Abstract
Pain can vary over the estrous cycle as a result of changes in estradiol concentration but the mechanism causing this variation is unclear. Because the thalamus is important in pain control, gene expression in the lateral thalamus (ventral posteromedial, ventral posterolateral, reticular thalamic nuclei) was screened at different phases of the estrous cycle. Gene expression changes in Sprague-Dawley rats were further analyzed by real-time PCR and ELISA and plasma estradiol levels were measured by RIAs at different phases of the estrous cycle. Our results indicated that both the RNA and protein expression of glutamate decarboxylase 1 and 2 (GAD1, GAD2), GABA(A) receptor-associated protein like 1 (GABARAPL1) and vesicular GABA transporter (VGAT) significantly increased in the lateral thalamus when plasma estradiol levels were elevated. Estradiol levels were elevated during the proestrus and estrus phases of the estrous cycle. Estrogen receptor α (ERα) was observed to be co-localized in thalamic cells and thalamic infusion of an ERα antagonist significantly reduced GAD1 and VGAT transcript. GAD1, GAD2 GABARAPL1 and VGAT have been shown to effect neuronal responses suggesting that modulation of pain during the estrous cycle can be dependent, in part, through estradiol induced changes in thalamic gene expression.
Introduction
Women often have a higher incidence of pain (Amandusson and Blomqvist, 2013; Fillingim and Maixner, 2000; Iacovides et al., 2015; Sherman and LeResche, 2006) and women report orofacial pain more often than men (Koopman et al., 2009). Polymorphisms in the estrogen receptor will increase the risk of women developing various orofacial pain conditions supporting a biological basis for estradiol (Kang et al., 2007; Ribeiro-Dasilva et al., 2009). Moreover, orofacial pain varies in intensity over the menstrual cycle (LeResche et al., 2003; Suenaga et al., 2001) and is marked by the attenuation of symptoms in post-menopausal women (Salonen et al., 1990; Von et al., 1988). However, the attenuation of symptoms in post-menopausal women is reversed when they take estrogen supplements (LeResche et al., 1994; LeResche et al., 1997). In support of a role for sex hormones, nociceptive responses are reduced in proestrus rats when estradiol levels are elevated (Fischer et al., 2008; Kramer and Bellinger, 2009). Interestingly, elevated pain sensitivity at diestrus and metestrus has been linked to sex steroid modulation of GABA activity (Taherianfard and Mosavi, 2011).
Gamma-aminobutyric acid (GABA) receptors are found in the thalamus and GABA release in the thalamus has been shown to regulate pain responses (Olausson et al., 1994; Oliveras and Montagne-Clavel, 1994; Pirker et al., 2000; Reyes-Vazquez et al., 1986; Roberts et al., 1992). Estradiol has been shown to modulate pain responses by altering thalamic pain signaling (Naderi et al., 2014; Reed et al., 2009). Moreover, estradiol has been shown modulate GABA production in the brain (Demling et al., 1985) where the estrogen receptor is present (Flugge et al., 1986; Herbison, 1994). Although estradiol has been shown to alter pain responses through altered GABA signaling centrally (Taherianfard and Mosavi, 2011) it is currently not clear estradiol’s effect on thalamic GABA genes expression and the role of the estrogen receptor. To begin to address these questions gene expression was measured in the thalamus of cycling female rats.
During the estrous cycle gene expression was quantitated in the thalamus using a gene array, that includes >20,000 probes. Gene expression in the thalamus of proestrus and diestrus rats was compared. Of all the genes analyzed we focused on those with the greatest change in gene expression and on genes that had a known pain function. Using these criteria four GABA related genes (GAD1, GAD2, GABARAPL1, VGAT) showed significantly altered gene expression in the lateral thalamus. GAD1 and GAD2 are enzymes that produce GABA, VGAT transports GABA into vesicles and GABARAPL1 functions in GABA receptor signaling (Erlander et al., 1991; Karlsen et al., 1991; Mansuy et al., 2004; McIntire et al., 1997). The four candidate genes from the array were further analyzed by real time PCR and ELISA. The changes in transcript were then correlated to the level of plasma estradiol. Co-expression of these genes with ERα was analyzed and the role of ERα was further tested by administering an antagonist in the lateral hypothalamus.
Materials and Methods
Animal Husbandry
The Texas A&M University Baylor College of Dentistry Institutional Animal Care and Use Committee approved the experimental protocol. Female Sprague-Dawley rats (280–300 grams) from Harlan Industries, Houston, TX were kept on a 14:10 light/dark cycle. The rats were given food and water ad libitum. After a 4 day acclimation period the rats were smeared daily to determine the stage of the estrous cycle.
Tissue collection
Vaginal smears were performed for at least three consecutive days, the stage of the estrous cycle was determined and the rat sacrificed. In this study four different experiments were performed using the cycled female rats, in experiment #1, #2 and in experiment #3 the rats were sacrificed by exposure to CO2 followed by decapitation. The brain was extracted using a rongeur and sliced on a slicer with 1 mm increments (Zivic, Pittsburgh, PA). Sections were 2 mm thick by skipping every other slot in the slicer and the sections between Bregma −3 to −5 were placed on glass slides and kept on dry ice. Lateral thalamic tissue was collected with punches 2 mm in diameter, punches included the ventral posteromedial, ventral posterolateral and reticular thalamic nuclei. Tissue was stored in liquid nitrogen until RNA or protein could be isolated. In experiment #1 and #2 trunk blood was collected from each animal after decapitation and the thalamic tissue was used for RNA isolation, transcript quantitation or gene array studies. In the experiment #3 the thalamic tissue was used for protein isolation. Trunk blood was collected in half of the animals in experiment #3. In Experiment #4 the rats were perfused with 9% sucrose followed by 4% paraformaldyhyde. The brain was extracted and stored in 25% sucrose at 4°C.
Array Analysis
Thalamic tissue from two diestrus and two proestrus rats was collected, pooled and the RNA isolated. The RNA was processed and analyzed using the Affymetrix Rat Gene 2.0 ST Array (Santa Clara, CA).
Cannulation and drug infusion
In experiment #1, adult female Sprague-Dawley rats (250–300 grams) were anesthetized with 2% isoflurane and an air flow of 2 liters per minute. Unilateral placement of a 22 gauge guide cannula (C313G, Plastics One, Roanoke, VA) was performed stereotaxically, with the tip placed in the lateral thalamic region (from Bregma posterior 3.6 mm, lateral 2.7 mm, depth 6.0 mm). After surgery analgesia was obtained with nalbuphine. At least one week following surgery proestrus was determined by a vaginal smear and 0.5 μl of the ERα antagonist MPP dihydrochloride (Tocris Bioscience, Bio-Techne, Minneapolis MN) was infused in the thalamus over a 5 minute period. MPP dihydrochloride was dissolved in 5% dextrose (30 nM solution) and control animals were infused with the same volume of 5% dextrose (i.e., vehicle). Infusion was performed using a 10 μl Hamilton syringe connected to PE-10 flexible tubing and a 28 gauge infusion cannula (C313I, Plastics One). After infusion the injection cannula was removed from the guide cannula and a dummy cannula cap was placed to seal off the opening (C313DC, Plastics One). Twenty-four hours after infusion a vaginal smear was completed to confirm estrus and the animal were euthanized by an overdose of CO2. Blood plasma was collected and estradiol quantitated. The lateral thalamic tissue was isolated with a 2 mm punch and frozen in liquid nitrogen as previously described.
Radio Immuno Assay
In experiments #1 and #2 plasma 17β-estradiol was quantitated in the trunk blood by first, centrifugation and collection of the upper plasma layer, vigorously mixing 0.5 ml of plasma with 5 ml of ether. Second, the solution was frozen in an ethanol, dry ice bath and the ether phase decanted. The ether phase contains the lipid soluble hormones. Third, the ether was evaporated by nitrogen gas and the remaining residue suspended in radioimmunoassay buffer supplied by the manufacturer and assayed according to the manufactures directions (Beckman Coulter, Pasadena, CA).
Real time PCR
In experiments #1 and #2 RNA extraction was performed using the RNA Lipid Tissue Kit from Qiagen (Valencia, CA). The RNA concentration in the resulting sample was determined on a Nanodrop2000. A one-step reverse transcription PCR reaction was performed on BioRAD C1000 Thermal Cycler using the SYBR-Green 1-Step RT-PCR kit from Qiagen. The thermal protocol was 30 min @ 50 °C for the reverse transcription reaction, 15 min @ 95 °C for DNA pol activation and 40x (15 s @ 94 °C melting, 30 s @ 56 °C annealing, 30 s @ 72 °C extension). A melting curve was obtained thereafter for quality assurance. Sample amount was adjusted according to total RNA concentration to obtain 20 ng of total RNA per well in the final reaction mix. All reactions were run in triplicate. PCR runs that did not exhibit a proper amplification profile were discarded. For the real time PCR data a line tangent to the inflection point of each amplification curve was determined and the cycle number that this line intersected was labeled Cγ0, as previously described in detail (Guescini et al., 2008). For each sample, the Cγ0 for GAPDH was subtracted from the Cγ0 of each gene of interest to normalize the results (ΔCγ0). In the non-cannulated rats a ΔCγ0 value at a particular estrous cycle phase was subtracted from the mean ΔCγ0 metestrus value (ΔCγ0 metestrus average − ΔCγ0 (cycle phase) = ΔΔCγ0). To get the fold change the ΔΔCγ0 was raised to the second power (2ΔΔCγ0). In the cannulated rats the ΔCγ0 on the contralateral, non-cannulated side was subtracted from the ΔCγ0 on the ipsilateral, cannulated side to calculate a ΔΔCγ0 value for each gene of interest. This ΔΔCγ0 was raised to the second power to determine the fold change. If the 2 ΔΔCγ0 value was less than one we used the formula (1/(2 ΔΔCγ0)) to determine a negative fold change.
Enzyme-linked immunosorbent assay
In experiment #3 each thalamic tissue plug was stored separately in liquid nitrogen until analysis. For analysis the tissue was placed in 300 μl of T-Per tissue protein extraction reagent containing Halt Protease Inhibitor and ground (Thermo Scientific, Rockford, IL). Ground samples were frozen and thawed, followed by centrifugation and decanting of the supernatant. Quantitation of protein in the supernatant was completed on duplicate 100 μl samples of supernatant using an ELISA following the manufacturer’s directions (MyBioSource, San Diego, CA). Total protein was determined in each sample using a BCA protein assay (Thermo Scientific, Waltham, WA). Values were given as the pg of GAD1, GAD2, VGAT or ng GABARAPL1 per mg of total protein.
Immuno-fluorescent Staining
In experiment #4 fixed whole brains stored in 25% sucrose (experiment #4) were frozen, cryo-sectioned and the 20 μm sections placed on Histobond slides (VWR international, Radnor, PA). The tissue was then blocked with a PBS solution containing 5% normal goat serum and 0.3% Triton-X 100 for 2 hours at room temperature. Following three rinses in PBS with 0.3% Triton-X 100 the slides were incubated in a primary antibody solution overnight at 4°C. Primary antibodies consisted of a 1:500 dilution of the mouse monoclonal ERα antibody (Millipore, Temecula, CA) and a 1:300 dilution of the rabbit polyclonal VGAT antibody (AB5062P, Millipore) or a 1:500 dilution of the rabbit polyclonal GABARAPL1 antibody (Millipore). Staining also included a 1:500 dilution of a rabbit polyclonal ERα antibody (C1355, Millipore) and a 1:300 dilution of the mouse-monoclonal GAD1 (AB26116 Abcam, Cambridge, MA) or a 1:500 dilution of the mouse-monoclonal GAD2 antibody (AB26113, Abcam). Primary antibodies were diluted with PBS, 5% BSA and 0.3% Triton X-100. After incubation in primary antibody the slides were rinsed three times in PBS and Triton-X 100 for a total of 60 min and placed for 2 hours in a 1:500 dilution of secondary antibody in PBS and 0.3% Triton X-100. Secondary antibodies included a mixture of goat anti-mouse 488 and goat anti-rabbit 568 (Invitrogen, Carlsbad, CA). After rinsing the slides three times in PBS for a total of 60 min, the slides were then mounted with Fluoromount-G mounting medium containing Hoechst 33342 stain (Electron Microscopy Sciences, Hatfield, PA). The fluorescent signal was imaged using a Nikon fluorescent microscope and NIS-Elements imaging software and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA).
Statistics
The data were analyzed using ANOVA to determine significant estrous cycle or antagonist effects. When significant (p<0.05), the test was followed by Tukey’s post-hoc test. A two-tailed Spearman correlation statistic was used to compare the RIA and real time PCR data.
Results
When comparing thalamic transcript at proestrus and diestrus the genes that increased at least two fold on a large gene array were mostly related to GABA function (Table 1).
Table 1.
Thalmic gene expression changes over 2 fold between proestrus and diestrus
| Fold change | Proestrus signal | Diestrus signal | Genbank# | Gene Description | Gene symbol |
|---|---|---|---|---|---|
| 3.46 | 311.32 | 180.74 | Similar to 40S ribosomal protein S3 | RGD1560831 | |
| 3.18 | 1519.41 | 678.48 | M72422 | Glutamate decarboxylase 2 | Gad2 |
| 2.8 | 881.36 | 366.14 | M34445 | Glutamate decarboxylase 1 | Gad1 |
| 2.05 | 502.57 | 275.51 | AF030253 | Solute carrier family 32 (GABA vesicular transporter) member1 | Slc32a1 (vGAT) |
| 2.05 | 392.20 | 145.97 | Meishomeobox2 | Meis2 | |
| 2.03 | 103.05 | 197.57 | FQ221570 | Thyrotropin releasing hormone | Trh |
| 2.01 | 222.72 | 185.77 | FQ221553 | Ubiquitin associated and SH3 domain containing, B | Ubash3b |
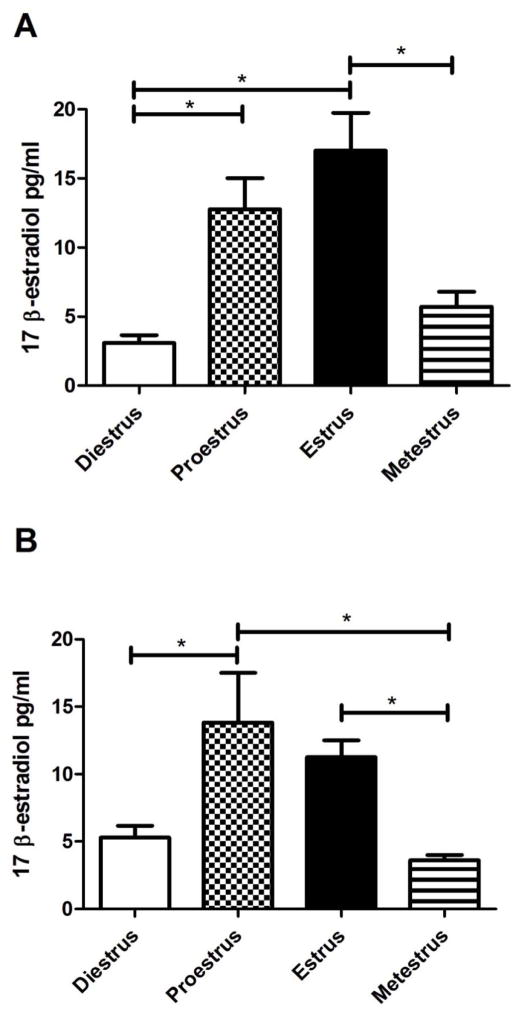
Measurement of plasma estradiol indicated rats in the proestrus or estrus phase of the estrous cycle had a significantly greater amount of estradiol in comparison to rats in either diestrus or metestrus. This elevated estradiol was observed in two groups of rats; one group in which transcript was measured (Fig. 1A) and another group in which protein was measured (Fig. 1B).
Figure 1. Plasma 17 β-estradiol concentration in rats during the estrous cycle.
Vaginal smears were performed and plasma was collected within one hour. Plasma estradiol was measured by RIA. Panel A is from Experiment #1 where transcript levels were measured in the thalamic tissue by RT-PCR. Panel B is from Experiment #3 where thalamic protein content was quantitated by ELISA. Significant differences of p<0.05 are indicated by an asterisk. Seven animals were in each of the four estrous cycle phases.
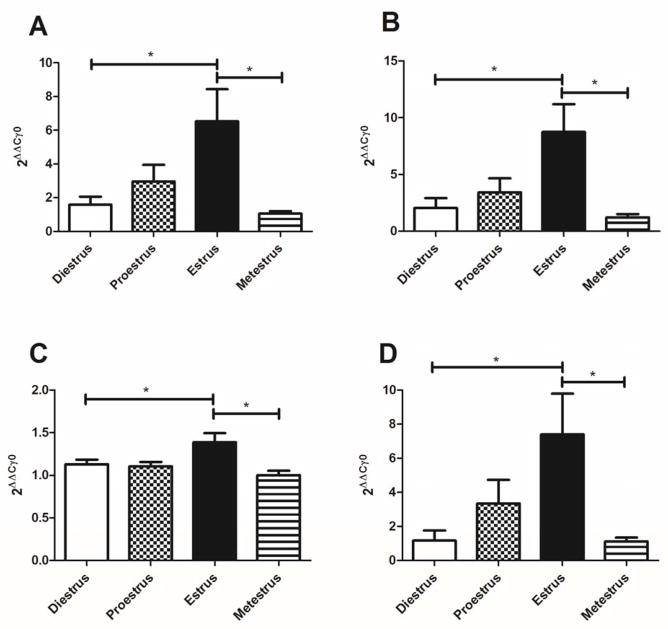
Transcript for the GABA related genes GAD1, GAD2, GABARAPL1 and VGAT was significantly elevated at the estrus phase of the estrous cycle (Fig. 2A, B, C and D).
Figure 2. Transcript levels of GAD1, GAD2, GABARAPL1 and VGAT in the thalamus of cycling female rats.
Estrous cycle phases were determined by vaginal smears and the thalamic tissue was isolated within one hour. RT-PCR analysis of the thalamic tissue was then performed. Panel A shows GAD1 transcript levels, Panel B shows GAD2 transcript expression, Panel C shows GABARAPL1 transcript expression and Panel D shows VGAT transcript expression. Significant differences of p<0.05 are indicated by an asterisk. There were 7 animals in each of the three estrous phases except estrus where there were 8 animals.
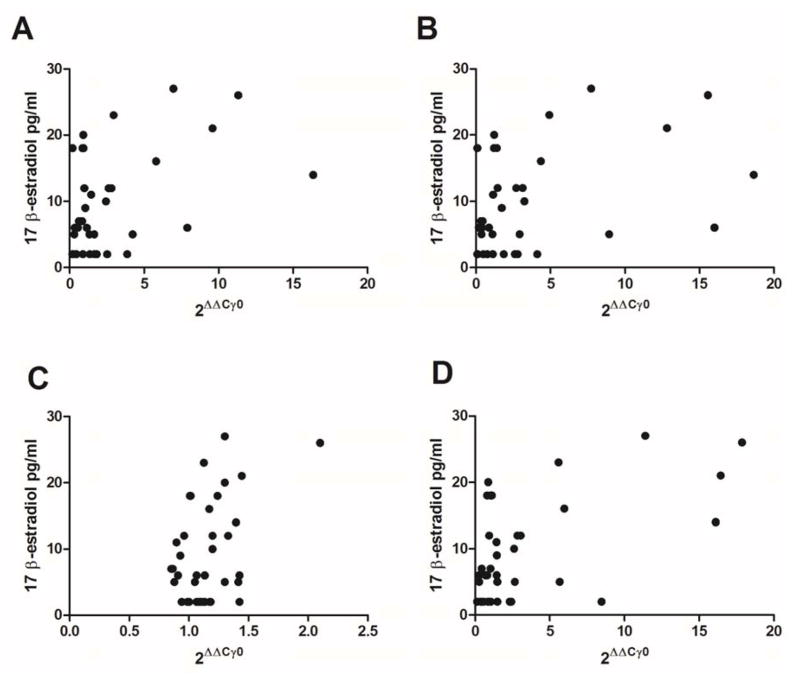
In Figure 3 there was a direct relationship between the amount of transcript and the plasma estradiol. A significant correlation between GAD1 (r=0.35, p<0.05, Fig. 3A), GAD2 (r=0.36, p<0.05, Fig. 3B), VGAT (r=0.42, p<0.01, Fig. 3D) and estradiol was measured but no significant relationship was observed for GABARAPL1 and estradiol (r=0.25, p>0.1, Fig. 3C).
Figure 3. Thalamic gene expression plotted against the plasma estradiol concentration.
Panel A shows GAD1 transcript levels, Panel B shows GAD2 transcript expression, Panel C shows GABARAPL1 transcript expression and Panel D shows VGAT transcript expression plotted on the x-axis. The estradiol concentration was plotted on the y-axis. There were 7 animals in each of the three estrous phases except estrus where there were 8 animals.
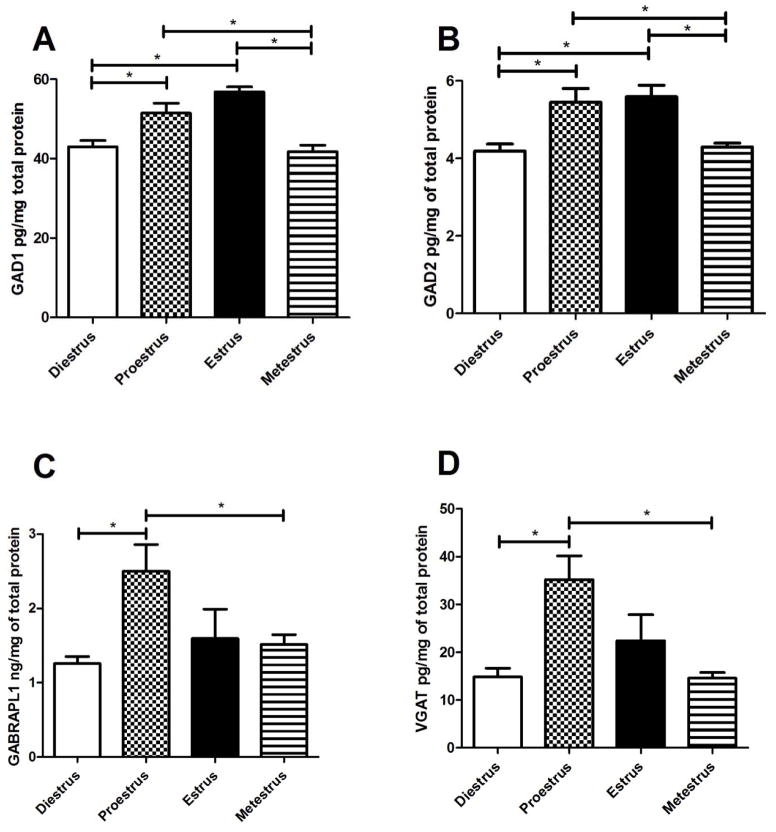
Protein levels were significantly greater in proestrus and estrus for GAD1 and GAD2 (Fig. 4A and B). GABARAPL1 and VGAT expression was greater in proestrus rats (Fig. 4C and D).
Figure 4. Protein content of GAD1, GAD2, GABARAPL1 and VGAT in the thalamus of cycling female rats.
Estrous cycle phases were determined by vaginal smears and the thalamic tissue was isolated within one hour. Protein content was measured by ELISA. Panel A shows GAD1 protein levels, Panel B shows GAD2 protein expression, Panel C shows GABARAPL1 protein expression and Panel D shows VGAT protein expression. Significant differences of p<0.05 are indicated by an asterisk. There were 5 animals in each of the 4 estrous phases.
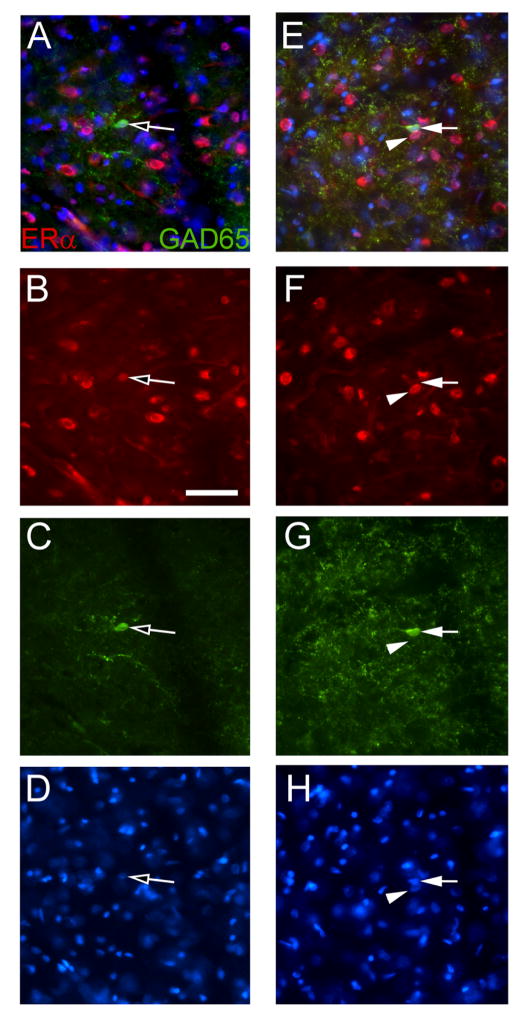
Immunostaining in the lateral thalamic region indicated that GAD2 and ERα co-localized to cells with neuronal morphology (Fig. 5A). Although GAD2 was observed in cells most of the GAD2 signal was present in varicosities (Fig. 5A, E, C and G). Thalamic ERα localized to the nucleus of most cells (Fig. 5B, F). Many ERα positive cells (Fig. 5B, F) were surrounded by GAD2 positive varicosities (Fig. 5A and E). There were cells that stained for GAD2 but did not stain for ERα (Fig. 5E). VGAT, GAD1 and GABARAPL1 also showed punctate staining along linear tracks, in between and adjacent to ERα positive cells, very similar to the GAD2 staining in Fig. 5 (data not shown).
Figure 5. Lateral thalamic immuno-fluorescent staining for ERα and GAD2 (GAD65).
A frozen section from a diestrus rat was stained for ERα (red) and GAD2 (green). Panel A shows a cell with ERα and GAD2 co-localized (open arrow) and panel E is a different region on the same section showing a cell expressing ERα (arrowhead) and a cell expressing GAD2 (arrow). The ERα signal is shown in panels B and F, the GAD2 signal is shown in panels C and G and in panels D and H the nuclei are stained with Hoechst 33342. Image is taken from along the border of the reticular thalamic nucleus and the ventral posterolateral nucleus. Bar equals 50 μm.
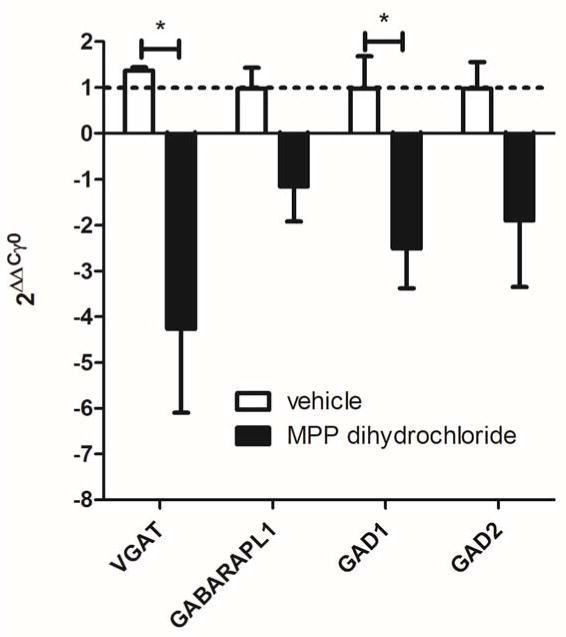
Thalamic injection of the ERα antagonist MPP dihydrochloride lead to a significant, greater than 2 fold, decrease in VGAT and GAD1 transcript on the ipsilateral side in comparison to the non-injected contralateral side (Fig. 6). Thalamic injection of vehicle did not significantly alter gene expression when comparing the injected to the non-injected side, a value of 1 would indicate no change between the injected (ipsilateral) and non-injected (contralateral) sides (Fig. 6)
Figure 6. Thalamic injection of an ERα antagonist reduced expression of VGAT and GAD1 during estrus.
The lateral region of the thalamus was cannulated unilaterally and two weeks after surgery vehicle or the ERα antagonist MPP dihydrochloride was infused on the day of proestrus. Rats were sacrificed the next day (i.e., estrus) and the injected thalamus (ipsilateral) and non-injected thalamus (contralateral) were isolated. The estrus phase was confirmed by measuring blood plasma estradiol levels and vaginal smears. Real time PCR was performed using the thalamic tissues and the amount of GAD1, GAD2, GABARAPL1 and VGAT was measured and reported as the fold change in transcript, contralateral thalamus versus ipsilateral thalamus. A value of less than 0 indicates that gene expression on the ipsilateral side was reduced versus the contralateral side. A value of 1 (dotted line) indicates the transcript levels were the same on the ipsilateral and contralateral sides. There were 4 vehicle injected rats and 4 MPP dihydrochloride injected rats.
Discussion
Screening of thalamic genes in proestrus and diestrus rats revealed changes in expression for GAD1, GAD2, GABARAPL1 and VGAT. Expression of these four genes was studied further at each stage of the estrous cycle using real time PCR and ELISA assays. The results indicate that all four genes significantly increased at the proestrus or estrus stage, a period when estradiol is elevated. GAD1, GAD2, GABARAPL1 and VGAT show a similar pattern of change across the estrous cycle and the pattern of transcript expression correlated to the change in estradiol during the cycle. Co-localization of ERα was observed in cells of the lateral hypothalamus and thalamic infusion of an ERα antagonist significantly decreased VGAT and GAD1 transcript suggesting estradiol could, in part, modulate expression by binding this receptor. Importantly, elevated estradiol was associated with elevation of these GABA genes in the thalamus; consistent with the idea that estradiol can modulate pain responses by increasing activity in the GABA pathway.
In this study we demonstrate ERα was expressed in cells of the lateral hypothalamus and that this expression co-localized with GABAergic neurons. One explanation for the increased gene expression during proestrus and estrus would be that a higher concentration of estradiol bound to ERα and signaled increased gene expression. Results showing infusion of an ERα antagonist inhibits this gene expression further supports this idea. In previous reports estradiol has been shown modulate GABA production in the brain (Demling et al., 1985), particularly in regions where GAD co-localizes with estrogen and the estrogen receptor (Flugge et al., 1986; Herbison, 1994). GAD1 and GAD2 synthesize GABA in neurons (Martin and Rimvall, 1993) potentially through binding of the estrogen receptor to the GAD promoter (Hudgens et al., 2009). Estradiol will increase GAD expression in one brain nuclei and estradiol will decrease GAD expression in a different nuclei but the mechanism for these differences are unknown (McCarthy et al., 1995; Weiland, 1992). Alternatively, estradiol can alter GABA levels through enhancing expression of the GABA transporter VGAT (Herbison et al., 1995; Ottem et al., 2004). The effect of estradiol on VGAT has been hypothesized to result from estrogen receptor binding to an estrogen response element in the VGAT promoter (Herbison et al., 1995). GABARAPL1 plays a role in intracellular GABA(A) receptor trafficking (Mansuy et al., 2004) and estradiol has been shown to alter GABARAPL1 expression through binding an estrogen response element within the promoter (Vernier-Magnin et al., 2001).
Transcript levels for GAD1, GAD2 GABARAPL1 and VGAT were not significantly elevated in proestrus rats but the protein content was elevated at proestrus. One explanation for this result would be that the transcript was measured in early proestrus when estradiol was less. Whereas the protein was isolated from rats in late proestrus and plasma estradiol was greater. The greater amount of estradiol could explain the higher amount of GAD1, GAD2 GABARAPL1 and VGAT protein versus transcript. Alternatively, estradiol did not have enough time to increase transcript in experiment #1 but did have sufficient time to increase protein content in experiment #3. Changes in gene expression typically takes several hours after binding ERα (Lee and Gorski; LeMeur et al.) and this delay, compounded with a lower level of estradiol, could have contributed to the lower amount of transcript observed at proestrus.
It is known that sex hormones (notably, estrogen) affect nociception and pain perception across mammalian species (Amandusson and Blomqvist, 2013; Fillingim and Maixner, 2000; Iacovides et al., 2015; Sherman and LeResche, 2006). For example, orofacial nociceptive responses are reduced in proestrus rats when estradiol levels are elevated (Fischer et al., 2008; Kramer and Bellinger, 2009). Elevated pain sensitivity at diestrus and metestrus has been linked to sex steroid modulation of GABA activity (Taherianfard and Mosavi, 2011). Viral introduction of GAD1 into the sciatic nerve has been shown to reduce neuropathic pain (Kanao et al., 2015). A recent study has shown that altering the activity of GAD1 positive cells in the cingulate cortex can effect nociceptive responses (Gu et al., 2015). Moreover, a link between GAD2 expression and pain was observed in the spinal cord (Lorenzo et al., 2014; Meisner et al., 2010). In mice where VGAT levels are reduced thermal nociception and inflammatory pain were enhanced (Yamada et al., 2012). Together these results suggest altered expression of GAD1, GAD2 and VGAT can affect pain responses.
Increased production of GABARAPL1, a GABAA receptor subunit recycling protein (Mansuy et al., 2004), during proestrus/estrus would increase turnover of the GABA receptor on the postsynaptic membranes. However, a higher turnover would decrease receptor density and reduce neuronal sensitivity to GABA resulting in attenuation of GABA’s inhibitory effect.
In conclusion, during estrus and proestrus, periods of high estradiol, GAD1, GAD2, VGAT and GABARAPL1 were elevated. GAD1, GAD2, VGAT and GABARAPL1 all have estrogen response elements within their promoters (Herbison et al.; Hudgens et al.; Vernier-Magnin et al.) and co-localization of GAD2 and ERα in the thalamus, as well as, ERα antagonist studies suggests regulation could involve the estrogen receptor. Elevated GAD1, GAD2, and VGAT expression has been shown to attenuate pain responses (Gu et al., 2015; Kanao et al., 2015; Lorenzo et al., 2014; Meisner et al., 2010; Yamada et al., 2012) and previous evidence indicates that elevated estradiol will reduce orofacial pain (Fischer et al., 2008; Kramer and Bellinger, 2009) consistent with the idea that elevated GAD1, GAD2, and VGAT, at proestrus/estrus contributes, in part, to the reduced pain response.
Acknowledgments
This study was supported by NIDCR grants DE016059 (LLB) and DE022129 (PRK). We would like to thank Jeremy Alfred, Fatemeh Nakhaie Sistani and Paulina Clendennen for their technical help in these studies.
References
- Amandusson A, Blomqvist A. Estrogenic influences in pain processing. Frontiers in neuroendocrinology. 2013;34(4):329–349. doi: 10.1016/j.yfrne.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Demling J, Fuchs E, Baumert M, Wuttke W. Preoptic catecholamine, GABA, and glutamate release in ovariectomized and ovariectomized estrogen-primed rats utilizing a push-pull cannula technique. Neuroendocrinology. 1985;41(3):212–218. doi: 10.1159/000124180. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W. Sex, Gender and Pain. Seattle: IASP Press; 2000. [Google Scholar]
- Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de AV, Tambeli CH. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J Pain. 2008;9(7):630–638. doi: 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43(1):1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- Gu L, Uhelski ML, Anand S, Romero-Ortega M, Kim YT, Fuchs PN, Mohanty SK. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PloS one. 2015;10(2):e0117746. doi: 10.1371/journal.pone.0117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guescini M, Sisti D, Rocchi MB, Stocchi L, Stocchi V. A new real-time PCR method to overcome significant quantitative inaccuracy due to slight amplification inhibition. BMC bioinformatics. 2008;9:326. doi: 10.1186/1471-2105-9-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Immunocytochemical evidence for oestrogen receptors within GABA neurones located in the perinuclear zone of the supraoptic nucleus and GABAA receptor beta 2/beta 3 subunits on supraoptic oxytocin neurones. J Neuroendocrinol. 1994;6(1):5–11. doi: 10.1111/j.1365-2826.1994.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Augood SJ, Simonian SX, Chapman C. Regulation of GABA transporter activity and mRNA expression by estrogen in rat preoptic area. J Neurosci. 1995;15(12):8302–8309. doi: 10.1523/JNEUROSCI.15-12-08302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens ED, Ji L, Carpenter CD, Petersen SL. The gad2 promoter is a transcriptional target of estrogen receptor (ER)alpha and ER beta: a unifying hypothesis to explain diverse effects of estradiol. J Neurosci. 2009;29(27):8790–8797. doi: 10.1523/JNEUROSCI.1289-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovides S, Avidon I, Baker FC. Does pain vary across the menstrual cycle? A review. European journal of pain (London, England) 2015 doi: 10.1002/ejp.714. [DOI] [PubMed] [Google Scholar]
- Kanao M, Kanda H, Huang W, Liu S, Yi H, Candiotti KA, Lubarsky DA, Levitt RC, Hao S. Gene Transfer of Glutamic Acid Decarboxylase 67 by Herpes Simplex Virus Vectors Suppresses Neuropathic Pain Induced by Human Immunodeficiency Virus gp120 Combined with ddC in Rats. Anesthesia and analgesia. 2015;120(6):1394–1404. doi: 10.1213/ANE.0000000000000729. [DOI] [PubMed] [Google Scholar]
- Kang SC, Lee DG, Choi JH, Kim ST, Kim YK, Ahn HJ. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. IntJ Oral Maxillofac Surg. 2007;36(5):391–394. doi: 10.1016/j.ijom.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Karlsen AE, Hagopian WA, Grubin CE, Dube S, Disteche CM, Adler DA, Barmeier H, Mathewes S, Grant FJ, Foster D, et al. Cloning and primary structure of a human islet isoform of glutamic acid decarboxylase from chromosome 10. Proc Natl Acad Sci U S A. 1991;88(19):8337–8341. doi: 10.1073/pnas.88.19.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman JSHA, Dieleman JP, Huygen FJ, de Mos M, Martin CGM, Sturkenboom MCJM. Incidence of facial pain in the general population. PAIN. 2009;147(1–3):122–127. doi: 10.1016/j.pain.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150(8):3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Gorski J. Estrogen-induced transcription of the progesterone receptor gene does not parallel estrogen receptor occupancy. Proc Natl Acad Sci U S A. 1996;93(26):15180–15184. doi: 10.1073/pnas.93.26.15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMeur M, Glanville N, Mandel JL, Gerlinger P, Palmiter R, Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- LeResche L, Dworkin SF, Saunders K, Vonkorff M, Barlow W. Is Postmenopausal Hormone Use A Risk Factor for Tmd. Journal of Dental Research. 1994;73:186–186. [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106(3):253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69(1–2):153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- Lorenzo LE, Magnussen C, Bailey AL, St Louis M, De Koninck Y, Ribeiro-da-Silva A. Spatial and temporal pattern of changes in the number of GAD65-immunoreactive inhibitory terminals in the rat superficial dorsal horn following peripheral nerve injury. Mol Pain. 2014;10:57. doi: 10.1186/1744-8069-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy V, Boireau W, Fraichard A, Schlick JL, Jouvenot M, Delage-Mourroux R. GEC1, a protein related to GABARAP, interacts with tubulin and GABA(A) receptor. Biochem Biophys Res Commun. 2004;325(2):639–648. doi: 10.1016/j.bbrc.2004.10.072. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. Journal of neurochemistry. 1993;60(2):395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Kaufman LC, Brooks PJ, Pfaff DW, Schwartz-Giblin S. Estrogen modulation of mRNA levels for the two forms of glutamic acid decarboxylase (GAD) in female rat brain. J Comp Neurol. 1995;360(4):685–697. doi: 10.1002/cne.903600412. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389(6653):870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I–III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma. 2010;27(4):729–737. doi: 10.1089/neu.2009.1166. [DOI] [PubMed] [Google Scholar]
- Naderi A, Asgari A, Zahed R, Ghanbari A, Samandari R, Jorjani M. Estradiol attenuates spinal cord injury-related central pain by decreasing glutamate levels in thalamic VPL nucleus in male rats. Metab Brain Dis. 2014:1–8. doi: 10.1007/s11011-014-9570-z. [DOI] [PubMed] [Google Scholar]
- Olausson B, Xu ZQ, Shyu BC. Dorsal column inhibition of nociceptive thalamic cells mediated by gamma-aminobutyric acid mechanisms in the cat. Acta physiologica Scandinavica. 1994;152(3):239–247. doi: 10.1111/j.1748-1716.1994.tb09803.x. [DOI] [PubMed] [Google Scholar]
- Oliveras JL, Montagne-Clavel J. The GABAA receptor antagonist picrotoxin induces a ‘pain-like’ behavior when administered into the thalamic reticular nucleus of the behaving rat: a possible model for ‘central’ pain? Neurosci Lett. 1994;179(1–2):21–24. doi: 10.1016/0304-3940(94)90925-3. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24(37):8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Reed WR, Chadha HK, Hubscher CH. Effects of 17beta-estradiol on responses of viscerosomatic convergent thalamic neurons in the ovariectomized female rat. J Neurophysiol. 2009;102(2):1062–1074. doi: 10.1152/jn.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Vazquez C, Enna SJ, Dafny N. The parafasciculus thalami as a site for mediating the antinociceptive response to GABAergic drugs. Brain Res. 1986;383(1–2):177–184. doi: 10.1016/0006-8993(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Dasilva MC, Peres L, Sr, LGdSMC, Arthuri MT, Hou W, Fillingim RB, Rizzatti Barbosa CM. Estrogen receptor-alpha polymorphisms and predisposition to TMJ disorder. J Pain. 2009;10(5):527–533. doi: 10.1016/j.jpain.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA, Eaton SA, Salt TE. Widely distributed GABA-mediated afferent inhibition processes within the ventrobasal thalamus of rat and their possible relevance to pathological pain states and somatotopic plasticity. Exp Brain Res. 1992;89(2):363–372. doi: 10.1007/BF00228252. [DOI] [PubMed] [Google Scholar]
- Salonen L, Hellden L, Carlsson GE. Prevalence of signs and symptoms of dysfunction in the masticatory system: an epidemiologic study in an adult Swedish population. J Craniomandib Disord. 1990;4(4):241–250. [PubMed] [Google Scholar]
- Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R245–256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- Suenaga S, Abeyama K, Indo H, Shigeta K, Noikura T. Temporomandibular disorders: MR assessment of inflammatory changes in the posterior disk attachment during the menstrual cycle. J Comput Assist Tomogr. 2001;25(3):476–481. doi: 10.1097/00004728-200105000-00023. [DOI] [PubMed] [Google Scholar]
- Taherianfard M, Mosavi M. Hippocampal GABA(A) Receptor and Pain Sensitivity during Estrous Cycle in the Rat. Iranian journal of medical sciences. 2011;36(4):289–295. [PMC free article] [PubMed] [Google Scholar]
- Vernier-Magnin S, Muller S, Sallot M, Radom J, Musard JF, Adami P, Dulieu P, Remy-Martin JP, Jouvenot M, Fraichard A. A novel early estrogen-regulated gene gec1 encodes a protein related to GABARAP. Biochem Biophys Res Commun. 2001;284(1):118–125. doi: 10.1006/bbrc.2001.4908. [DOI] [PubMed] [Google Scholar]
- Von KM, Dworkin SF, Le RL, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32(2):173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Glutamic acid decarboxylase messenger ribonucleic acid is regulated by estradiol and progesterone in the hippocampus. Endocrinology. 1992;131(6):2697–2702. doi: 10.1210/endo.131.6.1446611. [DOI] [PubMed] [Google Scholar]
- Yamada MH, Nishikawa K, Kubo K, Yanagawa Y, Saito S. Impaired glycinergic synaptic transmission and enhanced inflammatory pain in mice with reduced expression of vesicular GABA transporter (VGAT) Mol Pharmacol. 2012;81(4):610–619. doi: 10.1124/mol.111.076083. [DOI] [PubMed] [Google Scholar]