Abstract
Atrial fibrillation (AF) is the most common arrhythmia in adults and is associated with significant morbidity and mortality. Substantial interest has developed in the primary prevention of AF, and thus the identification of individuals at risk for developing AF. The electrocardiogram (ECG) provides a wealth of information, which is of value in predicting incident AF. The PR interval and P wave indices (including P wave duration, P wave terminal force, P wave axis, and other measures of P wave morphology) are discussed with regard to their ability to predict and characterize AF risk in the general population. The predictive value of the QT interval, ECG criteria for left ventricular hypertrophy, and findings of atrial and ventricular ectopy are also discussed. Efforts are underway to develop models that predict AF incidence in the general population; however, at present, little information from the ECG is included in these models. The ECG provides a great deal of information on AF risk and has the potential to contribute substantially to AF risk estimation, but more research is needed.
Keywords: atrial fibrillation, electrocardiogram, risk stratification, primary prevention, P wave indices
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in adult medicine. It affects 5.2 million individuals in the United States,1 and approximately one in four individuals will acquire this condition in their lifetimes.2 Moreover, asymptomatic AF is likely underdiagnosed in clinical practice: continuous monitoring in patients with implanted devices and in patients who have had cryptogenic stroke has revealed a substantial burden of subclinical AF not detected by conventional methods.3, 4, 5, 6, 7 AF is associated with a two‐ to three fold increased risk of cardiovascular mortality and sudden cardiac death,8, 9 a five fold increased risk of stroke,10 and a three fold increased risk of heart failure.11 Patients with AF are hospitalized twice as often as patients without AF, and there are more than 479,000 hospitalizations annually in the United States with AF listed as the primary discharge diagnosis.12 AF is associated with approximately $8700 of annual incremental healthcare costs per patient,13 and it accounts for 16 to 26 billion dollars of annual U.S. health care expenses.13, 14 Currently, there is no cure for AF. Thus, the development of a primary prevention strategy is of critical importance. Doing so will require the successful identification of individuals in the general population who are at higher risk for acquiring AF.
Although the pathophysiology of AF remains incompletely understood, it is clear that in most individuals the arrhythmia occurs in the context of adverse remodeling of the atrial electroanatomical substrate as well as abnormal triggered activity.15 The electrocardiogram (ECG) may be valuable in prevention efforts because of its ability to characterize electrophysiological changes as intermediate phenotypes along the pathway to AF. The purpose of this review is to describe efforts that have been made to identify electrocardiographic predictors of incident AF in the general population (Table 1).
Table 1.
Electrocardiographic Predictors of Incident Atrial Fibrillation in the General Population—Data from Prospective Cohorts
| Predictor | Risk Estimate (95% CI) | P Value | Study |
|---|---|---|---|
| Prolonged PR interval | HR, 1.11 (1.02–1.22)a | 0.02 | FHS (n = 7575)16 |
| HR, 1.41 (1.20–1.65)a | – | ARIC study (n = 15,429)17 | |
| HR, 1.13 (1.04–1.23)a | 0.005 | Health ABC study (n = 2722)18 | |
| HR, 1.26 (1.17–1.35)b | <0.001 | Copenhagen ECG study (n = 288,181)19 | |
| HR, 1.29 (0.68–2.44)c | 0.434 | Busselton Health study (n = 4267)25 | |
| Short PR interval | HR, 1.21 (1.06–1.37)b | 0.004 | Copenhagen ECG study (n = 288,181)19 |
| HR, 6.21 (1.52–25.31)c | 0.011 | Busselton Health study (n = 4267)25 | |
| Prolonged P wave duration | HR, 1.79 (1.51–2.14)a | – | ARIC study (n = 15,429)17 |
| HR, 1.15 (0.90–1.47)a | 0.27 | FHS (n = 1550)30 | |
| HR, 2.06 (1.89–2.23)d | <0.001 | Copenhagen ECG study (n = 285,933)31 | |
| Short P wave duration | HR, 1.60 (1.41–1.81)d | <0.001 | Copenhagen ECG study (n = 285,933)31 |
| P wave terminal force (PTFV1) | HR, 1.23 (1.04–1.46)a | – | ARIC study (n = 15,429)17 |
| P′ deep terminal negativity | HR, 5.02 (3.23–7.80) | <0.0001 | ARIC study (n = 15,376)37 |
| Prolonged QT interval | HR, 1.44 (1.24–1.66)e | < 0.001 | Copenhagen ECG study (n = 281,277)57 |
| HR, 1.99 (1.37–2.89)f | <0.001 | ARIC study (n = 14,538)58 | |
| HR, 1.57 (1.18–2.07)f | 0.002 | CHS (n = 4745)58 | |
| HR, 1.42 (1.003–2.02)f | 0.048 | Health ABC study (n = 2396)58 | |
| Short QT interval | HR, 1.45 (1.14–1.84)e | 0.002 | Copenhagen ECG study (n = 281,277)57 |
| HR, 0.84 (0.66–1.06)f | 0.14 | ARIC study (n = 14,538)58 | |
| HR, 1.09 (0.81–1.47)f | 0.57 | CHS (n = 4745)58 | |
| HR, 1.37 (0.92–2.04)f | 0.12 | Health ABC study (n = 2396)58 | |
| ECG‐LVH | HR, 1.4 (0.9–2.4) for men, HR 1.3 (0.9–2.1) for women | NS, NS | FHS (n = 2090 men, 2641 women)59 |
| HR, 1.39 (1.11–1.75)c | 0.05 | Niigata Preventive Medicine Study (n = 63,386)60 | |
| HR, 2.24 (1.33–3.76)g | 0.002 | MESA (n = 4942)61 | |
| Any ectopy (PACs or | HR, 2.52 (1.84–3.44)h | <0.001 | Niigata Preventive Medicine Study (n = 63,386)60 |
| PVCs) | HR, 3.49 (2.40–5.08)i | <0.001 | Niigata Preventive Medicine Study (n = 63,386)60 |
| Atrial ectopy (PACs) | HR, 1.49 (1.02 – 2.17)j | 0.038 | Copenhagen Holter Study (n = 678)65 |
| HR, 1.17 (1.13 – 1.22)k | <0.001 | CHS (n = 1260)66 | |
| HR, 1.38 (1.14 – 1.68)k | 0.001 | Malmö Diet and Cancer Study (n = 383)67 | |
| Ventricular ectopy (PVCs) | HR, 1.56 (1.30–1.87)l | – | ARIC study (n = 14,783)68 |
| HR, 1.38 (0.94–2.03)m | 0.101 | Taipei Veterans General Hospital Database (n = 3351)69 | |
| HR, 1.55 (1.06–2.26)n | 0.024 | Taipei Veterans General Hospital Database (n = 3351)69 |
All results presented are those of multivariable‐adjusted analyses. Covariates differ between studies.
HR per standard deviation change.
PR interval defined as prolonged if ≥200 milliseconds, and compared to reference group with PR interval 150–161 milliseconds (40th to 60th percentile). Short PR interval defined as PR interval <123 milliseconds (<5th percentile), compared to reference group with PR interval 150–161 milliseconds (40–60th percentile).
PR interval duration or LVH defined by Minnesota code.
P wave prolongation defined as P wave duration ≥130 milliseconds (≥95th percentile); short P wave duration defined as P wave duration ≤89 milliseconds (<5th percentile); both are compared to those with P wave duration 100–105 milliseconds (20th to <40th percentile).
QT interval corrected by Framingham formula with prolongation defined as QTc ≥ 464 milliseconds (≥99th percentile) and shortening defined as ≤372 milliseconds (≤1st percentile), compared to the reference group with QTc 411–419 milliseconds (40th to <60th percentiles).
QT interval corrected by Framingham formula: defined as prolonged if ≥460 milliseconds in women and ≥450 milliseconds in men, and defined short if ≤390 milliseconds.
LVH defined as Sokolow‐Lyon voltage product ≥371,000 μV·ms, where the Sokolow‐Lyon voltage is calculated as (SV1 + RV5/RV6) × QRS duration.
Low‐frequency ectopy, defined as <10% of total beats (taken from a single 10 seconds ECG recording).
High‐frequency ectopy, defined as >10% of total beats (taken from a single 10 seconds ECG recording).
HR for admission for AF, per each increment of 10 supraventricular ectopic complex per hour.
HR per log‐unit increase in ectopy: increased hazard per doubling in ectopic beats per hour.
Ventricular ectopy defined as any PVC detected on a 2‐minute rhythm strip.
Ventricular ectopy defined as uniform PVCs noted on a 24‐hour ambulatory ECG recording.
Ventricular ectopy defined as multiform PVCs noted on a 24‐hour ambulatory ECG recording.
THE PR INTERVAL
On the surface ECG, the PR interval is defined as the period of time between the onset of the P wave (atrial depolarization) and the onset of the QRS complex (ventricular depolarization), and in most cases it is determined by conduction delay in the atrioventricular (AV) node. It was thought that the PR interval might serve as a convenient indicator of atrial conduction system disease because it is routinely reported on most 12‐lead ECGs obtained in clinical practice. In the Framingham Heart Study (FHS), PR interval prolongation >200 milliseconds was shown to be associated with increased risk for AF [multivariable‐adjusted hazard ratio (HR), 2.06; 95% confidence interval (CI), 1.36–3.12], pacemaker implantation (multivariable‐adjusted HR, 2.89; 95% CI, 1.83–4.57), and all‐cause mortality (multivariable‐adjusted HR, 1.44; 95% CI, 1.09–1.91).16 Similar findings were reported by other investigators,17, 18, 19, 20 and the PR interval was subsequently incorporated into a risk prediction tool for AF.21, 22
However, the association between PR interval prolongation and adverse outcomes has not been replicated consistently.23 In the Finnish Coronary Heart Disease (CHD) Study, there was no evidence of a relationship between PR interval duration and mortality or AF‐related hospitalization.24 Furthermore, in multiple additional studies, increased risk for AF was seemingly contradictorily observed in those with shorter PR intervals but not in those with PR interval prolongation.25, 26 Soliman et al. presented data from the National Health and Nutrition Examination Survey (NHANES), suggesting that the discrepant associations between the PR interval and mortality may be because of variation in the degree of contribution of the P wave duration to the PR interval in the different populations studied.23 Thus, it seems unlikely that the PR interval will prove to be the ECG marker most reflective of abnormal atrial substrate and consequent AF risk.
THE P wave INDICES
The P wave indices are quantitative measures of atrial electrical function derived from the surface ECG (Fig. 1).27 These measurements have the advantage of characterizing atrial electrical activity during depolarization without assessing other features of cardiac electrophysiology such as conduction delay through the AV node.
Figure 1.
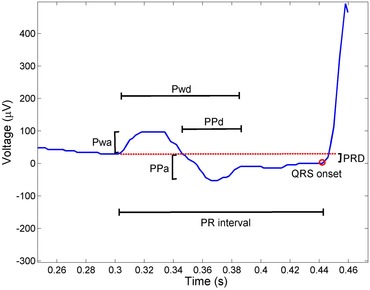
A representative P wave and PR segment in lead V1. The PR interval, P wave duration (Pwd), P wave amplitude (Pwa), P′ amplitude (PPa), P′ duration (PPd), and PR segment depression (PRD) are marked. The P wave terminal force (PTFV1) is derived by multiplication of PPa by PPd.
P Wave Duration
The P wave duration quantifies the time required for atrial depolarization: prolongation of this interval is indicative of delayed intra‐ or interatrial conduction. The finding of P wave prolongation on signal‐averaged ECG has been demonstrated to predict AF after cardiac surgery,28 and after cardioversion.29 Soliman et al. reported in the Atherosclerosis Risk in Communities (ARIC) Study that P wave duration was consistently associated with incident AF.17 Magnani et al. reported similar findings in a cohort from FHS: a maximum P wave duration >95th percentile was associated with an HR of 2.19 for the development of AF (95% CI, 1.13–5.57), and the association remained significant after adjustment for confounding risk factors, including the PR interval. Notably, the hazard was greater than that of a PR interval >95th percentile (HR, 1.65; 95% CI, 0.83–3.03).30 The association between P wave duration and incident AF has been confirmed in additional cohorts.20, 31 Again, however, there appears to be a nonlinear relationship between this P wave index and patient outcomes: several small case‐control studies reported a relationship between shorter P wave durations and AF, and a likely U‐shaped relationship between P wave duration and incident AF was further elucidated in the Copenhagen ECG Study (HR, 1.60 for P wave duration ≤89 milliseconds; 95% CI, 1.41–1.81).31 The investigators hypothesized that more rapid atrial conduction, as exhibited by a shorter P wave duration, might predispose to AF because of its greater likelihood to initiate reentry.
P Wave Terminal Force and Deep Terminal Negativity of P′ in V1
P wave terminal force in lead V1 (PTFV1) is a measurement of the terminal negative deflection in the P wave (i.e., P′), itself a reflection of left atrial activation. It is calculated by multiplying the P′ duration in lead V1 (PPdV1) by the P′ amplitude in lead V1 (PPaV1).32 Although commonly regarded as a sign of left atrial enlargement when abnormal (usually ≤–0.04 mm·s), the observation was made long ago that electrocardiographic left atrial abnormality is more consistently a sign of delayed interatrial conduction than of left atrial size.33 Indeed, PTFV1 had been shown to be associated with stroke, even when controlled for echocardiographic left atrial enlargement.34 In the PRIMERI study, a single‐center prospective cohort of hospitalized patients with ECG evidence of structural heart disease, PTFV1 was significantly associated with the degree of left ventricular fibrosis and indices of left atrial function, as assessed by cardiac magnetic resonance imaging. Interestingly, PPdV1 was most strongly associated with fibrosis and this association was significantly stronger than that of the P wave duration, whereas PPaV1 was most strongly associated with indices of atrial mechanical function such as left atrial volume and left atrial strain.35 PTFV1 has been validated as a predictor of incident AF in multiple large epidemiologic cohort studies. In the ARIC study, PTFV1 was significantly associated with incident AF in a multivariable model adjusted for major confounders (HR, 1.23 per SD change; 95% CI, 1.20–1.65).17 In the CHD study, PTFV1 ≤ −0.06 mm·s was significantly associated with both incident AF (HR, 1.91; 95% CI, 1.34–2.73) and all‐cause mortality (HR, 1.76; 95% CI, 1.45–2.12) in multivariable models adjusted for age, sex, BMI, and other confounding factors.36 Although PTFV1 was not significantly predictive of AF in FHS, this may be explained by differences in measurement: automated measurement of PPaV1 is more robust and reproducible than measurement of PPdV1 (unpublished observations). Deep terminal negativity of P′ in V1 is a simplified measure of interatrial conduction abnormality and of PPaV1, and it is defined as the presence of a biphasic P wave (positive/negative) in V1 with terminal negative phase amplitude (i.e., PPaV1) <−100 μV. In the ARIC and NHANES studies, it was independently associated with incident AF,37 cardiovascular mortality,38 and sudden cardiac death.37 Deep terminal negativity of P′ in V1 may be stronger predictor of adverse outcomes and incident AF than PTFV1.
Interatrial Block
Synchronous atrial systole requires unencumbered propagation of the electrical wave front from the right atrium to the left atrium. Bachmann's bundle is considered to be the most important pathway for interatrial conduction, and disruption of Bachmann's bundle (“advanced inter‐atrial block”) in animals has been shown to cause prolongation of the P wave and specific alterations in P wave morphology (P wave prolongation with biphasic P waves in the inferior leads).39 Disruption of posterior interatrial fibers is felt to cause partial interatrial block, leading to P wave prolongation alone (with or without increased PTFV1).40 As mentioned previously, the pattern of electrocardiographic left atrial abnormality (and thus also of abnormal PTFV1 or P′ deep terminal negativity) is more indicative of interatrial conduction disease than of anatomic or hemodynamic features of the left atrium.33 Furthermore, it appears that interatrial conduction delay may represent an intermediate phenotype on the pathway to AF: in a study of 612 patients referred for electrophysiological studies, invasively assessed interatrial conduction time was a strong predictor of incident AF.41
Although specific P wave morphologies on the surface ECG correlate reasonably well with patterns of interatrial conduction assessed by noninvasive electroanatomic mapping,42 it is unclear if these findings will be useful for predicting incident AF in the general population. In the ARIC study, advanced interatrial block was rare (0.55% of subjects), and not significantly associated with incident sudden cardiac death (HR, 1.20; 95% CI, 0.43–3.37).37
P Wave Area
P wave area has been proposed as a marker for abnormal atrial structure (e.g., left atrial enlargement), hence risk for AF. In the ARIC study, mean P wave area was found to predict incident AF (HR, 1.17 per SD change; 95% CI, 1.01–1.41, adjusted for multiple confounders).17 The association was not significant in FHS, and when data from the ARIC study and FHS were combined, the result did not meet statistical significance after adjustment for other clinical predictors of AF.43 It is likely that P wave area only weakly predicts incident AF in the general population.
P Wave Axis
Despite also being routinely reported on most 12‐lead ECGs obtained in clinical practice, the frontal P wave axis generally receives little attention and is known primarily for its correlation with the severity of pulmonary disease.44 Among 7501 participants of NHANES followed for a median of 13.8 years, an abnormal frontal P wave axis (outside of 0°–75°) was found to be associated with increased risk for all‐cause and cardiovascular mortality, and the association remained significant after adjustment for confounders, including history of asthma and COPD.45 Although the P wave axis is a reflection of anatomical features such as the positioning of the atria within the thoracic cavity and the relative size of the atria, P wave axis changes also reflect abnormal atrial electrical wave front propagation in a diseased myocardium. P wave axis was a significant predictor of incident AF in a large retrospective cohort of U.S. veterans.46 Importantly, only the frontal P wave axis is routinely reported on the 12‐lead ECG: atrial depolarization occurs in three‐dimensional space, and its true directionality is better by the three‐dimensional P loop (Fig. 2). Further studies are needed to more fully characterize spatial P loop vectors and morphology and the association between three‐dimensional depolarization vector changes and incident AF.
Figure 2.
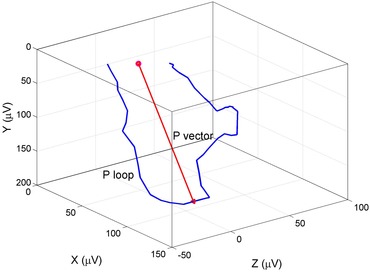
The vectorcardiographic P loop morphology and the spatial P loop vector characterize atrial conduction.
ASSESSMENT OF ATRIAL REPOLARIZATION
The P wave reflects atrial depolarization. However, it is clear from animal models and small human studies that abnormal atrial repolarization likely plays an important role in the pathogenesis of AF. Unfortunately, the surface ECG representation of atrial repolarization, the Ta wave, is poorly visualized except in cases of advanced heart block, because of its small amplitude and its coincidence with the much larger amplitude QRST complex.47 In a case‐control study of subjects with third‐degree AV block, those with paroxysmal AF had shorter PTa intervals and Ta waves peaked earlier and exhibited greater peak negative amplitudes.48 Invasive findings of abnormal atrial repolarization have been associated with AF inducibility. Unfortunately, these findings cannot be replicated in large cohorts or used as risk prediction tools in the general population, but they do suggest that markers of atrial repolarization might be informative.
Several lines of evidence suggest that the initial period of atrial repolarization occurs during the PR segment,49 and shortened atrial repolarization or dispersion of atrial repolarization might be visible on the surface ECG as PR segment depression. Patients with short QT syndrome, who are at increased risk for AF as well as sudden cardiac death, exhibit greater prevalence of electrocardiographic PR segment depression when compared to controls.50 Animal models of this disorder suggest that dispersion of atrial refractory periods may contribute to arrhythmogenesis in AF.51 Furthermore, patients with inferior myocardial infarctions with PR depression appear to be at higher risk for AF.52, 53 It is not clear if PR depression in the general ambulatory population is associated with AF or other adverse outcomes. More research is needed to characterize the invasive electrophysiologic correlates of PR segment depression and further characterize early atrial repolarization noninvasively. Additionally, investigation of whether PR depression is associated with relevant clinical outcomes such as AF in larger cohorts is also needed.
QT INTERVAL
The electrocardiographic QT interval is predominantly a reflection of the time required for ventricular repolarization. Prolongation of the QT interval (or heart rate‐corrected QT interval, QTc) is a well‐established risk factor for sudden cardiac death and all‐cause mortality.54 Because of the observation that hereditary short QT and long QT syndromes are associated with increased prevalence of AF,55, 56 and the assumption that the same pathophysiology which affects the ventricles also affects the atria, it has been suggested that the QT interval might be a suitable surrogate measure for assessment of abnormal atrial repolarization. The association between the QT interval and incident AF was initially noted in the PROSPER study, a randomized clinical trial investigating the use of pravastatin for the prevention of cardiovascular events in elderly individuals.20 Later, in an analysis of 281,277 subjects enrolled in the Copenhagen ECG Study who were followed for a median of 5.7 years (interquartile range, 3.2–8.4 years), risk for AF increased in a dose‐dependent fashion for those with QTc intervals ≥420 milliseconds, as well as for those with abnormally low QTc intervals (≤372 milliseconds).57 Data from ARIC, CHS, and the Health ABC cohorts confirmed the relationship between prolonged QT interval and risk for AF, but failed to demonstrate a statistically significant association between short QT and AF, perhaps because of more limited power.58
LEFT VENTRICULAR HYPERTROPHY
Despite their limited sensitivity and variable definitions, electrocardiographic criteria for left ventricular hypertrophy (LVH) are capable of identifying individuals at higher risk for multiple adverse outcomes. In FHS, electrocardiographic LVH was a significant predictor of incident AF before, but not after adjustment for confounders.59 However, other studies have reported a more consistent relationship. Watanabe et al. reported a significant association between ECG‐LVH and incident AF (HR, 1.39; 95% CI, 1.11–1.75) in a large Japanese cohort of community‐dwelling adults.60 In the Multi‐Ethnic Study of Atherosclerosis (MESA), 4942 individuals were assessed by cardiac magnetic resonance (CMR) imaging, as well as ECG, and followed for incident AF events. When assessed by Sokolow‐Lyon voltage, Sokolow‐Lyon voltage product, or Perugia score, ECG‐LVH was a significant predictor of incident AF even after adjustment for multiple confounders.61
ATRIAL AND VENTRICULAR ECTOPY
Premature atrial complexes (PACs) are the electrocardiographic manifestation of early atrial depolarization initiated from a site outside the sinoatrial node. They are commonly seen on extended ECG recordings in healthy individuals and have long been regarded as a benign ECG finding. However, when they occur frequently they can be harbingers of AF and adverse cardiovascular outcomes. It was previously demonstrated that PACs initiate episodes of AF in vulnerable individuals, and targeted ablation of atrial ectopy can reduce AF recurrence.62
Subsequent studies described an association between PACs and incident AF in stroke patients,63 and in those presenting with palpitations, dizziness, or syncope.64 In 2010, Binici et al. reported a significant relationship between excessive supraventricular ectopy and incident AF in 678 European middle‐aged to elderly adults selected to undergo 24‐hour ambulatory ECG monitoring.65 Dewland et al. confirmed that PAC burden is an independent predictor of incident AF in the general elderly population: among 1260 adults enrolled in CHS who underwent 24‐hour ambulatory ECG monitoring, median hourly PAC count was significantly predictive of incident AF (top quartile HR, 4.92; 95% CI, 3.39–7.16), cardiovascular mortality (top quartile HR, 1.50; 95% CI, 1.08–2.08), and all‐cause mortality (top quartile HR, 1.35; 95% CI, 1.10–1.66) over a follow‐up period of approximately 13 years.66 Similar findings were noted in the Swedish Malmö Diet and Cancer Study, confirming the value of PAC burden as a predictor of incident AF.67
Although PACs appear to be more related to arrhythmogenesis in AF, Watanabe et al. noted in 2006 that premature complexes are associated with incident AF regardless of their origin.60 In the ARIC study, the presence of premature ventricular complexes (PVCs) on a 2‐minute rhythm strip was a significant predictor of incident AF and stroke.68 Interestingly, in a large retrospective study of Taiwanese patients referred for ambulatory ECG monitoring, those with multiform PVCs but not uniform PVCs had increased risk for incident AF and heart failure.69 It is possible that the presence of PVCs corresponds to adverse changes throughout the atrial and ventricular myocardium which in the atria predispose to the development of AF.
INTEGRATION INTO CLINICAL PREDICTION TOOLS
The general goal of characterizing risk for incident AF in the general population has led to the production of several risk prediction tools,21, 22, 26, 70 with the intention that these models would serve clinicians and researchers seeking to identify effective primary prevention therapies for AF. The FHS AF risk score was developed in 2009 and included patient clinical characteristics as well as the PR interval as predictor variables. Although it performed moderately well in the middle‐aged to elderly white population from which it was derived (C‐statistic = 0.78),21 the performance was somewhat less impressive when the score was applied to two other more geographically and racially diverse cohorts (C‐statistic = 0.68).22 The ARIC risk prediction tool was derived from a population of American blacks and whites and included electrocardiographic P wave duration and LVH as predictors. It too performed relatively well, with a C‐statistic of 0.78,70 and has been shown to correlate with underlying structural changes in the atria, such as infiltrated atrial adipose tissue.71
The most recent tool, the CHARGE‐AF risk score, was derived from data from three large cohorts (ARIC, CHS, and FHS) and validated in two additional cohorts—the Age, Gene and Environment Study (AGES) and the Rotterdam Study (RS). It determines AF risk on the basis of age, race, height, weight, blood pressure, smoking status, antihypertensive medication use, and the presence or absence of diabetes, heart failure, and prior myocardial infarction. The C‐statistic was 0.765 for the pooled derivation cohort, 0.664 for AGES, and 0.705 for RS. Interestingly, although PR interval and electrocardiographic LVH were predictive of AF as individual risk factors, they did not add to the overall predictive ability of the model.26 Addition of PR interval, P wave duration, P wave area, and P wave terminal force to the CHARGE‐AF risk score did not significantly improve AF risk assessment.43 Other proposed electrocardiographic predictors of AF were not assessed. However, when Dewland et al. recently compared the discriminatory ability of the FHS AF risk score to the PAC count alone in a random selection of CHS participants, the results were similar (C‐statistic = 0.65, for the FHS AF score; and C‐statistic = 0.69, for PAC count), and addition of the PAC count to the Framingham model significantly enhanced its predictive power (C‐statistic = 0.72).66
CONCLUSION
Despite over 100 years of use in clinical practice, the ECG continually demonstrates its ability to yield new discoveries. It is a convenient, low‐cost, and ubiquitous clinical tool that holds promise for AF risk prediction. However, further work is needed to characterize abnormalities that are associated with abnormal atrial substrate (e.g., fibrosis) as well as propensity for triggered activity (e.g., atrial ectopy), and the association between those findings and incident AF in large cohorts. A number of areas remain relatively unexplored—ECG markers of atrial repolarization, vectorcardiographic P loops, and the role of changes in ECG predictors over time (data from serial ECGs). Current risk prediction models utilize little if any of the wealth of information available from the standard 12‐lead ECG, and additional work will need to be done to characterize the improvement of predictive models’ classification ability when novel ECG predictors are added, either in isolation, or in combination. Finally, because of their ability to demonstrate intermediate electrophysiological phenotypes on the pathway to AF, ECG markers could potentially serve as surrogate end points in future randomized clinical trials testing primary prevention interventions.
Conflict of Interest Statement: D.M. German, M.M. Kabir, T.A. Dewland, C.A. Henrikson, and L.G. Tereshchenko have no relevant financial and/or personal relationships with people or organizations that could inappropriately influence (bias) this work.
This work was partially supported by 1R01HL118277 (L.G. Tereshchenko).
REFERENCES
- 1. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 3. Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 4. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 5. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 6. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 7. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 8. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 9. Chen LY, Sotoodehnia N, Buzkova P, et al. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med 2013;173:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 11. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 12. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 13. Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 14. Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ 2008;11:281–298. [DOI] [PubMed] [Google Scholar]
- 15. Iwasaki YK, Nishida K, Kato T, et al. Atrial fibrillation pathophysiology: implications for management. Circulation 2011;124:2264–2274. [DOI] [PubMed] [Google Scholar]
- 16. Cheng S, Keyes MJ, Larson MG, et al. Long‐term outcomes in individuals with prolonged PR interval or first‐degree atrioventricular block. JAMA 2009;301:2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soliman EZ, Prineas RJ, Case LD, et al. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magnani JW, Wang N, Nelson KP, et al. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition study. Circ Arrhythm Electrophysiol 2013;6:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen JB, Pietersen A, Graff C, et al. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG Study. Heart Rhythm 2013;10:1249–1256. [DOI] [PubMed] [Google Scholar]
- 20. Macfarlane PW, Murray H, Sattar N, et al. The incidence and risk factors for new onset atrial fibrillation in the PROSPER study. Europace 2011;13:634–639. [DOI] [PubMed] [Google Scholar]
- 21. Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schnabel RB, Aspelund T, Li G, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med 2010;170:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soliman EZ, Cammarata M, Li Y. Explaining the inconsistent associations of PR interval with mortality: the role of P‐duration contribution to the length of PR interval. Heart Rhythm 2014;11:93–98. [DOI] [PubMed] [Google Scholar]
- 24. Aro AL, Anttonen O, Kerola T, et al. Prognostic significance of prolonged PR interval in the general population. Eur Heart J 2014;35:123–129. [DOI] [PubMed] [Google Scholar]
- 25. Knuiman M, Briffa T, Divitini M, et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur J Epidemiol 2014;29:181–190. [DOI] [PubMed] [Google Scholar]
- 26. Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol 2009;2:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E. Value of the P‐wave signal‐averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88:2618–2622. [DOI] [PubMed] [Google Scholar]
- 29. Budeus M, Hennersdorf M, Perings C, Wieneke H, Erbel R, Sack S. Prediction of the recurrence of atrial fibrillation after successful cardioversion with P wave signal‐averaged ECG. Ann Noninvasive Electrocardiol 2005;10:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons >/ = 60 years old (from the Framingham Heart Study). Am J Cardiol 2011;107:917–21e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nielsen JB, Kuhl JT, Pietersen A, et al. P‐wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm 2015;12(9):1887–95. [DOI] [PubMed] [Google Scholar]
- 32. Morris JJ, Jr. , Estes EH, Jr. , Whalen RE, Thompson HK, Jr. , McIntosh HD. P‐wave Analysis in Valvular Heart Disease. Circulation 1964;29:242–252. [DOI] [PubMed] [Google Scholar]
- 33. Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol 1977;39:967–971. [DOI] [PubMed] [Google Scholar]
- 34. Kohsaka S, Sciacca RR, Sugioka K, et al. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke 2005;36:2481–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tiffany Win T, Ambale Venkatesh B, Volpe GJ, et al. Associations of electrocardiographic P‐wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Heart Rhythm 2015;12:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eranti A, Aro AL, Kerola T, et al. Prevalence and prognostic significance of abnormal P terminal force in lead V1 of the ECG in the general population. Circ Arrhythm Electrophysiol 2014;7:1116–1121. [DOI] [PubMed] [Google Scholar]
- 37. Tereshchenko LG, Henrikson CA, Sotoodehnia N, et al. Electrocardiographic deep terminal negativity of the P wave in V(1) and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc 2014;3:e001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tereshchenko LG, Shah AJ, Li Y, Soliman EZ. Electrocardiographic deep terminal negativity of the P wave in V1 and risk of mortality: the National Health and Nutrition Examination Survey III. J Cardiovasc Electrophysiol 2014;25:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Campenhout MJ, Yaksh A, Kik C, et al. Bachmann's bundle: a key player in the development of atrial fibrillation? Circ Arrhythm Electrophysiol 2013;6:1041–1046. [DOI] [PubMed] [Google Scholar]
- 40. Platonov PG. P‐wave morphology: underlying mechanisms and clinical implications. Ann Noninvasive Electrocardiol 2012;17:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deftereos S, Kossyvakis C, Efremidis M, et al. Interatrial conduction time and incident atrial fibrillation: a prospective cohort study. Heart Rhythm 2014;11:1095–1101. [DOI] [PubMed] [Google Scholar]
- 42. Holmqvist F, Husser D, Tapanainen JM, et al. Interatrial conduction can be accurately determined using standard 12‐lead electrocardiography: validation of P‐wave morphology using electroanatomic mapping in man. Heart Rhythm 2008;5:413–418. [DOI] [PubMed] [Google Scholar]
- 43. Magnani JW, Zhu L, Lopez F, et al. P‐wave indices and atrial fibrillation: cross‐cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2015;169:53–61 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Incalzi RA, Fuso L, De Rosa M, et al. Electrocardiographic signs of chronic cor pulmonale: A negative prognostic finding in chronic obstructive pulmonary disease. Circulation 1999;99:1600–1605. [DOI] [PubMed] [Google Scholar]
- 45. Li Y, Shah AJ, Soliman EZ. Effect of electrocardiographic P‐wave axis on mortality. Am J Cardiol 2014;113:372–376. [DOI] [PubMed] [Google Scholar]
- 46. Perez MV, Dewey FE, Marcus R, et al. Electrocardiographic predictors of atrial fibrillation. Am Heart J 2009;158:622–628. [DOI] [PubMed] [Google Scholar]
- 47. Holmqvist F, Carlson J, Platonov PG. Detailed ECG analysis of atrial repolarization in humans. Ann Noninvasive Electrocardiol 2009;14:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holmqvist F, Carlson J, Waktare JE, et al. Noninvasive evidence of shortened atrial refractoriness during sinus rhythm in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2009;32:302–307. [DOI] [PubMed] [Google Scholar]
- 49. Ihara Z, van Oosterom A, Hoekema R. Atrial repolarization as observable during the PQ interval. J Electrocardiol 2006;39:290–297. [DOI] [PubMed] [Google Scholar]
- 50. Tulumen E, Giustetto C, Wolpert C, et al. PQ segment depression in patients with short QT syndrome: a novel marker for diagnosing short QT syndrome? Heart Rhythm 2014;11:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nof E, Burashnikov A, Antzelevitch C. Cellular basis for atrial fibrillation in an experimental model of short QT1: implications for a pharmacological approach to therapy. Heart Rhythm 2010;7:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nagahama Y, Sugiura T, Takehana K, et al. PQ segment depression in acute Q wave inferior wall myocardial infarction. Circulation 1995;91:641–644. [DOI] [PubMed] [Google Scholar]
- 53. Jim MH, Siu CW, Chan AO, et al. Prognostic implications of PR‐segment depression in inferior leads in acute inferior myocardial infarction. Clin Cardiol 2006;29:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Y, Post WS, Blasco‐Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta‐analysis. Epidemiology 2011;22:660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giustetto C, Schimpf R, Mazzanti A, et al. Long‐term follow‐up of patients with short QT syndrome. J Am Coll Cardiol 2011;58:587–595. [DOI] [PubMed] [Google Scholar]
- 56. Johnson JN, Tester DJ, Perry J, et al. Prevalence of early‐onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm 2008;5:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nielsen JB, Graff C, Pietersen A, et al. J‐shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol 2013;61:2557–2564. [DOI] [PubMed] [Google Scholar]
- 58. Mandyam MC, Soliman EZ, Alonso A, et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm 2013;10:1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA 1994;271:840–844. [PubMed] [Google Scholar]
- 60. Watanabe H, Tanabe N, Makiyama Y, et al. ST‐segment abnormalities and premature complexes are predictors of new‐onset atrial fibrillation: the Niigata preventive medicine study. Am Heart J 2006;152:731–735. [DOI] [PubMed] [Google Scholar]
- 61. Chrispin J, Jain A, Soliman EZ, et al. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2014;63:2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 63. Wallmann D, Tuller D, Wustmann K, et al. Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: an opportunity for a new diagnostic strategy. Stroke 2007;38:2292–2294. [DOI] [PubMed] [Google Scholar]
- 64. Chong BH, Pong V, Lam KF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace 2012;14:942–947. [DOI] [PubMed] [Google Scholar]
- 65. Binici Z, Intzilakis T, Nielsen OW, et al. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 2010;121:1904–1911. [DOI] [PubMed] [Google Scholar]
- 66. Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med 2013;159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnson LS, Juhlin T, Juul‐Moller S, et al. A prospective study of supraventricular activity and incidence of atrial fibrillation. Heart Rhythm 2015;12(9):1898–904. [DOI] [PubMed] [Google Scholar]
- 68. Agarwal SK, Heiss G, Rautaharju PM, Shahar E, Massing MW, Simpson RJ,Jr . Premature ventricular complexes and the risk of incident stroke: the Atherosclerosis Risk In Communities (ARIC) Study. Stroke 2010;41:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lin CY, Chang SL, Lin YJ, et al. Long‐term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol 2015;180:80–85. [DOI] [PubMed] [Google Scholar]
- 70. Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tereshchenko LG, Rizzi P, Mewton N, et al. Infiltrated atrial fat characterizes underlying atrial fibrillation substrate in patients at risk as defined by the ARIC atrial fibrillation risk score. Int J Cardiol 2014;172:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


