Abstract
Background
Corticotropin-releasing factor (CRF) mediates our body’s overall responses to stress. The role of central CRF in stress-stimulated colonic motility is well characterized. We hypothesized that transient perturbation in expression of enteric CRF is sufficient to change stress-induced colonic motor and secretory responses.
Methods
Sprague-Dawley rats (adult, male) were subjected to 1-h partial restraint stress (PRS) and euthanized at 0, 4, 8, and 24 h. CRF mRNA and peptide levels in the colon were quantified by real-time RT-PCR, enzyme immunoassay, and immunohistochemistry. Double-stranded RNA (dsRNA) designed to target CRF (dsCRF) was injected into the colonic wall to attain RNA interference (RNAi)-mediated inhibition of CRF mRNA expression. DsRNA for β-globin was used as a control (dsControl). Four days after dsRNA injection, rats were subjected to 1-h PRS. Fecal output was measured. Ussing chamber techniques were used to assess colonic mucosal ion secretion and transepithelial tissue conductance.
Key Results
Exposure to PRS elevated CRF expression and increased CRF release in the rat colon. Injection of dsCRF inhibited basal CRF expression and prevented the PRS-induced increase in CRF expression, whereas CRF expression in dsControl-injected colons remained high after PRS. In rats treated with dsControl, PRS caused a significant increase in fecal pellet output, colonic baseline ion secretion, and transepithelial tissue conductance. Inhibition of CRF expression in the colon prevented PRS-induced increase in fecal output, baseline ion secretion, and transepithelial tissue conductance.
Conclusions & Inferences
These results provide direct evidence that transient perturbation in peripherally expressed CRF prevents colonic responses to stress.
Keywords: stress, colon, CRF, motility, ion secretion
INTRODUCTION
Corticotropin releasing factor (CRF) is important for our body’s overall responses to stress.(1) Besides CRF, three other CRF-related peptides, urocortin (Ucn)1, Ucn2, and Ucn3, have been identified in mammals.(2–4) CRF and Ucns exert their biological actions via two CRF receptor subtypes, CRF1 and CRF2.(5) CRF signaling in the brain has been known to mediate colonic responses to stress. Exposure to an array of acute stressors stimulates colonic motor activity.(6–12) Intracerebral or intraventricular administration of CRF or Ucn1 simulates the effects of acute stress by enhancing colonic motility.(6,7,13–15) Intracerebral injection of non-selective peptide CRF receptor antagonists or selective CRF1 antagonists inhibits the effects of both acute stress and CRF/Ucn1 on colonic motor activity.(6,7,11,13–16) However, intracerebral administration of the CRF2-specific peptide antagonists does not inhibit stress or CRF-induced acceleration of colonic motility and transit.(11) These findings implicate the central CRF/CRF1 signaling pathway in mediating the colonic motor responses to acute stress.
Besides the brain, CRF peptides and CRF receptors are also found in the gut. We recently reported the expression of CRF/UCNs and their receptors in the enteric nervous system (ENS) of the rat and guinea pig gastrointestinal tract.(17–21) In guinea pig, the numbers of enteric neurons that express immunoreactivity for CRF increase progressively in the aboral direction, with the lowest numbers found in the stomach and the largest numbers in the distal colon.(18) CRF and Ucn1 cause excitation of myenteric neurons via activating CRF1.(17,19,22) CRF, CRF1, and CRF2 are also expressed in enterochromaffin cells, mononuclear cells, macrophages, and mast cells in the colon.(23–30) When injected peripherally, CRF enhances colonic motility in rodents and humans.(31–34) Peripherally injected non-selective peptide CRF receptor antagonists or selective CRF1 antagonists block the stimulation of colonic motility induced by acute stress or peripheral injection of CRF.(6,32,34) Peripheral activation of CRF2 by intraperitoneal (ip) injection of Ucn2 reduces acute stress or CRF-induced defecation and colonic motility in rodents; whereas ip injection of astressin2-B, a peptide CRF2 antagonist, enhances stress-induced increase in colonic motility.(35) These observations suggest that activation of peripheral CRF1 contributes to stress-related stimulation of colon motility, whereas activation of peripheral CRF2 counteracts the CRF1-mediated stimulation of colonic motility and initiates a stress coping response in rodents.
Stress greatly enhances intestinal ion secretion and disrupts intestinal epithelial barrier. Psychological stress has been found to increase Na+ and Cl− secretion and reduce H2O absorption in human jejunum.(36) Restraint stress has been found to induce watery diarrhea in rats.(37) Similarly, ip injection of CRF increases colonic baseline ion secretion and evokes watery diarrhea in rats, which is mimicked by peripheral injection of cortagine, a selective CRF1 agonist,(38) and antagonized by the selective CRF1 antagonists.(39,40) Peripherally applied CRF increases intestinal epithelial permeability for large organic molecules in human colon mucosal biopsies.(41) Pretreatment with α-helical CRF9–41, a non-selective peptide CRF receptor antagonist, abolished the actions of CRF on colonic mucosal permeability.(41)
The evidence implicates that CRF signaling takes place locally in the gut and affects colonic motility and secretion. However, no study has been done to determine the role of endogenous CRF in the gut in colonic responses to stress. We therefore aimed to investigate if transient perturbation in expression of endogenous CRF in the gut is sufficient to change stress-induced colonic motor and secretory responses. Preliminary reports of the results have been published in abstract form.(42,43)
MATERIALS AND METHODS
Animals
Adult Sprague-Dawley rats (male, 250–275 g) were purchased from Charles River, Wilmington, MA, USA, housed in the University of Wisconsin-La Crosse and/or University of California San Francisco (UCSF) animal facility at 22°C with a 12-h light/12-h dark cycle, and had free access to food and tap water. The animal care and experimental protocols were approved by the Laboratory Animal Care and Use Committees of University of Wisconsin-La Crosse and UCSF and complied with the Guide for the Care and Use of Laboratory Animals by U. S. National Institutes of Health.
Partial restraint stress protocol
Rats were lightly anesthetized with isoflurane prior to partial restraint stress (PRS). The movements of the rats were restricted by wrapping the adhesive tapes around their upper forelimbs, shoulders, and thoracic trunks.(6) Control animals were anesthetized but not wrapped to allow free movement after recovering from anesthesia. The PRS procedures were carried out between 9:00–11:00 AM on the days of the experiment to avoid the influence of circadian rhythm. After 1 h PRS, the fecal pellets excreted by the rats were collected. The rats were then euthanized by CO2 inhalation at different time points (0, 4, 8, and 24 h) after PRS (Fig. 1, inset). The distal colons were taken out of the abdominal cavity and flushed with chilled Krebs solution containing: NaCl (120.9 mM); KCl (5.9 mM); MgCl2 (1.2 mM); NaH2PO4 (1.2 mM); NaHCO3 (14.4 mM); CaCl2 (2.5 mM); and glucose (11.5 mM). The colonic specimens were then used for real-time reverse transcription-polymerase chain reaction (RT-PCR), enzyme immunoassay, and immunohistochemistry according to the following procedures.
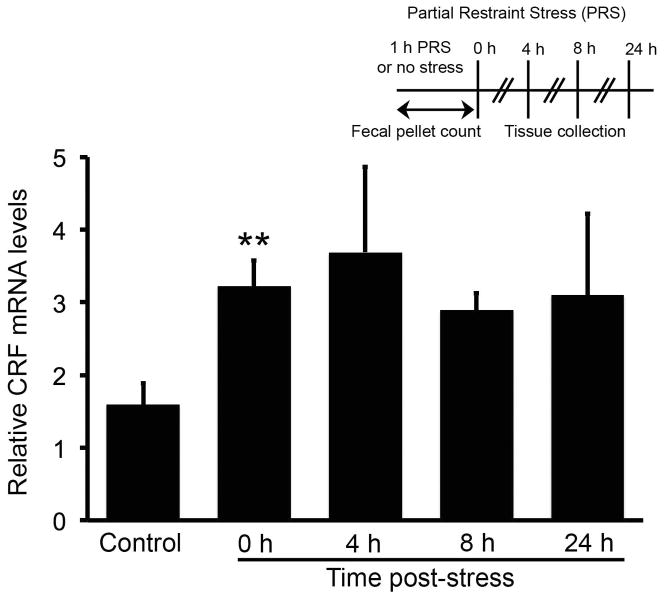
Fig. 1.
Time course of the effects of partial restraint stress on CRF mRNA levels in the rat colon. Real-time RT-PCR was performed to investigate the effect of partial restraint stress (PRS) on CRF mRNA expression in the rat colon. PRS increased CRF mRNA levels immediately after termination of PRS (0 h, P < 0.01). While CRF mRNA levels remained high at other time points examined, they did not attain statistical significance. N = 5–9/group. PRS and time points for tissue collection are illustrated in the inset.
Real-time RT-PCR
CRF mRNA relative expression in the rat colon was measured by real-time RT-PCR. Total RNA was extracted from the rat colon using the Absolutely RNA Miniprep Kit (Agilent Technologies, La Jolla, CA, USA) and was used to synthesize the first-strand cDNA by utilizing the AffinityScript QPCR cDNA Synthesis Kit (Agilent Technologies). Real-time PCR was implemented using a Brilliant® SYBR® Green QPCR Master Mix (Agilent Technologies) on a LightCycler Real-Time PCR machine (Roche Diagnostics, Indianapolis, IN, USA). Primers for CRF were designed according to the rat CRF mRNA sequence (NCBI Reference Sequence: NM_031019.1): 5′-TCT CTG GAT CTC ACC TTC CAC CTT-3′ (forward) and 5′-AGT TTC CTG TTG CTG TGA GCT TGC-3′ (reverse, with estimated PCR product size 92 bp). Expression of β-actin (a house-keeping gene) was measured for every sample in parallel with CRF for data normalization. Primers for β-actin were the same ones used in a previous study.(44) The corresponding no-RT RNA samples were included as negative controls. Data were analyzed using the 2−ΔΔCT method.(45)
Enzyme immunoassay
This assay was used to measure CRF peptide levels and CRF release from the rat colon. Segments of rat colon were homogenized in Tris buffer (50 mM, pH 7.5), NaCl (150 mM), phenylmethanesulfonyl fluoride (PMSF, 0.1 mM), aprotinin (2.8 μg/ml), and tablets of complete protease inhibitor cocktail (Roche Diagnostics). The homogenates were centrifuged (10,000g, 5 min, 4°C) and the supernatants were used to determine CRF peptide levels by using a commercial CRF enzyme immunoassay kit (Phoenix Pharmaceuticals, Belmont, CA, USA). To measure CRF release, segments of rat colon were incubated in 1.5 ml micro-centrifuge tubes containing 500 μl preoxygenated Kreb’s solution with PMSF (0.1 mM), aprotinin (2.8 μg/ml), and bovine serum albumin (2.5 μg/ml) at 37°C for 30 min. At the end of the incubation, the micro-centrifuge tubes were vortex mixed and centrifuged (3,000g, 10 min, room temperature). The supernatants were recovered and used for CRF enzyme immunoassay.
Immunohistochemistry
The whole-mount preparations of colonic myenteric and submucosal plexuses were treated with colchicine before fixation to enhance CRF immunoreactivity in the neuronal cell bodies according to previously described method.(18) The approximate dimension of the whole-mounts was 1.0 cm (length) × 1.0 cm (width). After 24-h incubation in colchicine, the preparations were washed with Krebs solution and fixed in Zamboni’s fixative (3 h, room temperature). The preparations were then washed in phosphate buffered saline (PBS) three times, 10 min each and used for immunofluorescence staining for CRF according to the method described previously.(18) CRF antibody (rabbit, 1:3,000, PBLrC70, Gift of W. Vale) specificity was tested previously.(18,46) Immunofluorescence labeling was visualized using a Nikon 80i fluorescence microscope. Numbers of CRF- immunoreactive (CRF-IR) neurons were counted from 30 ganglia in each whole-mount preparation from each animal, and five animals in each treatment group were studied. The 30 ganglia were selected randomly from the whole-mount preparation at six different locations, two on the left, two in the middle, and two on the right, to minimize the influence of ganglionic size on neuronal counting.
Transmural injection of dsRNA
Rats were anesthetized and their abdominal walls opened. The cecum and colon were exposed, and a suture was put in the muscular layer at a site 5 cm distal to the ileocecal junction to serve as a reference for double stranded RNA (dsRNA) injection sites. DsRNA for CRF (dsCRF) or β-globin (as a control dsRNA, dsControl) was mixed with Lipofectamine 2000 and injected into the muscularis externa at two sites (15 μg/site in 125 μl), 1.5 cm proximal and distal to the reference suture. We have earlier characterized the time course and spatial spread of dsRNA-mediated RNA interference (RNAi) effects in detail elsewhere.(47) As the colonic mucosa sheds every 3–5 days, the effect of dsRNA injection is most pronounced in the enteric neurons and spreads about 6 cm-length of the colon. Synthesis of dsRNAs for CRF and β-globin has been described previously.(48,49) The abdominal incision was closed with sutures, and the rats were monitored daily for four days following surgery. The total fecal pellets passed out over 24 h were collected the day before dsRNA injection and for four consecutive days following dsRNA injections. The 24-h fecal pellet outputs in rats after dsRNA injection were similar to the levels observed before surgery, thereby suggesting that rats did not show any overt signs of post-operative ileus after injection of dsControl or dsCRF.
Measurement of colonic motility and secretion
Four days after dsRNA injection, half of the rats in each group (n = 5) were subjected to a 1-h PRS as described above while the other half were not stressed. The fecal pellets expelled during the 1-h PRS were collected. The rats were euthanized and a 2-cm colonic segment in between the two-dsRNA injection sites were removed and used for real-time RT-PCR and immunofluorescence staining to confirm CRF knockdown. An adjacent 1-cm colonic segment was used to measure colonic secretion using the Ussing chamber techniques. The mucosa/submucosa preparations were obtained by removing the serosa and muscularis externa layers and subsequently mounted in modified Ussing chambers (cross-sectional area 0.5 cm2). The Ussing chambers were filled with 5 ml of Krebs solution, which is oxygenated with 95% O2 and 5% CO2 and maintained at 37°C by a temperature-controlled water bath. Transepithelial potential difference for each mucosa/submucosa preparation was measured by a pair of Ag/AgCl pellet electrodes (P2020S; Physiologic Instruments, San Diego, CA, USA) connected to a VCC MC6 multichannel voltage/current clamp (Physiologic Instruments). A pair of Ag wire electrodes were connected to the voltage/current clamp to inject short-circuit current (ISC) in order to maintain a zero transepithelial potential difference across the mucosa/submucosa preparation. The baseline ISC was expressed as current per unit area of tissue (μA/cm2) and used as an indicator of colonic secretory activity. Transepithelial conductance was determined by pulsing a small command voltage (2.5 mV) in voltage clamp mode and recording the change in the Isc, and calculated according to Ohm’s law (transepithelial conductance = ΔIsc/2.5 mV).
Statistical analysis
Data were shown as means ± SEM; n values represent the number of animals in each group. Student’s t-test or one-way/two-way ANOVA was used to determine statistical significance. Tukey’s HSD was used for post hoc test when statistical significance was found in one-way or two-way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Partial restraint stress increases CRF expression in the rat colon
Real-time RT-PCR was used to investigate the effect of PRS on CRF mRNA expression in the rat colon. CRF mRNA levels in the colon increased by two-fold immediately after PRS (control: 1.59 ± 0.30, n = 9; PRS: 3.22 ± 0.36, n = 5; P < 0.01), whereas the mRNA levels tended to remain high 4–24 h post PRS (Fig. 1).
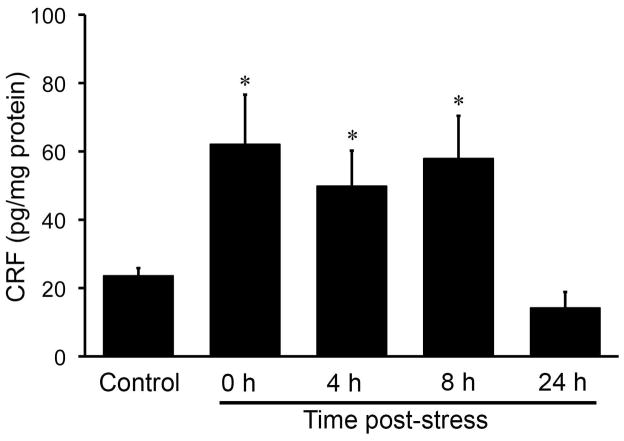
Next, we determine whether PRS increases CRF peptide levels. CRF peptide concentrations in the rat colon were measured by enzyme immunoassay. PRS increases CRF peptide concentrations in the colon compared with non-stressed controls (Fig. 2). CRF peptide levels in the rat colon were 2.6-fold higher immediately after stress was terminated (control: 23.58 ± 2.28 pg/mg protein, n = 10; PRS: 62.06 ± 14.55 pg/mg protein, n = 5; P < 0.05), continued elevated for 8 h (57.89 ± 12.51 pg/mg protein, n = 5; P < 0.05), and fell back to control levels 24 h post-stress (14.16 ± 4.72 pg/mg protein, n = 5; P > 0.05) (Fig. 2).
Fig. 2.
Time course of the effects of partial restraint stress on CRF peptide levels in the rat colon. Enzyme immunoassay was performed to determine CRF peptide levels in the rat colon. CRF peptide level was significantly elevated immediately after 1 h of PRS, remained at a higher level 8 h after stress, and returned to control level 24 h after stress. N = 5–10/group; * P < 0.05 compared to control.
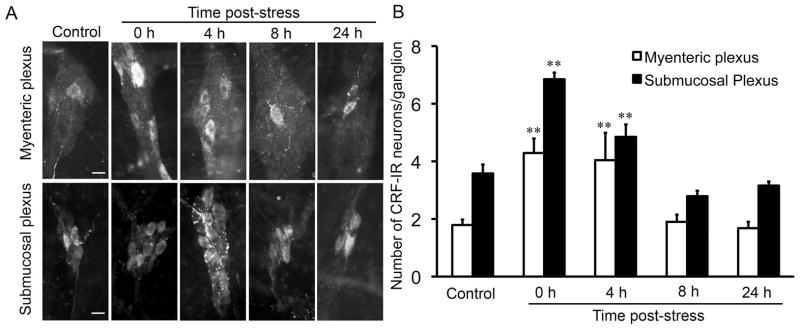
CRF immunoreactivity was found in the soma and nerve fibers of enteric neurons in both the myenteric and submucosal plexuses of the rat colon, consistent with previous reports.(18,29,50,51) In response to PRS, the number of CRF-IR neurons was increased immediately in both the myenteric (control: 1.77 ± 0.14 neurons/ganglion, n = 8; PRS: 4.33 ± 0.41 neurons/ganglion, n = 6; P < 0.01) and submucosal plexuses (control: 3.46 ± 0.19 neurons/ganglion, n = 8; PRS: 6.73 ± 0.22 neurons/ganglion, n = 6; P < 0.01) after stress was terminated (Fig. 3A, B). The number of CRF-IR neurons fell back to control levels 8 h post- stress (1.90 ± 0.25 neurons/myenteric ganglion, n = 5, P > 0.05; 2.79 ± 0.19 neurons/submucosal ganglion, n = 5, P > 0.05) (Fig. 3A, B).
Fig. 3.
Time course of the effects of partial restraint stress on the number of CRF-immunoreactive neurons in the enteric nervous system of the rat colon. Immunofluorescence staining was used to determine the number of CRF-immunoreactive (CRF-IR) neurons in the myenteric and submucosal plexuses of the rat colon. (A) Representative images showing the effects of PRS on the number of CRF-IR neurons in the enteric nervous system. In control rats, only one or two CRF-IR neurons were found in each myenteric or submucosal ganglion. Immediately after PRS, the number of CRF-IR neurons/ganglion increased significantly. The increase in CRF-IR neurons lasted for 4 h and returned to normal levels after 8 h. (B) Bar graph showing the effects of PRS on CRF-IR neurons. Scale bar = 20 μm. N = 5–8/group; ** P < 0.01 compared to control.
Partial restraint stress increases CRF release from the rat colon
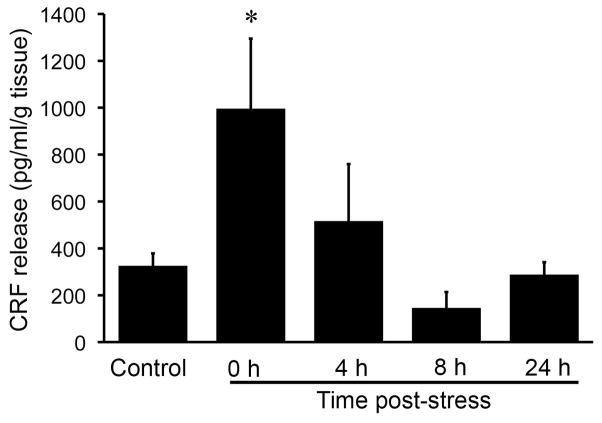
To investigate the effect of PRS on CRF release from the rat colon, CRF accumulation in the supernatant of rat colon specimens after PRS was measured by enzyme immunoassay. PRS significantly increased CRF concentrations in the supernatant. CRF concentrations in the supernatant were 3-fold higher immediately after stress was terminated (control: 325.43 ± 53.26 pg/g tissue, n = 10; PRS: 995.98 ± 299.05 pg/g tissue, n = 5; P < 0.05) and returned to control levels 4 h post-stress (516.51 ± 243.17 pg/g tissue, n = 5; P > 0.05) (Fig. 4).
Fig. 4.
Partial restraint stress increases CRF release from rat colon. CRF accumulation in the supernatant of rat colon specimens after 1 h of PRS was measured by enzyme immunoassay as CRF release. PRS significantly increased CRF release from the rat colon. CRF release returned to control levels 4 h post-stress. N = 5–10/group; * P < 0.05 compared to control.
Transmural injection of CRF dsRNA in the colon knocks down CRF expression and prevents partial restraint stress-induced increase in CRF expression
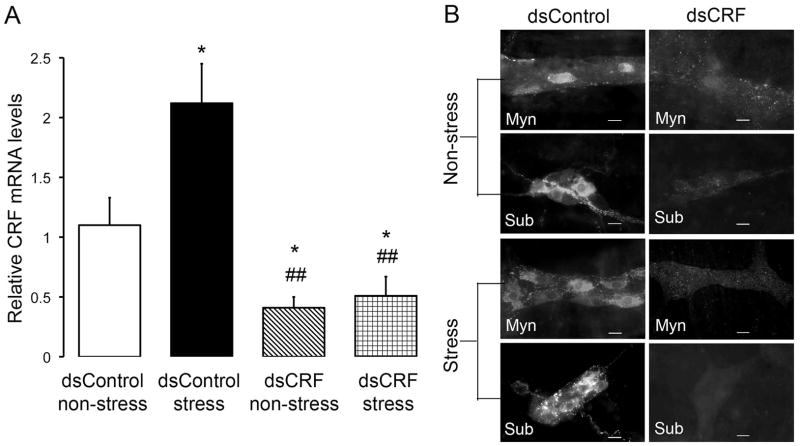
To ascertain whether transient perturbation in expression of endogenous CRF in the gut is sufficient to change stress-induced colonic motor and secretory responses, we silenced CRF expression in the rat colon by transmural injection of CRF dsRNA, which led to RNAi-mediated degradation of CRF mRNA. In dsControl-treated rats, PRS caused a two-fold increase in CRF mRNA levels in the rat colon (dsControl non-stress: 1.10 ± 0.23, n = 5; dsControl stress: 2.12 ± 0.33, n = 5, P < 0.05; Fig. 5A). Injection of dsCRF into the colonic muscularis externa suppressed basal CRF mRNA expression by 63% (dsCRF non-stress: 0.41 ± 0.09, n = 5; P < 0.05 compared to dsControl non-stress) and prevented PRS-induced increase in CRF mRNA level (dsCRF stress: 0.51 ± 0.16, n = 5; P > 0.05 compared to dsCRF non-stress) (Fig. 5A). In addition to suppressing CRF mRNA expression, dsCRF effectively silenced CRF peptide expression in the rat colon as assessed by immunohistochemistry. In dsControl-treated rats, the number of CRF-IR neuronal cell bodies was relatively small in the colonic myenteric (1.79 ± 0.19 neurons/ganglion; n = 5) and submucosal plexuses (3.58 ± 0.31 neurons/ganglion; n = 5). PRS increased the number of CRF-IR neurons in both the myenteric (4.29 ± 0.50 neurons/ganglion, n = 5; P < 0.05) and submucosal plexuses (6.85 ± 0.23 neurons/ganglion, n = 5; P < 0.05) (Fig. 5B). Treatment with dsCRF effectively achieved a colon-specific suppression of CRF immunoreactivity and prevented PRS-induced increase of CRF immunoreactivity. CRF immunoreactivity was very low or undetectable in myenteric and submucosal neurons in dsCRF-treated rats (Fig. 5B).
Fig. 5.
Inhibition of CRF expression by in vivo RNAi in the rat colon. Rats were treated with long dsRNA for CRF (dsCRF) or β-globin (dsControl). Four days after dsRNA injection, half of the rats in each group (n = 5) were subjected to 1 h PRS, while the other half were kept in their cages without stress. Rats were euthanized immediately after stress. (A) Real-time RT-PCR revealed that local dsCRF injection effectively inhibited expression of basal CRF mRNA and prevented PRS-induced increase in CRF mRNA levels. Local injection of dsControl did not prevent PRS-induced increase in CRF mRNA levels. (B) Representative images showing that local injection of dsCRF effectively silenced CRF immunoreactivity in the myenteric and submucosal plexuses in the rat colon and prevented PRS-induced increase in CRF immunoreactivity. Scale bar = 20 μm. N = 5/group; * P < 0.05 compared to dsControl non-stress; ## P < 0.01 compared to dsControl stress.
Transmural injection of CRF dsRNA in the colon has no effect on basal fecal pellet output
Knockdown of endogenous CRF expression in the colon did not affect daily fecal pellet output as measured for four consecutive days following dsRNA injection (Fig. S1). The total wet weight, dry weight, and water content of the fecal matter were similar in dsControl and dsCRF groups (data not shown).
Transmural injection of CRF dsRNA in the colon prevents partial restraint stress-stimulated fecal output
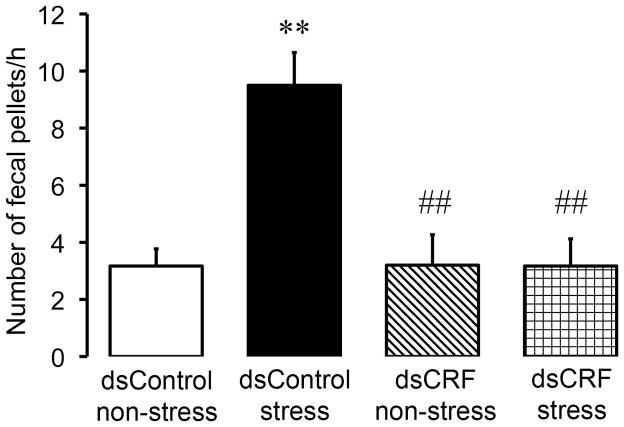
Control dsRNA-treated rats expelled 3.17 ± 0.60 fecal pellets (n = 5) during the 1-h observation period (Fig. 6). When dsControl-treated rats were exposed to PRS, the number of fecal pellets excreted increased significantly during the 1-h PRS compared with non-stressed control rats (9.50 ± 1.15 fecal pellets/h; n = 5; P < 0.01) (Fig. 6). Local injection of dsCRF in the colon did not change fecal pellet output in non-stressed rats (3.20 ± 1.07 fecal pellets/h; P > 0.05 compared with dsControl non-stress). As predicted, dsCRF treatment effectively prevented the PRS-induced increase in fecal excretion (3.17 ± 0.95 fecal pellets/h; P < 0.01 compared with dsControl stress and P > 0.05 compared with dsControl non-stress) (Fig. 6).
Fig. 6.
Inhibition of CRF expression in the rat colon prevents partial restraint stress-stimulated increase in fecal pellet output. Control dsRNA-treated rats defecated only occasionally during the 1-h observation period. Exposure to PRS for 1 h caused significantly increase in the number of fecal pellets excreted compared with non-stressed dsControl rats. Local injection of dsCRF in the colon did not change fecal pellet output in non-stressed rats, but effectively prevented PRS-induced increase in fecal excretion. N = 5/group; ** P < 0.01 compared to dsControl non-stress; ## P < 0.01 compared to dsControl stress.
Transmural injection of CRF dsRNA in the colon prevents partial restraint stress- induced increase in colonic ion secretion
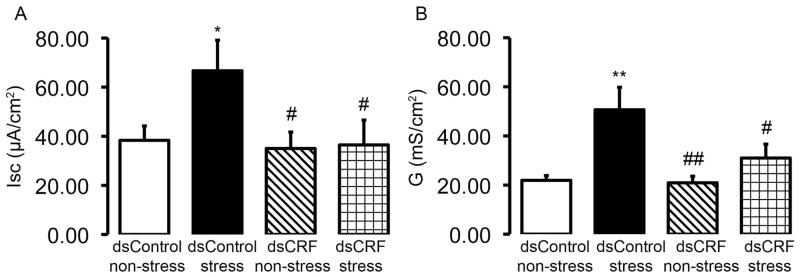
To test if transient perturbation in expression of endogenous CRF in the gut prevented stress-induced changes in colonic epithelial function, we measured baseline ISC and transepithelial tissue conductance four days post dsRNA injection. PRS induced a 1.7-fold increase in baseline ISC (dsControl non-stress: 38.33 ± 5.86 μA/cm2, n = 5; dsControl stress: 66.67 ± 12.45 μA/cm2, n = 5, P < 0.05 compared to dsControl non-stress) (Fig. 7A) and a 2.3-fold increase in transepithelial tissue conductance (dsControl non-stress: 21.92 ± 1.93 mS/cm2, n = 5; dsControl stress: 50.66 ± 9.15 mS/cm2, n = 5, P < 0.01 compared to dsControl non-stress) (Fig. 7B) in colonic mucosa from dsControl-treated rats. Local injection of dsCRF in the colon effectively prevented the PRS-induced increase in baseline ISC (dsCRF non-stress: 35.00 ± 6.72 μA/cm2, n = 5; dsCRF stress: 36.46 ± 10.12 μA/cm2, n = 5, P > 0.05 compared to dsCRF non-stress) (Fig. 7A) and transepithelial tissue conductance (dsCRF non-stress: 20.92 ± 2.67 mS/cm2, n = 5; dsCRF stress: 30.99 ± 5.67 mS/cm2, n = 5, P > 0.05 compared to dsCRF non-stress) (Fig. 7B).
Fig. 7.
Inhibition of CRF expression in the rat colon prevents partial restraint stress-induced increase in colonic ion secretion. Colonic ion secretion and transepithelial conductance were measure by the Ussing chamber technique. Colonic mucosa/submucoa preparations from dsControl-treated rats that were exposed to PRS had significantly elevated baseline ISC (A) as well as significantly elevated transepithelial tissue conductance (B). Local injection of dsCRF in the colon effectively prevented the PRS-induced increase in baseline ISC (A) and transepithelial tissue conductance (B). N = 5/group; * P < 0.05, ** P < 0.01 compared to dsControl non-stress; # P < 0.05, ## P < 0.01 compared to dsControl stress.
DISCUSSION
CRF signaling in the brain, in relation to stress-induced modification of gastrointestinal motility and mucosal secretion, has been extensively studied and documented.(6,7,11,13–15) Alteration of gastrointestinal functions by CRF and CRF receptor agonists and antagonists, when administrated in the periphery either by intravenous or intraperitoneal route, has been systematically described as well.(31–34) Nevertheless, prior to our study, the role of endogenous CRF in the gut in stress-induced changes in colonic motility and secretory activity had not received systematic study.
In view of current concepts for CRF signaling in the brain as well as in the ENS, we thought that endogenous CRF in the gut plays a critical role in stress-induced changes in colonic motor and secretory functions. The following observations from the present study support our hypothesis: 1) partial restraint stress disturbs colonic motility and mucosal secretion; 2) upregulation of CRF mRNA occurs in the colon during partial restraint stress and not in non-stressed controls; 3) concentrations of CRF peptide inside the colon become elevated during partial restraint stress and remain unchanged in non-stressed controls; 4) numbers of CRF-IR neuronal cell bodies increase in the myenteric and submucosal plexuses in stressed rats, but remain unchanged in non-stressed controls; 5) larger amounts of CRF is released from inside the colon of stressed rats as compared with amounts released from non-stressed controls; 6) transient knockdown of CRF expression in the rat colon is sufficient to prevent the effects of stress on colonic motility and mucosal secretion. Our results suggest that enteric CRF has a critical role in mediating colonic motor and secretory responses to stress.
Partial restraint stress stimulates intramural CRF production
We reported earlier that ENS neurons express CRF and its two receptor subtypes.(17–21) We found, in the present study, that exposure to partial restraint stress resulted in upregulation of intramural CRF, which was evidenced as elevated expression of CRF mRNA and associated synthesis of the peptide. Immunohistochemistry showed that the observed upregulation of CRF peptide took place in ENS neurons and most likely also in other kinds of intramural cells, such as enteric immune cells and mucosal enterochromaffin cells. The stress-induced increase in CRF mRNA and peptide levels was found to be variable in their individual time courses over the 24 h span (see Figs. 1–3). Nevertheless, recovery to control levels was complete at 24 h post stress, suggesting that the effect of acute stress on enteric CRF levels is transient.
We confirmed that cells in the colon, which may be ENS neurons or populations of other cell types, could release their CRF into the extracellular milieu in response to stress (see Fig. 4). CRF, once released, acts at the CRF1 receptor subtype on adjacent ENS neurons and causes membrane depolarization and action potential discharge in these neurons.(17,22) Interestingly, in the guinea pig, the ENS neurons that contain CRF do not express CRF1 or CRF2 receptors.(18) Instead, excitatory CRF1 receptors are present in ENS neurons adjacent to the CRF population. This indicates that CRF-containing neurons are not synaptically interconnected one-with-the-other in the ENS microcircuits. Instead, CRF immunoreactivity is found in presynaptic varicose terminals surrounding non-CRF-IR cell bodies in both the myenteric and submucosal plexuses. (18) CRF released from these nerve terminals is expected to activate CRF1 receptors expressed by neurons identified by one or the other of neurochemical codes, including choline acetyltransferase and calbindin in the myenteric plexus, and choline acetyltranferase, calbindin, substance P, and neuropeptide-Y in the submucosal plexus.(17) Actions of enteric CRF on ENS neurons might contribute to stress-stimulated colonic propulsive motor function and mucosal secretion.
Aside from release at neuron-neuron synapses, ENS neurons also release CRF at junctions with enteric mast cells. Activation of CRF receptors on mast cells causes mast cell degranulation and release of histamine, mast cell proteases, and other inflammatory mediators,(30,52,53) which implicates CRF as a major factor in neuro-immune and neuro-inflammatory interactions in the gastrointestinal tract.(49,54)
Knockdown of CRF in the colon prevents stress-induced increase in colonic motility and mucosal secretion
Results of our study show that CRF production and release in the colon are associated with stress-induced functional changes that include facilitated large intestinal transit, elevated ion and H2O secretion, and increased colonic mucosal permeability. Our finding that transient perturbation in expression of endogenous CRF in the gut prevents these stress-induced functional changes is strong evidence that peripheral CRF is key to understanding the changes in intestinal physiology that occur during stress. Stress-induced production of CRF in the ENS networks of the colon, like in circuitry of the brain,(55–57) is the possible initiating factor in the switch from normal to pathological behavior that occurs when an animal encounters adverse circumstances. However, it remains unclear as to mechanisms that connect the perception of detrimental stress by the conscious brain with activation of CRF production in the large intestine.
In future studies, it would be interesting to determine if transient perturbation in CRF expression alters gut function over long-term, or does function return to normal once CRF expression returns to baseline. If function does not recover, despite CRF expression returning to baseline, it would suggest that transient perturbation in CRF can have long-lasting effect on ENS plasticity and would require detailed investigation. Other studies that examined long-term recovery of pain behavior (58) or gut motility function (47) after RNAi suggest that RNAi effects are transient and long-term effects are unlikely.
Conclusion
Exposure to acute partial restraint stress enhances CRF expression and increases CRF release in the rat colon. Acute stress-evoked activation of CRF expression initiates a cascade of pathophysiological events that culminate in rapid fecal transit, diarrhea, and breach of the colonic epithelial barrier to luminal contents. Elevated CRF expression and its release in the colon underlie acute stress-induced abnormality in colonic motility, mucosal secretion, and epithelial barrier function.
Supplementary Material
Supplementary Fig. S1. Inhibition of CRF expression in the rat colon has no effect on basal fecal pellet output. Fecal pellets were counted daily for up to four days after dsRNA treatment. Knockdown of endogenous CRF expression in the rat colon did not affect daily fecal pellet output under non-stressed situation. N = 5/group.
Key Messages.
The role of central CRF in stress-stimulated large intestinal motility is well characterized, but the role of peripherally synthesized CRF in the colon in regulating colonic functions remains ambiguous.
We aimed to test a hypothesis that transient perturbation in expression of endogenous CRF in the gut is sufficient to change stress-induced colonic motor and secretory responses.
Double-stranded RNA (dsRNA) targeted to CRF (dsCRF) was injected into the rat colonic wall to attain RNAi-mediated inhibition of CRF expression. Rats were subjected to 1-h partial restraint stress four days after dsRNA injection.
Partial restraint stress elevated CRF expression and increased its release in the rat colon. Inhibition of CRF expression in the rat colon prevented the partial restraint stress-induced elevation of CRF expression and increase in fecal output, colonic ion secretion, and transepithelial tissue conductance, suggesting that peripherally synthesized CRF in the large intestine played a critical role in colonic responses to stress.
Acknowledgments
The authors thank Pamela Derish, Department of Surgery, UCSF, for critical reading of the manuscript.
FUNDING
This work was supported by grants from the National Institute of Health, USA, R15DK097460-01A1 (S. Liu) and R01DK080787 (A. Bhargava). Dr. S. Liu received a research starter award from the Pharmaceutical Research and Manufacturing (PhRMA) Foundation of America, a seed grant award from the University of North Carolina Center for Functional Gastrointestinal and Motility Disorders, and faculty research grants from the University of Wisconsin-La Crosse.
Abbreviations
- CRF
corticotropin releasing factor
- CRF1
corticotropin releasing factor receptor 1
- CRF2
corticotropin releasing factor receptor 2
- dsRNA
double-stranded RNA
- dsControl
control double-stranded RNA
- dsCRF
double-stranded RNA for CRF
- ENS
enteric nervous system
- ip
intraperitoneal
- ISC
short-circuit current
- PBS
phosphate buffered saline
- PRS
partial restraint stress
- RNAi
RNA interference
- RT-PCR
reverse transcription-polymerase chain reaction
- Ucn
urocortin
Footnotes
DISCLOSURE
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTION
SL and AB designed the experiments, collected and analyzed the data, drafted the manuscript, and critically revised the manuscript; JC, NL, KB, GT, JB, MQ, and WR performed the experiments, collected and analyzed the data, and revised the manuscript; JDW contributed to the design of the experiments and critically revised the manuscript; SC contributed to data analysis and manuscript revision.
References
- 1.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 3.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–8. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes T, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–5. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–6. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–21. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]
- 7.Gue M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964–70. doi: 10.1016/0016-5085(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 8.Morrow NS, Garrick T. Effects of intermittent tail shock or water avoidance on proximal colonic motor contractility in rats. Physiol Behav. 1997;62:233–9. doi: 10.1016/s0031-9384(97)00108-x. [DOI] [PubMed] [Google Scholar]
- 9.Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985–90. doi: 10.1111/j.1572-0241.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto O, Niida H, Tajima K, Shirouchi Y, Masui Y, Ueda F, Kise M, Kimura K. Inhibition of stress-stimulated colonic propulsion by alpha 2-adrenoceptor antagonists in rats. Neurogastroenterol Motil. 1998;10:523–32. doi: 10.1046/j.1365-2982.1998.00127.x. [DOI] [PubMed] [Google Scholar]
- 11.Martínez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556(Pt 1):221–34. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–64. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–23. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 14.Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 1988;95:1510–7. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- 15.Martínez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–60. [PubMed] [Google Scholar]
- 16.Martínez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29–35. doi: 10.1016/s0006-8993(00)03277-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Gao X, Gao N, Wang X, Fang X, Hu H-Z, Wang G-D, Xia Y, et al. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J Comp Neurol. 2005;481:284–98. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Gao N, Hu H-Z, Wang X, Wang G-D, Fang X, Gao X, Xia Y, et al. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol. 2006;494:63–74. doi: 10.1002/cne.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Ren W, Qu M-H, Bishop GA, Wang G-D, Wang X-Y, Xia Y, Wood JD. Differential actions of urocortins on neurons of the myenteric division of the enteric nervous system in guinea pig distal colon. Br J Pharmacol. 2010;159:222–36. doi: 10.1111/j.1476-5381.2009.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang J, Adams MR, Clifton MS, Liao M, Brooks JH, Hasdemir B, Bhargava A. Urocortin 1 modulates immunosignaling in a rat model of colitis via corticotropin-releasing factor receptor 2. Am J Physiol Gastrointest Liver Physiol. 2011;300:G884–94. doi: 10.1152/ajpgi.00319.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan S, Liao M, Barkan P, Takahashi K, Bhargava A. Urocortin 3 expression at baseline and during inflammation in the colon: corticotropin releasing factor receptors cross-talk. Peptides. 2014;54:58–66. doi: 10.1016/j.peptides.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanani M, Wood JD. Corticotropin-releasing hormone excites myenteric neurons in the guinea-pig small intestine. Eur J Pharmacol. 1992;211:23–7. doi: 10.1016/0014-2999(92)90256-4. [DOI] [PubMed] [Google Scholar]
- 23.Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, et al. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580(Pt 1):347–56. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–16. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, et al. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. 1994;106:859–65. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- 26.Moss AC, Anton P, Savidge T, Newman P, Cheifetz AS, Gay J, Paraschos S, Winter MW, et al. Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2alpha. Gut. 2007;56:1210–7. doi: 10.1136/gut.2006.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, et al. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 28.Yuan P-Q, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil. 2007;19:923–36. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan P-Q, Wu SV, Wang L, Taché Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–31. doi: 10.1016/j.peptides.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X-Y, Wang G-D, Yun X, Ren W, Mikam DJ, Needleman B, Melvin WS, Wood JD. Mast cell activation by corticotropin releasing factor (CRF) in guinea pig and human intestine. Gastroenterology. 2010;138:S–620. [Google Scholar]
- 31.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–9. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–79. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 33.Martínez V, Rivier J, Taché Y. Peripheral injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks peripheral CRF- and abdominal surgery-induced delayed gastric emptying in rats. J Pharmacol Exp Ther. 1999;290:629–34. [PubMed] [Google Scholar]
- 34.Martínez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–7. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 35.Gourcerol G, Wu SV, Yuan P-Q, Pham H, Miampamba M, Larauche M, Sanders P, Amano T, et al. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140:1586–96. e6. doi: 10.1053/j.gastro.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barclay GR, Turnberg LA. Effect of psychological stress on salt and water transport in the human jejunum. Gastroenterology. 1987;93:91–7. doi: 10.1016/0016-5085(87)90319-2. [DOI] [PubMed] [Google Scholar]
- 37.Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: a comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterol Motil. 2008;20:557–65. doi: 10.1111/j.1365-2982.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 38.Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, et al. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215–27. doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders PR, Maillot C, Million M, Taché Y. Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur J Pharmacol. 2002;435:231–5. doi: 10.1016/s0014-2999(01)01574-6. [DOI] [PubMed] [Google Scholar]
- 40.Saunders PR, Santos J, Hanssen NPM, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002;47:208–15. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- 41.Wallon C, Yang P-C, Keita AV, Ericson A-C, McKay DM, Sherman PM, Perdue MH, Söderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–8. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Qu M-H, Ren W, Bhargava A, Chang J, Hoy J, Wang G-D, Zuo F, et al. Silencing of gene expression for corticotropin-releasing factor (CRF) in the colon attenuates stress- induced acceleration of colonic transit in rats. Gastroenterology. 2008;134(4 Suppl 1):A49. [Google Scholar]
- 43.Beckwith B, Long N, Liu S. Effects of restraint stress on CRF expression in the enteric nervous system of the rat colon. Neurogastroenterol Motil. 2011;23(Suppl 1):49. [Google Scholar]
- 44.Li Y, Li X-F, Guo H, Xu J-D, Zhang X-H, Li L-S, Feng X-Y, Zhang Y, et al. Colonic submucosal 5-HT(3) receptor-mediated somatostatin-dependent secretoinhibitory pathway is suppressed in water-immersion restraint stressed rats. Eur J Pharmacol. 2011;656:94–100. doi: 10.1016/j.ejphar.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984;81:1883–7. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clifton MS, Hoy JJ, Chang J, Idumalla PS, Fakhruddin H, Grady EF, Dada S, Corvera CU, Bhargava A. Role of calcitonin receptor-like receptor in colonic motility and inflammation. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G36–44. doi: 10.1152/ajpgi.00464.2006. [DOI] [PubMed] [Google Scholar]
- 48.Bhargava A, Dallman MF, Pearce D, Choi S. Long double-stranded RNA-mediated RNA interference as a tool to achieve site-specific silencing of hypothalamic neuropeptides. Brain Res Brain Res Protoc. 2004;13:115–25. doi: 10.1016/j.brainresprot.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 49.La Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci U S A. 2005;102:7647–52. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T, Amano T, Uehara H, Ariga H, Ishida T, Torii A, Tajiri H, Matsueda K, et al. Urocortin I is present in the enteric nervous system and exerts an excitatory effect via cholinergic and serotonergic pathways in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2007;293:G903–10. doi: 10.1152/ajpgi.00066.2007. [DOI] [PubMed] [Google Scholar]
- 51.Sand E, Themner-Persson A, Ekblad E. Corticotropin releasing factor-distribution in rat intestine and role in neuroprotection. Regul Pept. 2011;166:68–75. doi: 10.1016/j.regpep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 53.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PloS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pothoulakis C, Castagliuolo I, Leeman SE. Neuroimmune mechanisms of intestinal responses to stress. Role of corticotropin-releasing factor and neurotensin. Ann N Y Acad Sci. 1998;840:635–48. doi: 10.1111/j.1749-6632.1998.tb09602.x. [DOI] [PubMed] [Google Scholar]
- 55.Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122:705–11. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- 56.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–9. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–10. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 58.Ohara PT, Vit J-P, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 2008;100:3064–73. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Inhibition of CRF expression in the rat colon has no effect on basal fecal pellet output. Fecal pellets were counted daily for up to four days after dsRNA treatment. Knockdown of endogenous CRF expression in the rat colon did not affect daily fecal pellet output under non-stressed situation. N = 5/group.