preschoolers (20 girls) were enrolled in the follow-up. Participants were largely non-Hispanic Caucasian (87.5%, 5.0% African-American, 5.0% Asian-American, 2.5% Hispanic). Nearly half (47.5%) of families reported an annual household income of more than $60,000 (range: <$15,000–$60,000+).
For the current analyses, four left-handed children were excluded. Four children did not provide usable baseline data. Data from one child were omitted due to excessive EEG artifact. Fearfulness could not be scored for 2 children because behavioral data were unavailable. Thus, the final sample included 30 children.
2.2. Procedures and measures
2.2.1. Participants
At age 2 years, children participated in a series of laboratory episodes designed to elicit a range of emotional responses in toddlers (Lab-TAB; Buss and Goldsmith, 2000). Given our hypotheses about dysregulated fear, we focused on two specific emotion episodes: one episode that was considered high threat and one episode that was considered low threat. As described in a previous report (Buss, 2011), putative threat levels were determined based on children's typical patterns of observed fear and engagement, with greater fear and less engagement being indicative of a high-threat episode and less fear and greater engagement being indicative of a low-threat episode.
At age 4.5, children returned to the laboratory for psychophysiological (EEG) data collection. This age was chosen for the EEG assessment given the well-documented difficulties of obtaining EEG data from toddlers (Gavin and Davies, 2008, Marshall and Fox, 2006). During the laboratory visit, children completed three tasks while EEG data were recorded: first resting baseline, an age-appropriate flanker task, second resting baseline. Delta and beta power were derived from baseline recordings. Data from the flanker task, which have been reported elsewhere (Brooker and Buss, 2014), were not associated with the hypothesis of the current study.
2.2.2. Fear in low-threat context
Fear in a low-threat context was assessed during a 3-min puppet show (Lab-TAB; Buss and Goldsmith, 2000). During the episode, the mother sat in a chair across the experimental room from a wooden puppet theater with her toddler on her lap. A trained female research assistant who was unseen by the child put on a puppet show in which a lion puppet and an elephant puppet played a series of three games, inviting the child to play in each activity: catch (1 min), fishing (1 min), and a final episode in which the puppets offering the toddler a sticker as a prize and before saying goodbye (1 min). The research assistant then revealed and introduced herself and asked if the child would like to play with the puppets. This time served as a debriefing period where the child could become acquainted with the puppeteer and the puppets.
2.2.3. Fear in high-threat context
Fearfulness in a high-threat context was assessed during a 2-min robot episode (Lab-TAB; Buss and Goldsmith, 2000). During the episode, the mother sat in a chair with her toddler in her lab across the experimental room from a wooden platform on which rested a remote-controlled robot. The robot, controlled by an experimenter in the next room, began moving around the platform making noises and lighting up for 1 min. The experimenter then entered the room and asked the child if s/he would like to touch the robot. The child was asked to touch the robot up to three times before the experimenter debriefed the toddler saying “It's just a funny toy robot.”
2.2.4. EEG recording
Children were fitted with a 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics, Inc.) for EEG collection. All of the electrodes used for analysis have demonstrated acceptable equivalence with 10/10 electrode positions (Luu and Ferree, 2005) per the standards of the American Board of Registration of Electroencphalographic and Evoked Potential Technologists (ABRET). Each child completed two baseline episodes in which s/he was instructed to alternate sitting for 1 min with eyes closed with 1 min for eyes open for a total of 5 min. Similar to procedures used in past research (Marshall and Fox, 2006), children were told to focus their attention on a slowly-moving shape on the computer screen to help minimize movement artifact during the baseline eyes-open period.
EEG data were recorded using NetStation (version 4.3.1) acquisition software (Electrical Geodesic, Inc.: Eugene, OR) and sampled at a rate of 500 Hz with a gain of 1000. Consistent with the manufacturer's instructions, impedances were reduced to less than 80 kΩ prior to data collection. EEG data were filtered during acquisition using a 0.10 Hz highpass filter and a 100 Hz lowpass filter. All channels were referenced to Cz during data collection. Data from each participant were submitted to an Independent Components Analysis (ICA) to extract eye blink and eye movement artifacts. ICAs were performed on the continuous EEG data in EEGLab Version 8.0.3b (Delorme and Makeig, 2004), which employs an automated version of the infomax ICA algorithm with enhancements to improve processing efficiency. The algorithm returns maximally independent sources of electrical activity in the neural recordings. Each component was plotted and components that were identified as having patterns consistent with eye blink or movement artifacts were deleted.
2.3. Data reduction
Videos of the puppet show and robot episodes were coded offline. Facial fear, bodily fear, freezing, and proximity to caregiver were micro-coded on a second-by-second basis for each episode (agreement: 86–91%). Facial fear was coded using the AFFEX system, which differentiates emotion expressions based on three regions of the face (Izard et al., 1983). Fear was coded when brows were straight and raised, eyes were open wide, and mouth was open with corners pulled back. Bodily expressions of fear were coded when activity was diminished, children remained still/rigid for more than two consecutive seconds, and/or muscles appeared tensed or trembling. Proximity to caregiver was scored when the child was within approximately 2 ft. of their caregiver. A Principal Components Analysis was conducted for each episode. In each analysis, a factor emerged which accounted for approximately 25% of the variance in the original variables and included the duration or timing of each fear behavior. Fear behaviors were standardized and composited into a single fearfulness variable for each episode (ICCs: 0.61–0.73) that indexed the proportion of time that children were engaged in fear behaviors. A mean split (M = 36.95, SD = 23.54) was used to create groups reflecting high (n = 14) and low (n = 16) fearfulness in Puppet Show, a low-threat context, and Robot (M = 67.76, SD = 23.82; high fear n = 11, low fear n = 19), a high-threat context. High fear group was unrelated to low fear group, supporting our assertion that dysregulated fear is unique from traditionally measured, overall high levels of fear (χ2(1) = 0.26, p > 0.05).
Offline EEG data processing was performed in Brain Vision Analyzer (BVA; Brain Products: Gilching, Germany). Data were re-referenced to the average of the right and left mastoids and high- and low-pass filtered at 0.10 Hz and 30 Hz, respectively. Segments of 1.024 s were extracted from the continuous EEG and baseline corrected using the entire data segment. Artifacts were identified when any of the following criteria were met: a voltage step of more than 75 μV/ms between data points, a voltage difference of 150 μV within a single segment, a voltage difference of less than 0.5 μV within a 50 ms interval, or an absolute voltage of ±100 μV.
Artifact-free data were submitted to a Fast-Fourier Transform using a Hamming window with 50% segment width overlap. Power (μV2) was derived in the delta (0.5–2.0 Hz) and beta (11.0–18.0 Hz) frequency bands for frontal (F3 and F4), central (C3 and C4), and parietal (P3 and P4) electrode sites. Cutoffs for delta and beta frequency bands and electrode sites were selected based on previous work with young children (Marshall and Fox, 2006, Miskovic et al., 2011a). Composite power values were transformed using the natural logarithm to correct for positive skew. Pre- and post-baseline data were combined to form a single baseline measure (mean r = 0.76). Finally, consistent with previous work, frontal (F3/4), central (C3/4), and parietal (P3/4) sites were combined, resulting in three composite measures of baseline delta–beta coupling.
Delta and beta power were largely uncorrelated with the total number of baseline segments (Mean |r|s = 0.10 and 0.06, respectively) and the total number of rejected segments (Mean |r|s = 0.29 and 0.20), respectively). The only exception to this was that the total number of rejected segments was moderately correlated with parietal delta power such that greater delta power at parietal electrodes was associated with a greater number of rejected segments. Delta and beta power were similarly unrelated to low threat fear groups (all ts < 1.20, ps > 0.10) and high threat fear groups (all ts < 1.21, ps > 0.10).
3. Results
Similar to previous research, Pearson correlation coefficients served as estimates of delta–beta coupling (Miskovic et al., 2010, Miskovic et al., 2011a, Miskovic et al., 2011b). We examined levels of coupling during baseline at frontal, central, and parietal electrodes and investigated associations between coupling and high- and low-threat contexts. Differences in coupling between high and low-fear groups in each episode were tested using Fisher's r-to-z transformation.
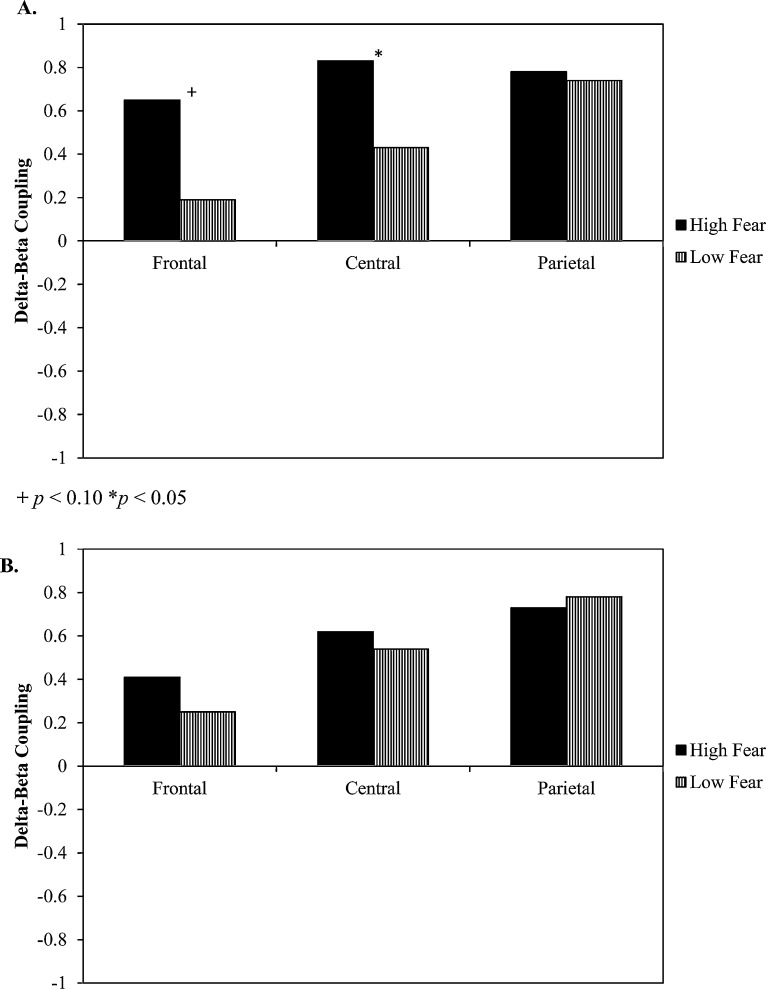
We first examined differences in coupling associated with high versus low levels of fear in a low-threat context. High levels of fear in a low-threat context (i.e., dysregulated fear) were associated with significant delta–beta coupling at frontal (r = 0.65, p < 0.05), central (r = 0.83, p < 0.01), and parietal (r = 0.78, p < 0.01) electrodes. In contrast, low levels of fear in a low-threat context were associated with significant coupling only at parietal sites (frontal: r = 0.19, p > 0.10, central: r = 0.43, p > 0.10, parietal: r = 0.74, p < 0.01). Differences in coupling between dysregulated fear and low fear groups fear were observed at frontal (z = 1.42, p < 0.10) and central (z = 1.81, p < 0.05) sites. No group differences were observed at parietal (z = 0.28, p > 0.10) electrodes (Fig. 1A).
Fig. 1.
Group differences in delta–beta coupling based on levels of fear in (A) low-threat and (B) high-threat contexts.
Next, we examined differences in coupling associated with high versus low levels of fear in a high-threat context. Note that high levels of fear under conditions of high-threat reflect a match between contextual incentives and fear responses. Thus, while some children may show high levels of fear in this context, this type of response reflects high fear, but not dysregulated fear. High levels of fear in a high-threat context were associated with significant coupling at central (r = 0.62, p < 0.01), and parietal (r = 0.73, p < 0.01), but not frontal (r = 0.41, p > 0.10) sites. Low levels of fear in a high-threat context were associated with significant coupling only at parietal sites (frontal: r = 0.25, p > 0.10, central: r = 0.54, p > 0.10, parietal: r = 0.78, p < 0.05). Importantly, coupling did not differ between low and high fear children at frontal (z = 0.43, p > 0.10), central (z = 0.28, p > 0.10), or parietal sites (z = −0.28, p > 0.10; Fig. 1B).
4. Discussion
Greater levels of baseline coupling were observed for children who showed dysregulated fear, or high levels of fear in a low-threat episode, relative to children who showed low levels of fear in a low-threat context. Differences in coupling were not apparent based on levels of fear in a high-threat context. Results are consistent with the notion that anxiety risk, indexed by dysregulated fear, is associated with trait-level propensities for over-control during early childhood. Because fear and coupling, both thought to reflect stable individual differences, were measured nearly 2 years apart, it is unlikely that links between these measures were due to state fluctuations of affect in young children. Rather, findings are consistent with behavioral (Eisenberg et al., 2001, Kochanska et al., 1996) and physiological studies (Brooker and Buss, 2014, Meyer et al., 2012) suggesting stable associations between tendencies for over-control and early risk for anxiety problems. The implications of these results are discussed below.
We tested two competing hypotheses about the propensities, at the neural level, that may underlie dysregulated fear. Our results provided initial evidence that trait level propensities for over-control, as indexed by delta–beta coupling, were most pronounced in association with fearfulness in low-threat environments. As previously noted, baseline coupling is believed to reflect increased cortical-subcortical crosstalk (Knyazev and Slobodskaya, 2003, Knyazev, 2007) and may indicate active control over emotion systems in a by more regulatory processes (Knyazev, 2007, Knyazev et al., 2006, Robinson, 1999). Although moderate levels of baseline coupling likely index adaptive regulatory efforts, enhanced baseline coupling has been associated with increased risk for anxiety problems in both children and adults (Miskovic et al., 2010, Miskovic et al., 2011a) as well as physiological indices of a heightened readiness to respond to environmental challenges (Schutter and van Honk, 2005, Van Peer et al., 2008). Similarly, both trait and state levels of anxious apprehension have been linked to enhanced delta–beta coupling during baseline (Knyazev, 2011, Knyazev et al., 2006). Cumulatively, past work and the current results provide support for the notion that baseline coupling may reflect the propensity for hypervigilance and chronic anticipation of threat that has long-standing links to fear-based anxiety risk (Bar-Haim et al., 2007, Reeb-Sutherland et al., 2014).
The pattern of results was not replicated when coupling was examined relative to levels of fear in a high-threat context. This is consistent with previous work suggesting that contextual incentives offer critical information for understanding fear responses (Buss, 2011, Buss et al., 2013, Buss et al., 2004). In the current study, similar group differences in coupling based on both high-threat and low-threat fearfulness would have suggested associations between coupling and tendencies to react with high levels of fear overall. Instead, our work supports the idea of a specific association between coupling and levels of dysregulated fear (i.e., high fear in a low-threat context). Our use of baseline measures allows us to conclude that early observations of fear, despite low levels of contextual threat, are associated with trait levels of cortical–subcortical crosstalk, perhaps reflecting propensities to engage neural systems of regulation, two years later. It will be important to understand, in future research, whether similar associations exist for non-baseline (i.e., task) measures, which may provide unique information about state-levels responses to one's environment (Coan et al., 2006). It will be similarly important to understand whether developmental changes in dysregulated fear are reflected in fluctuating levels of baseline coupling. Previous research suggests that, although coupling likely reflects a trait-level propensity, reductions in anxiety symptoms following treatment are associated with reduced levels of baseline coupling (Miskovic et al., 2011b). It is not yet clear whether similar changes may be visible in association with risk factors following intervention with young children. Testing this possibility will be an important area for future research.
It should be noted that our analyses were conducted in a between-subjects fashion. Previous work with adults has suggested that within- and between-subjects estimates of coupling reflect similar constructs (Schutter and Knyazev, 2012). However, this work also presents the possibility that coupling mechanisms develop over time, impacting observed associations between coupling and behavior. As age is an imperfect proxy for developmental stage, this suggests that there may be broad individual differences in coupling, and the within-subject link between coupling and dysregulated fear in young children. This should be kept in mind when interpreting the current results.
It is also noteworthy that our findings diverge from the adult literature in ways that might be expected given the developmental stage of the participants. Namely, coupling in both high and low fear groups was not specific to frontal recording sites, as is often seen in work with adults. Rather, significant levels of coupling were seen across frontal, central, and parietal recording sites. A lack of specialization of neural processes involved in emotion processing and self-regulation to frontal brain regions is frequently observed in neuroscience work with young children (e.g., Brooker and Buss, 2014, Solomon et al., 2014). Such findings are consistent with descriptions of neurodevelopment, which describe early-developing posterior regions involved in cognitive and regulatory processing being progressively overtaken by later-maturing, more anterior structures (Bachevalier and Mishkin, 1984, Goldman et al., 1971). This shift in primary processing centers is likely associated with patterns of change in neural activation during cognitive and emotional tasks from posterior to anterior areas.
We similarly report a lack of specialization of regulatory processing, as indexed by delta–beta coupling, to frontal regions. As preschoolers, both high and low fear children evidenced some level of posterior coupling in addition to coupling in more traditional, frontal areas. Broadly distributed coupling was most evident in association with fear the high-threat context, which may reflect the relevance of broad systems of regulation and coping for adaptive fear responses. Interestingly, group differences in coupling were only apparent at frontal electrode sites, potentially highlighting the importance of this region for trait-level propensities for regulation even at early ages. It will be important for future research to track the development of delta–beta coupling in association with children's growing regulatory capacities and long-term risk for disorder.
A final notable aspect of the current work is the strength of the longitudinal design. Given our emphasis on trait-level associations between dysregulated fear and delta–beta coupling, our efforts to eliminate state-level confounds is critical to the interpretation of the current results. While it will be important for future work to assess the relevance of possible state-related changes in coupling to early anxiety risk, our results suggest a long-term link between dysregulated fear and neural systems of regulation as indexed by delta–beta coupling. That is, we show a link between greater dysregulated fear and putative trait-level propensities for over-control that are stable across a two-year period of early childhood. Research is in progress that will establish the stability of dysregulated fear, further identifying its utility as an early marker of anxiety risk. Stable associations between dysregulated fear and neural systems of regulation provide possible targets for identifying those individuals most at risk for disorder. Similar to other domains and investigations of coupling with adults, these differences may also be used to assess the effectiveness of intervention and treatment programs for at-risk children (Lewis et al., 2008, Miskovic et al., 2011b).
Although our results extend a growing literature on dysregulated fear, the current study is not without limitations. Given our small sample size, a replication of these results is needed. A second study is currently underway in a larger sample that will allow for a direct replication of tests for group differences in delta–beta coupling based on fearfulness in a low-threat context. In addition, it is important to note that the current study did not include assessments of clinical outcomes, including diagnoses, in children. Given the age of the current sample, it is likely that the presence of clinical disorders will become more evident over time. Thus, additional longitudinal investigations that can directly test delta–beta coupling as one mechanism of association between dysregulated fear and anxiety diagnoses will be highly valuable for this line of research.
In addition, experimental evidence that that delta–beta coupling is associated with cortical-subcortical crosstalk is derived primarily from an animal literature (Guyton, 1976). To date, we remain limited in our understanding of the precise processes that are reflected by delta–beta coupling in humans, and in young children in particular. Therefore, although the limited behavioral work to date is consistent with a theory suggesting that delta–beta coupling is reflective of links between cortical and subcortical networks, our conclusions remain somewhat exploratory in nature. Future research that identifies the source generators of delta and beta oscillations will be critical for understanding the directionality and overall nature of the processes that are isolated using measures of delta–beta coupling.
In conclusion, we have provided initial evidence that dysregulated fear is related to a neural system for over-control in young children. This work is consistent with previous research suggesting that hypervigilance for threat and anxious apprehension are key risk factors for the development of anxiety problems. Our findings suggest one possible mechanism by which dysregulated fear is associated with long-term anxiety risk and contribute to a growing literature on the early identification of biological and behavioral markers of risk in young children.
Author notes
Data collection for this project was supported by a RGSO dissertation support award from the Penn State College of the Liberal Arts to the second author and R01 MH075750 from the National Institute of Mental Health (PI: Buss). The preparation of this manuscript was partially supported by K01 MH100240 (PI: Brooker). The first author was supported by P20 GM103474 during the writing of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All work was conducted with the ethical approval of all relevant bodies.
We thank the children and families who participated in this study and staff of the Emotion Development Lab and the Social, Life, and Engineering Sciences Imaging Center who provided assistance and resources for recruitment, data collection, and data analysis.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.09.007.
Appendix A. Supplementary data
References
- Bachevalier J., Mishkin M. An early and a late developing system for learning and retention in infant monkeys. Behav. Neurosci. 1984;98(5):770–778. doi: 10.1037//0735-7044.98.5.770. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. http://doi.org/10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bell M.A. Neuroimaging in Chold Neuropsychiatric Disorders. Springer-Verlag; Berlin: 1998. The ontogeny of the EEG during infancy and childhood: implications for cognitive development; pp. 97–111. [Google Scholar]
- Biederman J., Hirshfeld-Becker D.R., Rosenbaum J.F., Herot C., Friedman D., Snidman N. Further evidence of association between behavioral inhibition and social anxiety in children. Am. J. Psychiatry. 2001;158(10):1673–1679. doi: 10.1176/appi.ajp.158.10.1673. http://doi.org/10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Brooker R.J., Buss K.A. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Dev. Neuropsychol. 2014;39(1):1–8. doi: 10.1080/87565641.2013.826661. http://doi.org/10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R.J., Neiderhiser J.M., Kiel E.J., Leve L.D., Shaw D.S., Reiss D. The association between infants’ attention control and social inhibition is moderated by genetic and environmental risk for anxiety. Infancy. 2011;16(5):490–507. doi: 10.1111/j.1532-7078.2011.00068.x. http://doi.org/10.1111/j.1532-7078.2011.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss K.A. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Dev. Psychol. 2011;47(3):804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss K.A., Davidson R.J., Kalin N.H., Goldsmith H.H. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Dev. Psychol. 2004;40(4):583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Buss K.A., Davis E.L., Kiel E.J., Brooker R.J., Beekman C., Early M.C. Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. J. Clin. Child Adolesc. Psychol. 2013;42(5):603–616. doi: 10.1080/15374416.2013.769170. http://doi.org/10.1080/15374416.2013.769170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss K.A., Goldsmith H.H. University of Wisconsin-Madison, Department of Psychology; Madison, WI: 2000. Manual and Normative Data for the Laboratory Temperament Assessment Battery—Toddler Version. [Google Scholar]
- Byars A.W., Holland S.K., Strawsburg R.H., Schmithorst V.J., Dunn R.S., Ball W.S. Practical aspects of conducting large-scale fMRI studies in children. J. Child Neurol. 2002;17(12):885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J.J., Mumme D.L., Kermoian R., Campos R.G. A functionalist perspective on the nature of emotion. Monogr. Soc. Res. Child Dev. 1994;59(2–3):284–303. http://doi.org/10.1111/j.1540-5834.1994.tb01289.x. [PubMed] [Google Scholar]
- Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(10) doi: 10.1016/j.jaac.2012.08.002. 1066-1075 e1, http://doi.org/10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B., McKnight P.E. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. http://doi.org/10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P.M., Martin S.E., Dennis T.A. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. http://doi.org/10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole P.M., Michel M.K., Teti L.O. The development of emotion regulation and dysregulation: a clinical perspective. Monogr. Soc. Res. Child Dev. 1994;59(2–3):73–102. http://doi.org/10.1111/j.1540-5834.1994.tb01278.x. [PubMed] [Google Scholar]
- Delorme A., Makeig S. EELAB: an open source toolbox for analysis of single-trial EEG dynamics including independent components analysis. J. Neurosci. Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Anxiety and affective style: role of prefrontal cortex and amygdala. Soc. Anxiety. 2002;51(1):68–80. doi: 10.1016/S0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Cumberland A., Spinrad T.L., Fabes R.A., Shepard S.A., Reiser M. The relations of regulation and emotionality to children's externalizing and internalizing problem behavior. Child Dev. 2001;72(4):1112–1134. doi: 10.1111/1467-8624.00337. http://doi.org/10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2(10):704–716. doi: 10.1038/35094565. http://doi.org/10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Gavin W.J., Davies P.L. Obtaining reliable psychophysiolgocal data with child participants. In: Schmidt L.A., Segalowitz S.J., editors. Developmental Psychophysiology: Theory, Systems, and Methods. Cambridge University Press; New York, NY: 2008. pp. 424–447. [Google Scholar]
- Goldman P.S., Rosvold H.E., Vest B., Galkin T.W. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J. Comp. Physiol. Psychol. 1971;77(2):212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- González J., Gamundi A., Rial R., Nicolau M.C., de Vera L., Pereda E. Nonlinear, fractal, and spectral analysis of the EEG of lizard, Gallotia galloti. Am. J. Physiol.—Regul., Integr. Comp. Physiol. 1999;277(1):R86–R93. doi: 10.1152/ajpregu.1999.277.1.R86. [DOI] [PubMed] [Google Scholar]
- Gray A. Oxford University Press; Oxford: 1982. The Neuropsychology of Anxiety: An Enquiry Into the Septo-Hippocampal System. [Google Scholar]
- Gross J.J., Thompson Ross A. Emotion regulation: conceptual foundations. In: Gross James J., editor. Handbook of Emotion Regulation. The Guilford Press; New York, NY: 2007. pp. 3–24. [Google Scholar]
- Gunnar M.R. Reactivity of the hypothalamic–pituitary–adrenocortical system to stressors in normal infants and children. Pediatrics. 1992;90(3):491–497. [PubMed] [Google Scholar]
- Guyton A.C. W.B. Saunders; London: 1976. Organ Physiology: Structure and Fucntion of the Nervous System. [Google Scholar]
- Hirshfeld D.R., Rosenbaum J.F., Biederman J., Bolduc E.A., Faraone S.V., Snidman N. Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Izard C.E., Dougherty L.M., Hembree E.A. Instructional Resources Center, University of Delaware; Newark, DE: 1983. A System for Identifying Affect Expressions by Holistic Judgments (AFFEX) [Google Scholar]
- Kagan J., Reznick J.S., Snidman N. Biological bases of childhood shyness. Science. 1988;240(4849):167–171. doi: 10.1126/science.3353713. http://doi.org/10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. http://doi.org/10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. Cross-frequency coupling of brain oscillations: an impact of state anxiety. Int. J. Psychophysiol. 2011;80(3):236–245. doi: 10.1016/j.ijpsycho.2011.03.013. http://doi.org/10.1016/j.ijpsycho.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G., Schutter D.J.L.G., van Honk J. Anxious apprehension increases coupling of delta and beta oscillations. Int. J. Psychophysiol. 2006;61(2):283–287. doi: 10.1016/j.ijpsycho.2005.12.003. http://doi.org/10.1016/j.ijpsycho.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G., Slobodskaya H.R. Personality trait of behavioral inhibition is associated with oscillatory systems reciprocal relationships. Int. J. Psychophysiol. 2003;48(3):247–261. doi: 10.1016/s0167-8760(03)00072-2. http://doi.org/10.1016/S0167-8760(03)00072-2. [DOI] [PubMed] [Google Scholar]
- Kochanska G., Murray K., Jacques T.Y., Koenig A.L., Vandegeest K.A. Inhibitory control in young children and its role in emerging internalization. Child Dev. 1996;67(2):490–507. http://doi.org/10.1111/j.1467-8624.1996.tb01747.x. [PubMed] [Google Scholar]
- Levenson R.W. The intrapersonal functions of emotion. Cogn. Emotion. 1999;13(5):481–504. http://doi.org/10.1080/026999399379159. [Google Scholar]
- Lewis M.D., Granic I., Lamm C., Zelazo P.D., Stieben J., Todd R.M. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Dev. Psychopathol. 2008;20(03):913–939. doi: 10.1017/S0954579408000448. http://doi.org/10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Luu P., Ferree T. Technical Report. Eugene, OR: Electrical Geodesics, Inc; 2005. Determination of the HydroCel Geodesic Sensor Net's average electrode positions and their 10-10 international equivalents. [Google Scholar]
- Marshall P.J., Fox N.A. Infant EEG and ERP in relation to social and emotional development. In: DeHaan M., editor. Infant EEG and Event-Related Potentials. Psychology Press; New York, NY: 2006. pp. 227–250. [Google Scholar]
- Meyer A., Weinberg A., Klein D.N., Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Dev. Cogn. Neurosci. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. http://doi.org/10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V., Ashbaugh A.R., Santesso D.L., McCabe R.E., Antony M.M., Schmidt L.A. Frontal brain oscillations and social anxiety: a cross-frequency spectral analysis during baseline and speech anticipation. Biol. Psychol. 2010;83(2):125–132. doi: 10.1016/j.biopsycho.2009.11.010. http://doi.org/10.1016/j.biopsycho.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Campbell M.J., Santesso D.L., Ameringen M.V., Mancini C.L., Schmidt L.A. Frontal brain oscillatory coupling in children of parents with social phobia: a pilot study. J. Neuropsychiatry Clin. Neurosci. 2011;23(1):111–114. doi: 10.1176/jnp.23.1.jnp111. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Moscovitch D.A., Santesso D.L., McCabe R.E., Antony M.M., Schmidt L.A. Changes in EEG cross-frequency coupling during cognitive behavioral therapy for social anxiety disorder. Psychol. Sci. 2011;22(4):507–516. doi: 10.1177/0956797611400914. http://doi.org/10.1177/0956797611400914. [DOI] [PubMed] [Google Scholar]
- Murray K., Kochanska G. Effortful control: factor structure and relation to externalizing and internalizing behaviors. J. Abnorm. Child Psychol. 2002;30(5):503–514. doi: 10.1023/a:1019821031523. http://doi.org/10.1023/A:1019821031523. [DOI] [PubMed] [Google Scholar]
- Pfeifer M., Goldsmith H.H., Davidson R.J., Rickman M. Continuity and change in inhibited and uninhibited children. Child Dev. 2002;73(5):1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- Putman P. Resting state EEG delta–beta coherence in relation to anxiety, behavioral inhibition, and selective attentional processing of threatening stimuli. Int. J. Psychophysiol. 2011;80(1):63–68. doi: 10.1016/j.ijpsycho.2011.01.011. http://doi.org/10.1016/j.ijpsycho.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Ray W.J., Cole H.W. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. http://doi.org/10.2307/1694556. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland B.C., Rankin Williams L., Degnan K.A., Pérez-Edgar K., Chronis-Tuscano A., Leibenluft E. Identification of emotional facial expressions among behaviorally inhibited adolescents with lifetime anxiety disorders. Cogn. Emotion. 2014;29(2):372–382. doi: 10.1080/02699931.2014.913552. http://doi.org/10.1080/02699931.2014.913552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.L. The technical, neurological and psychological significance of “alpha”, “delta” and “theta” waves confounded in EEG evoked potentials: a study of peak latencies. Clin. Neurophysiol. 1999;110(8):1427–1434. doi: 10.1016/s1388-2457(99)00078-4. http://doi.org/10.1016/S1388-2457(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G., van Honk J. Salivary cortisol levels and the coupling of midfrontal delta–beta oscillations. Int. J. Psychophysiol. 2005;55(1):127–129. doi: 10.1016/j.ijpsycho.2004.07.003. http://doi.org/10.1016/j.ijpsycho.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L., Knyazev G.G. Cross-frequency coupling of brain oscillations in studying motivation and emotion. Motiv. Emotion. 2012;36:46–54. doi: 10.1007/s11031-011-9237-6. http://doi.org/10.1007/s11031-011-9237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B., O’Toole L., Hong M., Dennis T.A. Negative affectivity and EEG asymmetry interact to predict emotional interference on attention in early school-aged children. Brain Cogn. 2014;87(0):173–180. doi: 10.1016/j.bandc.2014.03.014. http://doi.org/10.1016/j.bandc.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R.M., Ray W.J., Quigley K.S. Oxford University Press; New York, NY: 2001. Psychophysiological Recording. [Google Scholar]
- Thompson R.A. Emotion and self-regulation. In: Thompson, Ross A., editors. vol. 36. University of Nebraska Press; Lincoln, NE: 1990. pp. 367–467. (Socioemotional Development. Nebraska Symposium on Motivation). [Google Scholar]
- Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. http://doi.org/10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Van Peer J.M., Roelofs K., Spinhoven P. Cortisol administration enhances the coupling of midfrontal delta and beta oscillations. Int. J. Psychophysiol. 2008;67(2):144–150. doi: 10.1016/j.ijpsycho.2007.11.001. http://doi.org/10.1016/j.ijpsycho.2007.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.