Abstract
Background & Aims
The interferon-free regimen of simeprevir plus sofosbuvir was recommended by professional guidelines for certain patients with hepatitis C virus (HCV) genotype 1 infection based on the findings of a phase 2 trial. We aimed to evaluate the safety and efficacy of this regimen in clinical practice settings in North America.
Methods
We collected demographic, clinical, and virologic data, as well as reports of adverse outcomes, from sequential participants in HCV-TARGET—a prospective, observational cohort study of patients undergoing HCV treatment in routine clinical care settings. From January through October 2014, 836 patients with HCV genotype 1 infection began 12 weeks of treatment with simeprevir plus sofosbuvir (treatment duration of up to 16 weeks); 169 of these patients received ribavirin. Most patients were male (61%), Caucasian (76%), or black (13%); 59% had cirrhosis. Most had failed prior treatment with peginterferon and ribavirin without (46%) or with telaprevir or boceprevir (12%). The primary outcome was sustained virologic response (SVR), defined as level of HCV RNA below quantification at least 64 days after the end of treatment (beginning of week 12 after treatment—a 2 week window). Logistic regression models with inverse probability weights were constructed to adjust for baseline covariates and potential selection bias.
Results
The overall rate of SVR rate was 84% (675/802 patients, 95% CI: 81–87%). Model-adjusted estimates indicate patients with cirrhosis, prior decompensation, and previous protease inhibitor treatments were less likely to achieve an SVR. The addition of ribavirin had no detectable effects on SVR. The most common adverse events were fatigue, headache, nausea, rash, and insomnia. Serious adverse events and treatment discontinuation occurred in only 5% and 3% of participants, respectively.
Conclusions
In a large, prospective observational cohort study, a 12 week regimen of simeprevir plus sofosbuvir was associated with high rates of SVR and infrequent treatment discontinuation. ClinicalTrials.gov: NCT01474811
Keywords: chronic hepatitis, direct-acting agent, NS3/4A protease inhibitor, NS5B
Introduction
Globally, chronic hepatitis C virus (HCV) is a major cause of morbidity and mortality, infecting an estimated 170 million persons.1, 2 Successful treatment results in cure of HCV, which has been linked to significant reductions in the risk of HCV-related complications including liver failure, hepatocellular carcinoma, need for transplantation and death.3–5 Until recently, the effectiveness of interferon-based treatments for HCV genotype 1 has been substantially limited by host-dependent HCV suppression and by significant safety and tolerability issues.6, 7 As many as 60% of patients evaluated in real world settings have been considered ineligible for HCV treatment with such interferon containing regimens.6, 8 Importantly, patients with advanced liver disease or decompensated cirrhosis were among those patients in whom the effectiveness of interferon-based therapy has been severely limited.9
In early 2014, the first highly effective, interferon-sparing HCV treatment regimen, simeprevir plus sofosbuvir entered clinical practice in the United States for the treatment of patients with HCV genotype 1 infection.10–12 In the phase 2 COSMOS study, this regimen was investigated with and without ribavirin for durations of 12 and 24 weeks in treatment naïve and prior null responders to peginterferon/ribavirin. Overall, more than 95% of the 167 patients treated in the COSMOS study achieved SVR and the addition of ribavirin did not appear to increase the likelihood of SVR. Based on the findings of this study, the American Association for the Study of Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) HCV Guidance Panel recommended the interferon-free, oral combination of simeprevir plus sofosbuvir with or without ribavirin for 12 weeks for the treatment of patients with chronic HCV infection who cannot take interferon and for those for whom prior treatment with peginterferon/ribavirin was ineffective.13 In the context of significant unmet medical need, the combination of simeprevir plus sofosbuvir as two pills once daily was rapidly adopted for the treatment of patients with HCV genotype 1 infection in North America.
The translation of novel HCV therapeutics from clinical trials to clinical practice has been associated with substantially lower SVR rates and higher rates of adverse events than observed in clinical trials.14, 15 Thus, the present study analyzed data from the HCV-TARGET, an observational cohort study designed to evaluate the safety, tolerability and effectiveness of simeprevir plus sofosbuvir in patients undergoing HCV treatment in routine clinical care settings.
Patients
HCV-TARGET is a consortium of academic (n=39) and community (n=15) centers that provide medical care and antiviral treatment to HCV-infected patients. Since 2011, patients prescribed HCV treatment as part of routine clinical practice have been enrolled in a longitudinal, prospective observational cohort study. In the current study, data from sequential patients treated with the interferon-free, combination of simeprevir plus sofosbuvir were prospectively collected within a centralized database utilizing standardized source data abstraction as previously described14 and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at UNC Chapel Hill. REDCap is a secure, web-based application designed to support data capture for research studies16. Patients were eligible if they were 18 years old or greater and were treated with the oral regimen with or without ribavirin. The cohort of patients included in this analysis started treatment prior to 10 October 2014, were infected with HCV genotype 1, did not have a prior liver transplantation, and were on treatment less than 16 weeks. During the study period, an additional 300 patients meeting these criteria were treated with alternative HCV regimens including sofosbuvir plus ribavirin alone (n=11) or with peginterferon (n=282). These patients were not included in the analysis since these alternatives were not potent, interferon-free HCV regimens. In light of the rapidly changing treatment options for HCV, the inclusion window for this cohort was defined a priori without reference to power/sample size calculations in order to provide a timely description of efficacy and safety of simeprevir plus sofosbuvir in clinical practice settings.
Treatments
The decision to initiate HCV treatment and the selection of the HCV treatment regimen (with or without ribavirin) was solely the responsibility of the treating clinician and his or her patient; this was a non-random process in which a regimen was selected for an individual patient. Patients were treated with sofosbuvir administered as a single 400 milligram tablet taken by mouth daily and simeprevir administered as a single 150 milligram capsule taken by mouth daily. Ribavirin dosing was variable across patients and treatment centers; however, for most patients, ribavirin was administered according to body weight (< 75 kilograms, 1000 milligrams/day in two divided doses; ≥ 75 kilograms, 1200 milligrams/day in two divided doses). The duration of HCV therapy for all patients in this analysis was 12 weeks with post-treatment follow-up of at least 64 days.
Measurements
Demographic, clinical, adverse events and virologic data were collected throughout treatment and post-treatment follow-up. Routine laboratory data collected included levels of serum creatinine, albumin, total bilirubin, alanine and aspartate aminotransferase, INR, hemoglobin, platelet count and HCV RNA. Liver disease stage (cirrhosis or no cirrhosis) was defined at the time of enrollment by biopsy and/or imaging and clinical criteria established a priori.14 The criteria for the diagnosis of cirrhosis were either confirmation by staging liver biopsy or a combination of clinical, laboratory, histologic, and imaging criteria features. Patients with METAVIR stage 3 fibrosis by liver biopsy, were defined as cirrhotic if they had any of the following: platelet count <140,000 per µL, presence of esophageal varices on esophagogastroduodenoscopy, nodular liver, portal hypertension, or ascites by radiologic imaging, non-invasive serum panels such as FibroSURE® (Laboratory Corporation of America) consistent with stage 4 fibrosis, or liver stiffness measurement by elastography (FibroScan®) with consistent with stage 4 fibrosis (kPa ≥ 14).17, 18 In the absence of liver biopsy, cirrhosis was defined by exhibiting two or more of the above non-histologic criteria. History of hepatic decompensation was defined as evidence of prior or current diagnosis of ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, or variceal hemorrhage or baseline concomitant medications with a specific indication for ascites, hepatic encephalopathy, hepatic hydrothorax, spontaneous bacterial peritonitis, or variceal hemorrhage.
Outcomes
Treatment efficacy was measured as sustained virologic response (SVR12) defined as HCV RNA level below level of quantitation or undetected recorded at least 64 days after treatment was discontinued. For those who did not achieve SVR, the frequency of relapse, virological breakthrough, and non-response was reported, as were those lost-to-follow-up or had early treatment discontinuations due to adverse events or other factors. Adverse events (AEs) were captured as follows: i) Any event that required a HCV medication dose reduction or discontinuation or the addition of a concomitant medication for management; ii) any event of special interest during treatment (e.g. rash, photosensitivity, anemia, jaundice, hepatic decompensation) regardless of management. Anemia was defined as presence of one of the following: i) anemia reported as an AE; ii) administration of red blood cell growth factors; or iii) blood transfusion. Serious adverse events (SAEs) were any AEs that met these criteria: i) Required hospitalization, or ii) met criteria for expedited reporting per FDA form MEDWATCH 35000.
Analysis Strategy
(1) The two treatment groups were described and compared in terms of baseline covariates. (2) Crude SVR rates (SVR calculated without adjustment for treatment choice or other covariates) and their relationships to patient characteristics were explored for each treatment group and for the groups combined. (3) The estimation and comparison of model-adjusted SVR rates relied on a two-step approach 27. In the first step, a logistic regression “selection model” was used to estimate the probability of treatment without ribavirin (i.e., the propensity score) as a function of potential confounders measured at baseline: fibrosis status, genotype, baseline AST, treatment start date, previous treatment with telaprevir or boceprevir).
In the second step, the estimated propensity scores were used to compute inverse of probability of treatment weights (IPTW), and the weights were used in the fitting of a logistic regression ‘response model’ for achievement of SVR as a function of baseline laboratory measures and the interaction of regimen with age, sex, cirrhosis status, HCV genotype/subtype 1a or 1b, previous telaprevir or boceprevir treatment failure, previous peginterferon/ribavirin treatment failure, and history of decompensating events. The set of baseline variables was selected a priori to the analysis. Standard errors for the resulting estimated odds ratios (ORs) were computed using the Huber-White sandwich estimator 28.
Model-adjusted estimates of SVR for sub-populations were calculated by averaging the patient-level estimates of SVR over the relevant sub-population. The corresponding standard error estimates for SVR were calculated from the standard error estimates of the odds ratios with numerical integration. To investigate the adequacy of the selection model, the weighted comparability of the two treatment groups was examined for its balance on the distributions of the baseline covariates. To evaluate the robustness of the main results to reasonable perturbations of assumptions and methods, analysis using matching as an alternative method for estimating treatment effects with observational data was performed.
Missing Data
Multiple imputation methods30 were used in order to investigate the potential bias related to missing outcome data. For each patient missing SVR12, we constructed a pool of similar patients not missing SVR12 by selecting patients with the same treatment regimen, cirrhosis status, previous treatment experience, and history of prior decompensation. We imputed the missing SVR12 by randomly selecting the response from a patient in the pool of similar patients. In order to incorporate information from the available post treatment HCV RNA measures, we gave a greater selection probability to patients in the pool whose post-treatment HCV RNA measures more closely resembled the available post-treatment HCV RNA measures of the patient missing SVR12. We generated 25 imputed datasets with this procedure. Estimates of SVR12 and estimates of odds ratios involving SVR12 were calculated by analyzing individually the 25 dataset and then combining the results into a single estimate and associated standard error using multiple imputation methods.31 To investigate the adequacy of the imputation model, the estimates of SVR12 to two other SVR12 estimates were compared: (a) the ‘complete-case’ estimate in which observations with missing SVR12 were omitted and (b) the ‘worst-case scenario’ estimate in which observations with missing SVR12 were marked as failures. The multiply imputed estimates appeared to be adequate.
Informed Consent
The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The independent ethics committee at each participating study center or a central IRB approved the protocol if a local IRB was not in place. All patients provided written informed consent for their participation. All authors had complete access to the study data, reviewed and approved the final manuscript.
Results
Patient population
Between January 1, 2014 and October 10, 2014, 836 patients with chronic HCV genotype 1 without history of liver transplant initiated treatment with simeprevir plus sofosbuvir for 12 weeks; of whom, 169 patients received concurrent ribavirin. The majority of patients were males (n=509, 60.9%), Caucasian (n=638; 76.3%) or Black (n=112; 13.4%), and between the ages 40 and 64 years (n=603; 72.1%) or older than 65 (n=204, n=24.4%) (Table 1). HCV genotype 1a and 1b infection was present in 60.9% and 28.9%, respectively; of note, subtype was not reported in 10.2% of patients. Testing for the presence of HCV variants with the Q80K polymorphism prior to treatment was performed in only 90 patients (10.7%). At baseline, cirrhosis was found in 491 patients (58.7%); of whom, 45% (n=221) had a prior episode of hepatic decompensation and 141 (40.3% of those with available scores) had a pre-treatment MELD score greater than or equal to 10. A high proportion of patients had previously failed to respond to treatment with peginterferon plus ribavirin (n=387, 46.3%), and 12% (n=100) had failed to respond to treatment that included first generation protease inhibitors, telaprevir or boceprevir. At baseline, the clinical and demographic characteristics of patients who received ribavirin were similar to those who were not prescribed ribavirin (Table 1).
Table 1.
Baseline demographics of all patients who started treatment with simeprevir plus sofosbuvir with or without ribavirin
| Characteristic | SOF SMV (n=667) |
SOF SMV RBV (n=169) |
Total (n=836) |
|---|---|---|---|
| Mean age (range), years | 59 (20–83) | 59 (29–80) | 59 (20–83) |
| 18–39 | 23 (3.4) | 6 (3.6) | 29 (3.5) |
| 40–64 | 470 (70.5) | 133 (78.7%) | 603 (72.1) |
| 65+ | 174 (26.1) | 30 (17.8) | 204 (24.4) |
| Male sex, n (%) | 405 (60.7) | 104 (61.5) | 509 (60.9) |
| Race, n (%) | |||
| White | 504 (75.6) | 134 (79.3) | 638 (76.3) |
| Black | 88 (13.2) | 24 (14.2) | 112 (13.4) |
| Asian | 13 (1.9) | 1 (0.6) | 14 (1.7) |
| Other/missing | 62 (9) | 10 (6) | 72 (9) |
| Hispanic ethnicity, n (%) | 31 (4.6) | 11 (6.5) | 42 (5.0) |
| Mean ALT (range), IU/l | 80 (8.0–409) | 86 (12.0–337) | 81 (8.0–409) |
|
Mean total bilirubin (range) mg/dl |
1.0 (0.1–8.0) | 1.2 (0.2–10.7) | 1.1 (0.1–10.7) |
| Mean albumin (range), g/dl | 3.8 (1.2–5.3) | 3.8 (1.8–4.7) | 3.8 (1.2–5.3) |
| Mean hemoglobin (range), g/dl | 14.0 (7.3–18.8) | 14.1 (9.7–18.6) | 14.0 (7.3–18.8) |
|
Mean platelet count (range) (×103) per µl |
152 (24.0–595) | 137 (24.0–521) | 149 (24.0–595) |
| History of cirrhosis, n (%) | 381 (57.1) | 110 (65.1) | 491 (58.7) |
|
History of liver decompensation (among cirrhotics), n (%) |
168 (44.1) | 53 (48.2) | 221 (45.0) |
| MELD among cirrhotics | |||
| 0–9 | 162 (42.5) | 47 (42.7) | 209 (42.6) |
| 10–15 | 101 (26.5) | 21 (19.1) | 122 (24.8) |
| 16–21 | 10 (2.6) | 3 (2.7) | 13 (2.6) |
| 21–39 | 5 (1.3) | 1 (0.9) | 6 (1.2) |
| Pending | 103 (27.0) | 38 (34.5) | 141 (28.7) |
| Presence of diabetes, n (%) | 147 (22.0) | 46 (27.2) | 193 (23.1) |
| HCV genotype, n (%) | |||
| 1a | 381 (57.1) | 128 (75.7) | 509 (60.9) |
| 1b | 221 (33.1) | 21 (12.4) | 242 (28.9) |
| 1, subtype unspecified | 65 (9.7) | 20 (11.8) | 85 (10.2) |
| Q80K mutation tested | 67 (10.0) | 23 (13.6) | 90 (10.7) |
| Q80K mutation present | 28 (4.2) | 14 (8.3) | 42 (5.0) |
| Prior HCV treatment, n (%) | |||
| Treatment naïve | 263 (39.4) | 63 (37.3) | 326 (39.0) |
| Prior Peg/Rbv treatment failures |
317 (47.5) | 70 (41.4) | 387 (46.3) |
| Prior telaprevir or boceprevir treatment failure |
66 (9.9) | 34 (20.1) | 100 (12.0) |
Efficacy
Outcomes were available for 802 of 836 patients (including 32 patients lost to follow-up) who started therapy with simeprevir plus sofosbuvir. Of those, 675 patients (84.2%) achieved SVR12. Of the patients who failed to achieve SVR12, the most frequent reason for not achieving SVR was post-treatment relapse (n=91 of 127 failures). In addition, three patients had lack of efficacy during treatment (2.4% of 127 failures) and 30 were lost to follow up after completing treatment (24% of 127 failures) (Table 2). An additional 34 patients (4%) remain in post treatment follow up. The SVR12 rate was similar in persons who received ribavirin (132 of 163 patients, 81%) and those who did not receive ribavirin (543 of 639 patients, 85%) (Table 3).
Table 2.
Patient disposition
| SOF SMV (n=667) |
SOF SMV RBV (n=169) |
Total (n=836) |
|
|---|---|---|---|
| Patient disposition during treatment, n (%) | |||
| Completed therapy | 648 (97.2) | 163 (96.4) | 811 (97.0) |
| Discontinued therapy early | 19 (2.8) | 6 (3.6) | 25 (3.0) |
| Adverse event | 13 (1.9) | 4 (2.4) | 17 (2.0) |
| Lack of efficacy | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Withdrawal by subject | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Loss to follow up during treatment | 1 (0.1) | 1 (0.6) | 2 (0.2) |
| Other | 3 (0.4) | 1 (0.6) | 4 (0.5) |
| Completed SVR 12 Follow-up | 639 (96) | 163 (98) | 802 (96) |
| Lost to post treatment follow-up* | 21(3) | 9 (5) | 30 (4) |
| HCV RNA result not available** | 28 (4) | 6 (4) | 34 (4) |
Included in the “Completed SVR12 follow up”
HCV RNA result not yet available as of last follow-up appointment
Table 3.
Unadjusted SVR12 rates among patients with available outcomes
| Group/ Subgroup | SOF/SMV | SOF/SMV/RBV | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | SVR12 | (95% CI) | n/N | SVR1 2 |
(95% CI) | n/N | SVR12 | (95% CI) | |||
| 543/639 | 85 | (82.0, 87.7) | 132/163 | 81 | (74.1, 86.7) | 675/802 | 84.2 | (81.5, 86.6) | |||
| Cirrhotics Non-cirrhotics |
296/367 247/272 |
80.7 90.8 |
(76.2, 84.6) (86.7, 94.0) |
88/110 44/53 |
80 83 |
(71.3, 87.0) (70.2, 91.9) |
384/477 291/325 Difference : |
80.5 89.5 9 |
(76.7, 84.0) (85.7, 92.6) (3.9, 14.2) |
||
| Prior PI Failure No Prior PI |
49/65 494/574 |
75.4 86.1 |
(63.1, 85.2) (83.0, 88.8) |
22/31 109/131 |
71 83.2 |
(52.0, 85.8) (75.7, 89.2) |
71/96 603/705 Difference : |
74 85.5 11.6 |
(64.0, 82.4) (82.7, 88.0) (1.8, 21.3) |
||
| Genotype 1a Genotype 1b |
308/371 185/206 |
83 89.8 |
(78.8, 86.7) (84.8, 93.6) |
102/125 16/19 |
81.6 84.2 |
(73.7, 88.0) (60.4, 96.6) |
410/496 201/225 Difference : |
82.7 89.3 6.7 |
(79.0, 85.9) (84.5, 93.0) (1.1, 12.2) |
||
| 1a | Experienced | Cirrhotic Non-cirrhotic |
97/129 77/86 |
75.2 89.5 |
(66.8, 82.4) (81.1, 95.1) |
41/50 15/21 |
82 71.4 |
(68.6, 91.4) (47.8, 88.7) |
138/179 92/107 |
77.1 86 |
(70.2, 83.0) (77.9, 91.9) |
| Naive | Cirrhotic Non-cirrhotic |
73/87 61/69 |
83.9 88.4 |
(74.5, 90.9) (78.4, 94.9) |
29/35 16/18 |
82.9 88.9 |
(66.4, 93.4) (65.3, 98.6) |
102/122 77/87 |
83.6 88.5 |
(75.8, 89.7) (79.9, 94.3) |
|
| Unk | Cirrhotic | 0/0 | 1/1 | 100 | (2.5, 100.0) | 1/1 | 100 | (2.5, 100.0) | |||
| 1b | Experienced | Cirrhotic Non-cirrhotic |
64/78 51/54 |
82.1 94.4 |
(71.7, 89.8) (84.6, 98.8) |
5/8 7/7 |
62.5 100 |
(24.5, 91.5) (59.0, 100.0) |
69/86 58/61 |
80.2 95.1 |
(70.2, 88.0) (86.3, 99.0) |
| Naive | Cirrhotic Non-cirrhotic |
33/36 37/38 |
91.7 97.4 |
(77.5, 98.2) (86.2, 99.9) |
2/2 2/2 |
100 100 |
(15.8, 100.0) (15.8, 100.0) |
35/38 39/40 |
92.1 97.5 |
(78.6, 98.3) (86.8, 99.9) |
|
| Unk | Experienced | Cirrhotic Non-cirrhotic |
21/24 13/16 |
87.5 81.2 |
(67.6, 97.3) (54.4, 96.0) |
7/10 3/3 |
70 100 |
(34.8, 93.3) (29.2, 100.0) |
28/34 16/19 |
82.4 84.2 |
(65.5, 93.2) (60.4, 96.6) |
| Naive | Cirrhotic Non-cirrhotic |
8/13 8/9 |
61.5 88.9 |
(31.6, 86.1) (51.8, 99.7) |
3/4 1/2 |
75 50 |
(19.4, 99.4) (1.3, 98.7) |
11/17 9/11 |
64.7 81.8 |
(38.3, 85.8) (48.2, 97.7) |
|
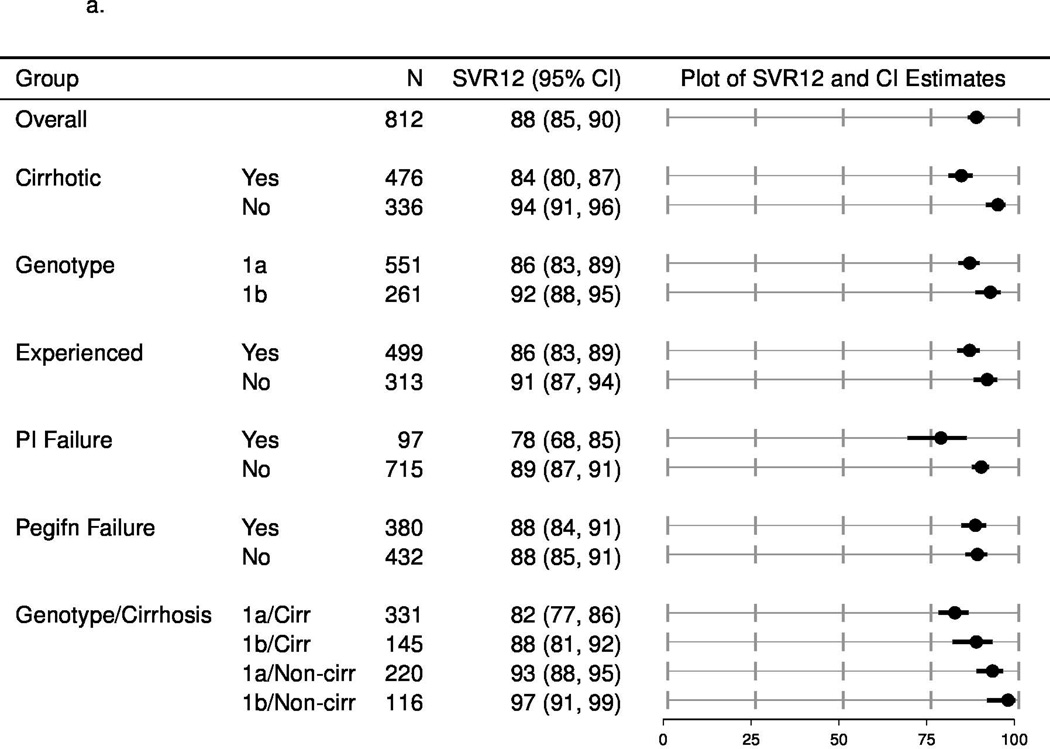
Among patients with outcomes available (n=802), the crude SVR12 rate varied according to the presence or absence of baseline cirrhosis, prior treatment experience, and subtype 1a or 1b (Table 3). In patients with genotype 1a infection treated with simeprevir plus sofosbuvir the crude SVR12 rate was 83% (308 of 371 patients) and 82% (102 of 125 patients) for patients who received this combination plus ribavirin. The crude SVR12 rate in patients with genotype 1b infection treated with simeprevir plus sofosbuvir without ribavirin was 90% (185 of 206 patients) and 84% (16 of 19 patients) with use of ribavirin. The highest crude SVR12 rates were observed among patients who were treatment-naïve, non-cirrhotic and infected with genotype 1b (39 of 40 patients, 98%) whereas the lowest crude SVR12 rates were observed patient who were treatment-experienced, cirrhotic and infected with genotype 1a (138 of 179, 77%) (Table 3).
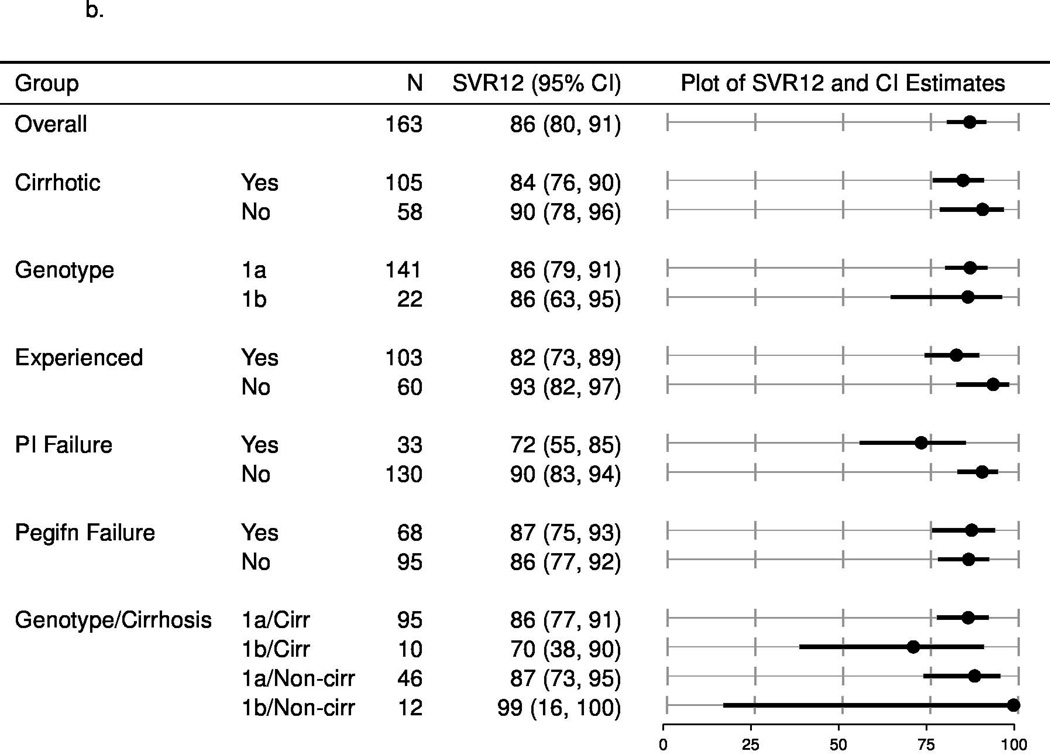
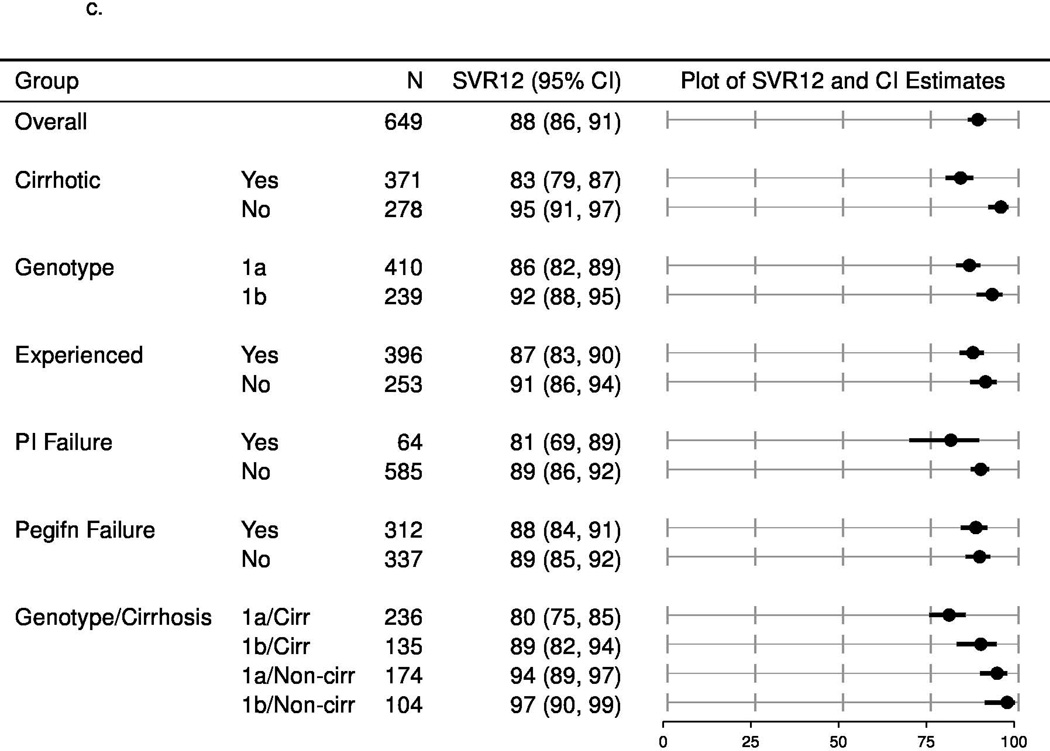
The SVR rates (estimated with multiple imputation methods to account for missing outcomes) among patients categorized by each of these factors (cirrhosis/no cirrhosis; treatment-naïve/treatment experienced and subtype 1a/1b) and other populations of interest, such as prior failures of protease inhibitors are shown in Figure 1a (overall population), Figure 1b (with ribavirin) and 1c (without ribavirin). In patients who completed treatment or discontinued early due to lack of efficacy, the SVR12 rate was lower among patients with cirrhosis compared to those without cirrhosis, higher in treatment naïve patients compared to those who had been treated previously and did not vary according to the use of ribavirin. The estimated SVR rates for patients who failed to respond to prior protease inhibitor-based regimens were 72% (95% CI: 55, 85) and 81% (95% CI: 69, 89) for simeprevir plus sofosbuvir with or without ribavirin, respectively. Due to limited pre-treatment testing, the impact of the presence of the Q80K or other baseline resistance associated variants could not be assessed.
Figure 1.
a. Unadjusted SVR12, multiply imputed for patients treated with simeprevir plus sofosbuvir with or without ribavirin
b. Unadjusted SVR12, multiply imputed for patients treated with simeprevir plus sofosbuvir with ribavirin
c. Unadjusted SVR12, multiply imputed for patients treated with simeprevir plus sofosbuvir without ribavirin
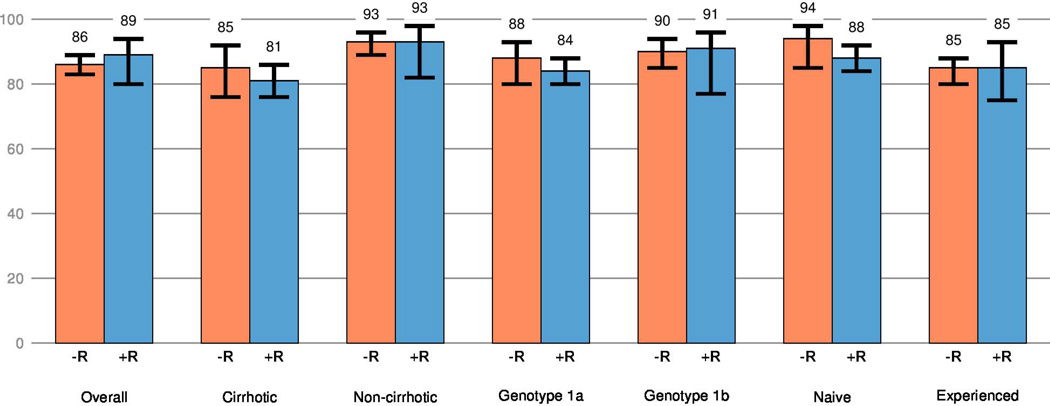
In the comparison of the two treatment regimens in terms of the model-adjusted SVR12 rates (with inverse probability of treatment weighting and adjusting for baseline factors), the overall SVR12 rate for patients treated without ribavirin was similar to those treated with ribavirin; the estimated difference in SVR12 was −2.3% (95%CI: −10.1, 6.4). Further, the difference in SVR rates for with and without ribavirin was similar (varying by no more than ± 6%) in a variety of subpopulations such as patients with cirrhosis versus no cirrhosis, genotype 1a versus 1b infection, treatment naïve versus experienced, or combinations of two of these factors. (Figure 2).
Figure 2.
Model adjusted estimates of SVR12 among patients treated with simeprevir plus sofosbuvir with or without ribavirin.
Population: patients which completed a full course of treatment or prematurely discontinued treatment for virological reasons.
Adjusted SVR12 estimates derived from a logistic regression model which simultaneously adjusted for regimen, subtype (1a versus 1b), cirrhosis status, previous treatment experience, prior PI failure, history of prior decompensation, sex, age, and baseline chemistry measures of albumin, aspartate aminotransferase, creatinine, HCV RNA, HGB, and total bilirubin.
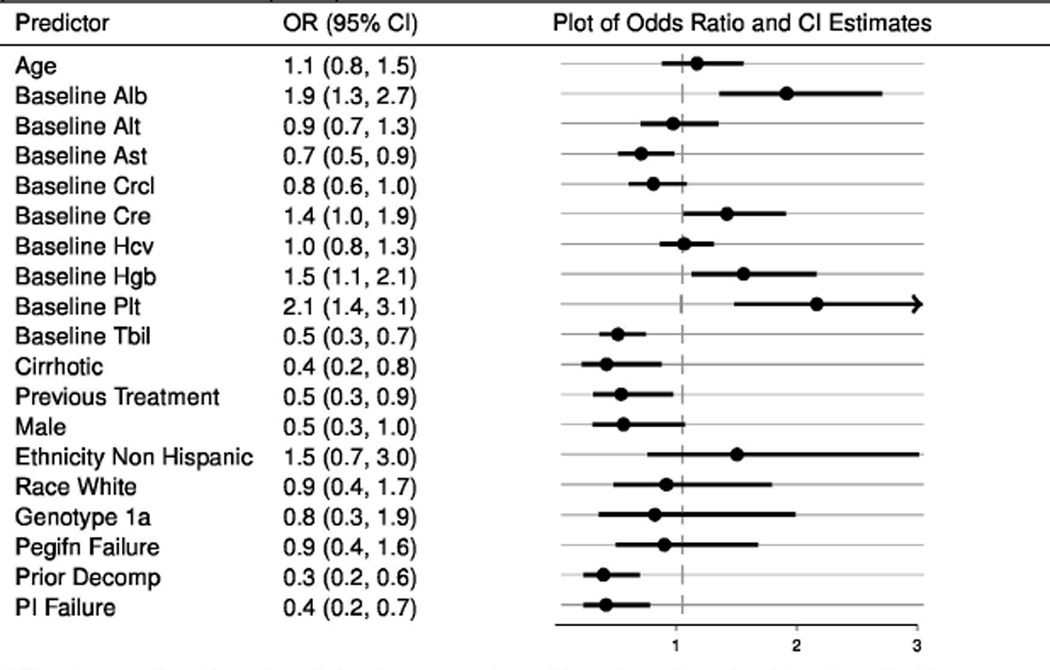
In exploratory analyses of the two groups combined with covariate adjustment for treatment regimen, genotype, gender, and age, several baseline patient and/or disease characteristics exhibited association with lower likelihood of SVR. The estimates suggest that patients with cirrhosis or historical evidence of hepatic decompensation and those with prior non-response following treatment with HCV protease inhibitors, telaprevir or boceprevir were less likely to achieve SVR. Further, the exploratory analysis indicates that higher total bilirubin or higher aspartate aminotransferase may be associated with lower SVR rates. In contrast, patients with higher baseline serum levels of albumin, total platelet count and hemoglobin were more likely to have an SVR. Interestingly, after adjusting for regimen, gender, and age, the estimate of association between genotype/subtype (1a versus 1b infection) and SVR was not as precise as the crude association discussed earlier (Figure 3).
Figure 3.
Odds ratio estimates from logistic regression models of SVR12 and baseline factors for patients treated with simeprevir plus sofosbuvir with or without ribavirin.
Odds ratios are adjusted for regimen (+R v. −R), genotype (1a v. 1b), gender (male vs. female), and age. Each line represents a unique model. Observations are weighted with the inverse of probability of treatment weights (IPTW)
Population: patients which completed a full course of treatment or prematurely discontinued treatment for virological reasons.
Safety and tolerability
Ninety seven percent of patients completed the prescribed treatment course. Discontinuation of simeprevir plus sofosbuvir with or without ribavirin occurred in only 25 patients (3%), and the rate of discontinuation for adverse events was only 2% (Table 2). The discontinuation rate for adverse events was similar in patients taking ribavirin (2.4%) and in those taking simeprevir plus sofosbuvir alone (1.9%). During the course of observation, five deaths were observed (Table 4); two deaths due to hepatic failure occurred in patients with advanced liver disease, and one death each was reported due to cerebral vascular accident, vascular shock, and unknown causes. Serious adverse events were recorded in 44 patients (5.3%), and incidence of such events was similar in patients taking or not taking ribavirin (7.1% and 4.8% respectively). Notable serious adverse events included gastrointestinal bleeding in 4 patients (0.5%), hepatic failure or encephalopathy in 10 patients (1.2%), infections in 9 patients (1.1%). No serious adverse events related to skin rash or photosensitivities were reported; however, one patient stopped simeprevir plus sofosbuvir alone due to photosensitivity.
Table 4.
Safety profile
| SOF SMV (n=667) |
SOF SMV RBV (n=169) |
Total (n=836) |
|
|---|---|---|---|
| Most common Adverse Events, n (%) | |||
| Fatigue | 162 (24.3) | 60 (35.5) | 222 (26.6) |
| Headache | 106 (15.9) | 31 (18.3) | 137 (16.4) |
| Nausea | 90 (13.5) | 32 (18.9) | 122 (14.7) |
| Influenza like illness | 73 (10.9) | 20 (11.8) | 93 (11.1) |
| Rash/ Pruritus | 60 (9.0) | 27 (16.0) | 87 (10.4) |
| Insomnia | 53 (8.0) | 28 (16.6) | 81 (9.7) |
| Pruritus | 53 (8.0) | 27 (16.0) | 80 (9.6) |
| Anaemia | 8 (1.2) | 50 (29.6) | 58 (6.9) |
| Photosensitivity reaction | 45 (6.8) | 13 (7.7) | 58 (6.9) |
| Total patients with any AE | 499 (74.81) | 150 (88.76) | 649 (77.63) |
| Anemia | |||
| AE Anemia, n (%) | 8 (1.2) | 50 (29.6) | 58 (6.9) |
| SAE Anemia, n (%) | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Hemoglobin change, delta mean (range) | −0.4 (−6.0,+5.1) | −2.1 (−5.7,+1.3) | −0.8 (−6.0,+5.1) |
| Ribavirin dose reduction/ interruption | 0 (0.0) | 35 (20.7) | 35 (4.2) |
| Ribavirin discontinuation | 0 (0.0) | 10 (5.9) | 10 (1.2) |
| Erythropoietin use | 0 (0.0) | 9 (5.3) | 9 (1.1) |
| Blood transfusion | 5 (0.7) | 5 (3.0) | 10 (1.2) |
| Most common Serious Adverse Events, n (%) | |||
| Decompensating events | 9 (1.4) | 2 (1.2) | 10 (1.2) |
| Infections and infestations | 8 (1.2) | 1 (0.6) | 9 (1.1) |
| Cardiac failure | 4 (0.6) | 0 (0.0) | 4 (0.5) |
| Gastrointestinal hemorrhage | 3 (0.5) | 1 (0.6) | 4 (0.5) |
| Arrhythmia | 2 (0.3) | 1 (0.6) | 3 (0.4) |
| Abdominal pain | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Renal failure acute | 0 (0.0) | 2 (1.2) | 2 (0.2) |
| Dyspnea | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Thromboembolic events | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Total patients with any SAE | 32 (4.80) | 12 (7.10) | 44 (5.26) |
| Creatinine changea, delta mean (range)mg/dL | 0.0 (−5.6,+0.9) | 0.0 (−0.8,+0.8) | 0.0 (−5.6,+0.9) |
| Total Bilirubin changea, delta mean (range) mg/dL | 0.1 (−3.3,+3.4) | 0.5 (−10.1,+8.3) | 0.2 (−10.1,+8.3) |
| Deaths, n | 4 (0.6) | 1 (0.6) | 5 (0.6) |
The change in creatinine and bilirubin is defined as the change in value from study initiation to the end of treatment (EOT or the date closest to EOT, not exceeding 2 weeks post EOT and not preceding 8 weeks post start of treatment).
The majority of serious adverse events occurred in patients with cirrhosis at baseline (37 of 44 reported events, 84%). Ten patients with cirrhosis underwent liver transplantation during the treatment period and all remained HCV RNA uninfected post-transplant. Approximately 40% of patients with cirrhosis and laboratory data available had a MELD score greater than 10 prior to therapy (n=141). Among those with repeat MELD determination during treatment, only 35 (19%) had an increase in MELD, and the majority were stable or decreased.
In overall population the most common adverse events were fatigue, headache, nausea, flu like symptoms, rash/ pruritus and insomnia (Table 4). Fatigue was reported in 222 patients (26.5%), and was more common in patients taking ribavirin (36%) compared to those taking simeprevir plus sofosbuvir alone (24%). Photosensitivity was observed in 58 patients (7%) and the incidence was similar in persons with and without ribavirin exposure. Patients taking ribavirin were more likely to develop anemia (29.6%) compared to patients not taking ribavirin (1.2%). The mean change in hemoglobin from baseline levels to on-treatment nadir was −2.1 grams/dL in patients taking ribavirin compared to −0.4 grams/dL in those taking only simeprevir plus sofosbuvir. Blood transfusions and use of erythropoietin agonists were uncommon.(Table 4).
Discussion
Following the individual regulatory approval of sofosbuvir, a nucleoside analogue NS5B polymerase inhibitor, and simeprevir, a NS3 protease inhibitor, each in combination with peginterferon/ribavirin, the AASLD/IDSA guidance panel provided recommendations for treatment of HCV with the “off-label” combination of these oral antivirals in patient for whom interferon was ineffective or contraindicated. In this context, more than 800 patients with HCV genotype 1 infection treated with the interferon-free, once-daily, oral combination of simeprevir and sofosbuvir were prospectively enrolled in the HCV TARGET prospective cohort study. Consistent with the recommendations by this panel, the majority of patients treated in the cohort study had an urgent need for HCV treatment, characterized by cirrhosis (58.7%) and non-response to prior therapy (61%). In this setting, the oral combination of simeprevir plus sofosbuvir was well tolerated, with only ∼ 2% of patients discontinuing treatment due to adverse events. Further, following 12 weeks regimen with (20%) or without ribavirin (80%), HCV cure was achieved in 84% of patients, many of whom had no options for effective HCV treatment prior to availability of these drugs. The successful translation of this novel, oral combination regimen from the clinical trial setting to clinical practice has several important implications for expectations the era of interferon-free therapy.
The AASLD/IDSA panel recommendation for use of this oral regimen in patients ineligible for interferon and those who had failed prior therapies was based on the absence of drug interactions between these antivirals and a relatively small (n=167) phase 2 COSMOS study in which this combination with and without ribavirin was examined for 12 or 24 weeks duration for the treatment of HCV genotype 1-infected patients including peginterferon/ribavirin experienced patients with compensated cirrhosis. In the final analysis of the phase 2 COSMOS study, Lawitz and coworkers report that simeprevir plus sofosbuvir was well tolerated and associated with high rates of SVR in all patient groups, and response rates were similar with or without ribavirin and with 12 or 24 weeks of therapy.10 More recently, the combination of simeprevir plus sofosbuvir was evaluated in two phase 3 clinical trials in HCV genotype 1 infected patients with (OPTIMIST-2 study) and without cirrhosis (OPTIMIST-1 study). Among patients treated for 12 weeks, SVR12 was achieved in 83% of 103 patients with compensated cirrhosis and 97% of patients with no evidence of cirrhosis.19, 20 Interestingly, the findings from these phase 3 studies were not available to the AASLD/IDSA panel members when recommendations to use the combination of simeprevir pus sofosbuvir were first published in January 2014. Since many of the patients treated in clinical practice settings may fall outside the spectrum of those patients treated in clinical trials, the translation from trials to community may be associated with a substantial decrease in clinical effectives as was the case with telaprevir or boceprevir in combination with peginterferon/ribavirin.15, 21, 22
Indeed, many of the patients enrolled in this HCV TARGET cohort would likely not have met enrollment criteria for the phase 2 and 3 studies due to prior hepatic decompensation (n=233) or prior treatment with NS3 protease inhibitors (n=100). Overall, the crude SVR rate among patients with cirrhosis was 81% and, although the SVR rate was lower among patients with prior hepatic decompensation (75%), those patients had no safe options for treatment prior to the availability of these combinations. Despite population with more advanced disease, this SVR rate is similar to that observed in patient with compensated cirrhosis in the OPTIMIST-2 study. Of equal importance, discontinuation due to adverse events and serious adverse advents was uncommon in this relatively unstable group of patients. Nonetheless, we did observe virologic relapse after stopping therapy in 15% of cirrhotic patients and 20% of those with prior decompensation (not shown in tables). This observation is consistent with the relatively high rate of post-treatment virologic relapse in patients with compensated cirrhosis treated for 12 weeks with simeprevir plus sofosbuvir in the OPTIMIST-2 study. In hindsight, these patients treated for 12 weeks in HCV TARGET may have benefited from longer treatment duration (24 weeks) as was ultimately recommended by the FDA based on the observation in the COSMOS study of higher rates of post-treatment relapse in patients with advanced fibrosis who were randomized to 12 weeks of treatment (3 patients with relapse) compared to those treated for 24 weeks (no patients with relapse).23
We also found that patients with prior exposure to HCV protease inhibitors and those with genotype 1a infection were less likely to achieve SVR compared to other patient populations. Similar to the findings reported in the phase 2 COSMOS study, the addition of ribavirin did not appear to increase the likelihood of SVR in this patient population. However, in our cohort, more than 74% of patients who had failed telaprevir or boceprevir plus peginterferon/ribavirin achieved SVR. This finding was somewhat unanticipated since the AASLD/IDSA guidance panel recommended that such patients not be treated with simeprevir, a NS3 protease inhibitor, due to the possibility that persistent variants selected and enriched by the previous protease inhibitor exposure would confer resistance to simeprevir.24, 25 Although not definitive, this observation supports the hypothesis that some HCV-infected patients who fail DAA therapy may be successfully re-treated with combination regimens that include antivirals that target the same HCV non-structural proteins as drugs included in the failed regimen.
Similarly, patients with genotype 1a infection had lower SVR rates compared to those with 1b infection, suggesting the potential impact of baseline simeprevir resistance associated variants, namely the Q80K variant which is found in up to 50% of patients with genotype 1a infection in the US and is known to confer inferior response to simeprevir plus peginterferon/ribavirin.10, 12, 26 Unfortunately, baseline testing for the Q80K polymorphism was uncommon (∼ 10% of the cohort) which limits the ability to assess SVR in this specific group of patients. Recent data from the phase 3 OPTIMIST studies suggest that presence of Q80K was associated with a decreased response in HCV genotype 1a infected patients with cirrhosis treated with simeprevir plus sofosbuvir but had no impact in non-cirrhotic patients treated for 12 weeks.19, 20 These observations suggest that the presence of Q80K may explain the lower SVR rates observed in our study among patients with genotype 1a infection compared with 1b infection. Further studies are underway to assess baseline blood samples for patients in this cohort to estimate the prevalence of baseline resistance-associated variants and their relationship with the likelihood of SVR.
Our study is subject to several limitations. First, the absence of randomization limits our ability to make definitive conclusions regarding the role of ribavirin. However, our results suggest that the presence of ribavirin had minimal or no impact the efficacy of simeprevir-sofosbuvir for all subgroups evaluated. Recent phase 3 trials of the combination of simeprevir plus sofosbuvir did not include ribavirin; as such, our analysis, in which we did not detect evidence of greater treatment efficacy with ribavirin, provides useful clinical data. Whether the addition of ribavirin to a 12 week regimen would be competitive with a 24 week regimen of simeprevir and sofosbuvir without ribavirin, as suggested by recent studies of the NS5A inhibitor ledipasvir plus sofosbuvir would require further study. Second, we enrolled a heterogeneous patient group including some patients for whom simeprevir is not recommended. While this limits our ability to compare outcomes in our study to clinical trial settings, these data provide valuable insights into the safety, tolerability and efficacy of these agents in the real world. Since this cohort was enrolled, two additional HCV oral regimens have been approved by the FDA, although simeprevir plus sofosbuvir remains one of several first-line options recommended by the AASLD/IDSA HCV Guidance Panel for the treatment of patients with genotype 1 infection. Furthermore, the combination of sofosbuvir and simeprevir may be although the efficacy in this population and the roles of extended treatment duration and the addition of ribavirin must be further studied.
In this large, prospective observational cohort study, the oral, interferon-free combination of simeprevir plus sofosbuvir for 12 weeks was associated with high rates of SVR and low rates of treatment discontinuation. This represents one of the first applications of a highly effective HCV regimen outside clinical trials; consequently, many patients treated had relatively urgent medical need for therapy due to advanced liver disease and/or the failure of all available treatment options. In this context, these data provide evidence that such regimens will translate effectively from the trial setting to clinical practice. Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations.
Acknowledgments
Financial support: HCV-TARGET is an investigator-initiated study jointly sponsored by The University of Florida, Gainesville, FL (PI: Nelson), and The University of North Carolina at Chapel Hill, Chapel Hill, NC (PI: Fried). It was funded in part by AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, and Vertex. Funded in part by CTSA UF UL1TR000064 and UNC 1UL1TR001111. Dr. Fried was funded in part by NIH Mid-Career Mentoring Award K24 DK066144. Dr. Sulkowski was funded in part by NIH K24DA034621
APPENDIX. HCV-TARGET investigators
The members of the HCV-TARGET study were as follow: N. Afdhal, Harvard University, Boston, MA; I. Alam, Austin Hepatitis Center, Austin, TX; Z. Ben-Ari, Sheba Medical Center, Tel Hashomer, Israel; J. Bredfeldt, Virginia Mason Medical Center, Seattle, WA; R.S. Brown, Columbia University Medical Center, New York, NY; R.T. Chung, Massachusetts General Hospital, Boston, MA; J. Darling, University of North Carolina at Chapel Hill, Chapel Hill, NC; W. Harlan, Asheville Gastroenterology, Asheville, NC; A. M. Di Bisceglie, Saint Louis University, Saint Louis, MO; R. C. Dickson, Dartmouth-Hitchcock Medical Center, Lebanon, NH; H. A. Elbeshbeshy, Baptist Medical Center, Oklahoma City, OK; G. Everson, University of Colorado, Aurora, CO; J. Feld, University Health Network- Toronto, Toronto, ON- Canada; J. M. Fenkel, Thomas Jefferson University, Philadelphia, PA; M. W. Fried, University of North Carolina at Chapel Hill, Chapel Hill, NC; J. Galati, Research Specialists of Texas, Houston, TX; S. C. Gordon, Henry Ford Hospital, Detroit, MI; M. Hassan, University of Minnesota, Minneapolis, MN; T.N. Hawkins, Southwest CARE Center, Sante Fe, NM; F. Hinestrosa, Orlando Immunology Center, Orlando, FL; I. M. Jacobson, Weill Cornell, New York City, NY; C. A. Kerr, Hudson River Health Care, Peekskill, NY; A. Kuo, University of California-San Diego, San Diego, CA; P. Y. Kwo, Indiana University, Indianapolis, IN; J. Levitsky, Northwestern University Feinberg School of Medicine, Chicago, IL; J. Lim, Yale University, New Haven, CT; A.S. Lok, University of Michigan, Ann Harbor, MI; M. Mailliard, University of Nebraska, Omaha, NE; M.P. Manns, Hannover Medical School, Hannover, Germany; G. Morelli, University of Florida, Gainesville, FL; A. J. Muir, Duke University, Durham, NC; D. Nelson, University of Florida, Gainesville, FL; J. G. O’Leary, Baylor University Medical Center, Dallas, TX; B. L. Pearlman, Atlanta Medical Center, Atlanta, GA; P. Pockros, Scripps Health, La Jolla, CA; A. Ramani, Mountain View Medical, Catskill, NY; N. Reau, University of Chicago, Chicago, IL; K. R. Reddy, University of Pennsylvania, Philadelphia, PA; E. R. Schiff, University of Miami, Miami, FL; K. E. Sherman, University of Cincinnati, Cincinnati, OH; M.L. Shiffman, Liver Institute of Virginia, Richmond, VA; C. Smith, University of Minnesota, Minneapolis, MN; J. R. Spivey, Emory University, Atlanta, GA; R. K. Sterling, Virginia Commonwealth University, Richmond, VA; M. S. Sulkowski, Johns Hopkins, Baltimore, MD; G. Szabo, University of Massachusetts, Worcester, MA; N. A. Terrault, University of California-San Francisco, San Francisco, CA; C. Trautwein, RWTH University Hospital, Aachen, Germany; H. E. Vargas, Mayo Clinic, Phoenix, AZ; K. Watts, Mayo Clinic, Rochester, MN; A. Williams, Liver Wellness Center, Little Rock, AR.; S. Zeuzem, Goethe University Hospital, Frankfurt, Germany.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contributions
All authors provided final approval of the manuscript. Drs. Sulkowski and Jacobson contributed to the conception of the work, the acquisition and interpretation of the data for the work, drafted the first version of manuscript, provided critical revisions to subsequent versions of the manuscript and approved the final submitted version. Drs. Vargas, Di Bisceglie, Kuo, Reddy, Lim, Morelli, Darling, Feld, Brown, and Frazier contributed to the acquisition and interpretation of the data for the work, provided critical revisions to draft versions of the manuscript and approved the final submitted version. Mr. Stewart conducted the primary data analysis, contributed to the interpretation of the data, provided critical revisions to draft versions of the manuscript and approved the final version. Dr. Fried contributed to the conception of the work, the acquisition and interpretation of the data for the work, provided critical revisions to draft versions of the manuscript and approved the final submitted version.
Conflict of interest
Dr. Sulkowski reports grants and personal fees from Gilead, grants and personal fees from Janssen, during the conduct of the study; personal fees from Achillion, grants and personal fees from AbbVie, grants and personal fees from Merck, grants and personal fees from BMS, outside the submitted work.
Dr. Vargas reports grant funding from AbbVie, Gilead, Merck, and BMS during the conduct of the study.
Dr. Di Bisceglie reports grant funding from Gilead, AbbVie, Janssen during the conduct of the study; consultant funds from Gilead and AbbVie outside the submitted work.
Dr. Kuo reports grant funding from Gilead during the conduct of the study.
Dr. Reddy reports grant funding from AbbVie, Merck, Gilead, Janssen and Vertex during the conduct of the study.
Dr. Lim reports grant funding from AbbVie, Achillion, Bohringer-Ingelheim, Bristol-Myers Squibb, Gilead, Glaxo-Smith Kline, Janssen, and Vertex during the conduct of the study; consultant funds from Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
Dr. Morelli reports grant funding from AbbVie, BMS, Gilead, Merck, Janssen, Vertex, Idenix, Conatus, and Salix during the conduct of the study.
Dr. Darling reports grant funding from Bristol-Myers Squibb during the conduct of the study.
Dr. Feld reports grant funding from AbbVie, Boehringer-Ingelheim, Gilead, Janssen, Merck, and Santaris during the conduct of the study.
Dr. Brown reports grant and consultant funding from Gilead and Janssen during the conduct of the study.
Lynn Frazier, ARNP reports grant and consultant funding from AbbVie, Gilead, Janssen, and Merck during the conduct of the study.
Thomas G. Stewart, MS reports no disclosures during the conduct of the study.
Dr. Fried reports grant funding from AbbVie, Bristol-Myers Squibb, Gilead, Glaxo, Merck, Vertex, Genentech/Roche and consultant funding from Genentech/Roche, Tibotec/Janssen, Vertex, Merck, Glaxo, Novartis, AbbVie, Gilead, Bristol-Myers Squibb during the conduct of the study along with funding from the NIH for research.
Dr. Nelson reports grant funding from AbbVie, Gilead, BMS, Janssen, Merck, Vertex, and GSK during the conduct of the study.
Dr. Jacobson reports grant funding from AbbVie, BMS, Gilead, Janssen, Merck, and Tobira during the conduct of the study. He reports consultant and advisor funding from AbbVie, Achillion, Alnylam, BMS, Enanta, Gilead, Janssen, Merck during the time of the study. He also reports funding from AbbVie, BMS, Gilead, and Janssen for Speakers’ Bureau.
References
- 1.Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nat Med. 2013;19:850–858. doi: 10.1038/nm.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin.Gastroenterol.Hepatol. 2011;9:509–516. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370–378. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 6.Kramer JR, Kanwal F, Richardson P, et al. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J.Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J.Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melia MT, Muir AJ, McCone J, et al. Racial Differences in Hepatitis C Treatment Eligibility. Hepatology. 2011;54:70–78. doi: 10.1002/hep.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 11.Hagan LM, Sulkowski MS, Schinazi RF. Cost Analysis of Sofosbuvir/Ribavirin Versus Sofosbuvir/Simeprevir for Genotype 1 Hepatitis C Virus in Interferon-Ineligible/Intolerant Individuals. Hepatology. 2014;60:37–45. doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 13.Panel AIHG. Hepatitis C Guidance: AASLD-IDSA Recommendations for Testing, Managing, and Treating Adults Infected with Hepatitis C Virus. Hepatology. 2015 doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 14.Gordon SC, Muir AJ, Lim JK, et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol. 2015;62:286–293. doi: 10.1016/j.jhep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hezode C, Fontaine H, Dorival C, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132–142. doi: 10.1053/j.gastro.2014.03.051. e4. [DOI] [PubMed] [Google Scholar]
- 16.Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 18.Boursier J, Zarski JP, de LV, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 19.Kwo P, Gitlin N, Nahass R, et al. Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver. Vienna, Austria: 2015. Apr 22–26, A phase 3, randomised, open-label study to evaluate the efficacy and safety of 12 and 8 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naive and -experienced patients with chronic HCV genotype 1 infection without cirrhosis: OPTIMIST-1. Abstract LB14. [Google Scholar]
- 20.Lawitz E, Matusow G, DeJesus E, et al. Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver. Vienna, Austria: 2015. Apr 22–26, A phase 3, open-label, single-arm study to evaluate the efficacy and safety of 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naive or –experienced patients with chronic HCV genotype 1 infection and cirrhosis: OPTIMIST-2. Abstract LP04. [Google Scholar]
- 21.Manns MP, McCone J, Davis MN, et al. Overall safety profile of boceprevir plus peginterferon alfa-2b and ribavirin in patients with chronic hepatitis C genotype 1: a combined analysis of 3 phase 2/3 clinical trials. Liver International. 2014;34:707–719. doi: 10.1111/liv.12300. [DOI] [PubMed] [Google Scholar]
- 22.Park C, Jiang S, Lawson KA. Efficacy and safety of telaprevir and boceprevir in patients with hepatitis C genotype 1: a meta-analysis. Journal of Clinical Pharmacy and Therapeutics. 2014;39:14–24. doi: 10.1111/jcpt.12106. [DOI] [PubMed] [Google Scholar]
- 23.OLYSIO® prescribing information. Janssen Therapeutics, Division of Janssen Products, LP. Titusville NJ: 08560. [Google Scholar]
- 24.Sullivan JC, De Meyer S, Bartels DJ, et al. Evolution of treatment-emergent resistant variants in telaprevir phase 3 clinical trials. Clin Infect Dis. 2013;57:221–229. doi: 10.1093/cid/cit226. [DOI] [PubMed] [Google Scholar]
- 25.Romano KP, Ali A, Aydin C, et al. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS.Pathog. 2012;8:e1002832. doi: 10.1371/journal.ppat.1002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 27.Curtis L, Hammill B, Eisenstein E, Kramer JM, Anstrom KJ. Using Inverse Probability-Weighted Estimators in Comparative Effectiveness Analyses With Observational Databases. Medical Care. 2007;45(10 Suppl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 28.White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980;48(4):817–838. [Google Scholar]
- 30.Andridge RR, Little RJA. A Review of Hot Deck Imputation for Survey Non-response. Internation statistical review. 2010;78(1):40–64. doi: 10.1111/j.1751-5823.2010.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little RJA, Rubin D. Statistical Analysis with Missing Data. 2nd edition. John Wiley & Sons; 2002. [Google Scholar]