Highlights
-
•
Spatial working memory load modulates activation in fronto-parietal regions in youth.
-
•
Performance correlates with load-dependent deactivation in default network regions.
-
•
Performance correlates with functional coupling between fronto-parietal regions.
-
•
Executive function scores at age 3, 6 and 9 predict performance and brain activity.
Keywords: Neurocognitive development, Spatial working memory, Fronto-parietal network, Default network, Preadolescence, fMRI
Abstract
Spatial working memory is a central cognitive process that matures through adolescence in conjunction with major changes in brain function and anatomy. Here we focused on late childhood and early adolescence to more closely examine the neural correlates of performance variability during this important transition period. Using a modified spatial 1-back task with two memory load conditions in an fMRI study, we examined the relationship between load-dependent neural responses and task performance in a sample of 39 youth aged 9–12 years. Our data revealed that between-subject differences in task performance was predicted by load-dependent deactivation in default network regions, including the ventral anterior cingulate cortex (vACC) and posterior cingulate cortex (PCC). Although load-dependent increases in activation in prefrontal and posterior parietal regions were only weakly correlated with performance, increased prefrontal–parietal coupling was associated with better performance. Furthermore, behavioral measures of executive function from as early as age 3 predicted current load-dependent deactivation in vACC and PCC. These findings suggest that both task positive and task negative brain activation during spatial working memory contributed to successful task performance in late childhood/early adolescence. This may serve as a good model for studying executive control deficits in developmental disorders.
1. Introduction
As a central form of executive function, working memory is required for the optimal performance of goal directed behaviors. Spatial working memory refers to the temporary maintenance of object location and the ability to manipulate this information (Logie, 1995). Neuroimaging studies have shown that the neural architecture of spatial working memory in young adults involves a group of frontal and parietal regions including the dorsolateral prefrontal cortex (DLPFC), frontal eye fields (FEF), pre-supplementary motor area (pre-SMA), superior (SPL) and inferior parietal lobules (IPL) and intraparietal sulcus (IPS) (Courtney et al., 1997, Leung et al., 2004). Similar frontal and parietal regions are found to be involved in spatial working memory in children and adolescents (Geier et al., 2009, Nelson et al., 2000, Thomas et al., 1999), though younger individuals (aged 9–12) appeared to show weaker neural responses to increased memory load compared to older individuals (aged 13–18) (Klingberg et al., 2002) and even weaker in comparison to adults (aged 20–29) (Thomason et al., 2009). This weaker load-related activation is thought to be related to lower working memory capacity and immature fronto-parietal function in youth (Giedd and Rapoport, 2010, Klingberg et al., 2002, Scherf et al., 2006).
Several cross-sectional neuroimaging studies have examined the relationship between frontal and parietal activation and spatial working memory performance in youth, but yielded mixed findings. An earlier study showed that activations in the left superior frontal sulcus and left posterior parietal cortex (PPC) during spatial delayed recognition were associated with better working memory capacity, measured outside the scanner, in a group of 9–18 year olds (Klingberg et al., 2002). Greater activation in the left PPC was also associated with better performance on a spatial 1-back task during fMRI in a group of youth aged 12–17, with the inferior parietal areas further associated with better executive function measured outside of the scanner using the Trail Making Task (Nagel et al., 2005). However, the same study found a positive correlation between ventral anterior cingulate cortex (vACC) activation and working memory performance (measured by a backward digit span and an arithmetic task outside the scanner), while many other frontal areas including the medial superior frontal gyrus and bilateral inferior frontal gyri showed a negative correlation. In contrast, Olesen and colleagues (2007) found no significant correlation between spatial working memory performance and activation in the prefrontal or parietal areas in a group of 13 year olds, though a significant positive correlation between performance and parietal activation was found when adults (mean age 22.8) were included in the sample. These previous studies showed some evidence that better spatial working memory performance is associated with greater frontal and parietal activation in samples with a wider age range or in older individuals, though this may not extend to a narrower age range or younger individuals. Thus, the relationship between neuropsychological measures of working memory and executive function with frontal and parietal activation during working memory remains unclear.
Along with increases in fronto-parietal activation, task-related decreases are frequently seen in a set of regions including the vACC and posterior cingulate cortex (PCC) commonly known as the default network (Greicius and Menon, 2004, Greicius et al., 2003, Raichle and MacLeod, 2001, Shulman et al., 1997). Previous neuroimaging studies showed that the magnitude of vACC and PCC deactivation increases with increasing task demand as compared to rest/baseline conditions (Li et al., 2007, McKiernan et al., 2003, Thomason et al., 2008), though both task-positive and task-negative networks are based on contrasts relative to baseline. While the specific role of vACC and PCC in working memory remains unclear, it has been suggested that their deactivation is related to the ability to ignore task irrelevant information (Chadick and Gazzaley, 2011). Few studies have examined the role of default network regions in spatial working memory performance, though positive correlations between the degree of deactivation in default network regions and performance during non-spatial working memory tasks have been observed in adults (Anticevic et al., 2010, Prakash et al., 2012). It is less clear whether similar deactivation patterns exist in younger individuals. The default network is known to undergo functional and structural changes through adolescence (Fair et al., 2008, Supekar et al., 2010; for review see Power et al., 2010). In a recent fMRI study with a large group of youth (ages 8–21) it was found that greater task-related deactivation in default network regions predicted better performance on a non-spatial n-back task with fractal visual stimuli (Satterthwaite et al., 2013). However, an earlier fMRI study of spatial working memory in youth (age 12–17) reported both positive and negative correlations between performance and deactivation of different default network regions (Nagel et al., 2005). The mixed findings could be due to parameter differences across studies and in participant age ranges. Thus, studying task-related deactivation during working memory in comparison with task-related activation with a more restricted age range would extend our understanding of their contribution to performance variability in youth.
Our goal in this study was to examine the relationship between spatial working memory and activation of the fronto-parietal and default network regions during late childhood and early adolescence, as working memory ability varies widely across individuals in this age range (Farrell Pagulayan et al., 2006, Gathercole et al., 2004) and large variability in the development of various cortical systems have been reported (Giedd and Rapoport, 2010). We used a modified spatial 1-back task with two load conditions to investigate the relationship between neural responses to spatial working memory load and performance variability in a sample of youth with a narrow age range (9–12). We used whole brain multiple regression, regions of interest (ROI), and functional connectivity to analyze the data.
It is well recognized that pronounced individual differences in working memory performance exist throughout childhood and adolescence. Previous longitudinal studies suggested that frontal and parietal activity predicts cognitive abilities later in development (Darki and Klingberg, 2014, Dumontheil and Klingberg, 2012, Ullman et al., 2014), it is therefore plausible that executive function abilities at earlier stages of development predict performance and brain activation during a later stage. To address this latter issue, we examined whether executive function ability measured at prior time points (ages 3, 6 and 9) predicted current spatial working memory performance and load-related neural activation at time of scanning.
2. Methods
2.1. Participants
Participants were a subsample of a larger sample of 559 subjects enrolled in the Stony Brook Temperament Study. Families were recruited from the community using commercial mailing lists, and participants were evaluated at 3, 6, and 9 years of age. A subgroup of 80 participants were recruited for a neuroimaging study 1–3 years after the age 9 assessment. 68 of these participants (85%) completed the working memory task during fMRI. These participants had no history of major medical conditions and had at least one English speaking biological parent. 29 subjects were excluded from the analyses: 17 had missing behavioral data, 4 appeared to have misunderstood the task instructions (performing the task as a delayed match-to-sample task instead), 4 were behavioral outliers (with accuracy 3 standard deviations below average), and another 4 had excessive head motion during fMRI. Thus, 39 participants (age 9–12 [mean = 11.1 ± 0.7], 43.6% female) were included in the final analysis. The Pubertal Development Scale (PDS) (Petersen et al., 1988) was used to measure puberty score at the time of fMRI. The participants had a mean puberty score of 8.37 ± 2.36; one male participant did not complete the PDS. There was no significant difference in PDS scores between males (8.14 ± 2.13) and females (8.65 ± 2.67) (t < 1). Age was not correlated with puberty scores (r = 0.28, p = 0.089), probably due to the restricted age range. Informed consent was obtained from parents of the participants in accordance with the Stony Brook University Institutional Review Board.
2.2. Spatial working memory task paradigm and behavioral data analysis (current age)
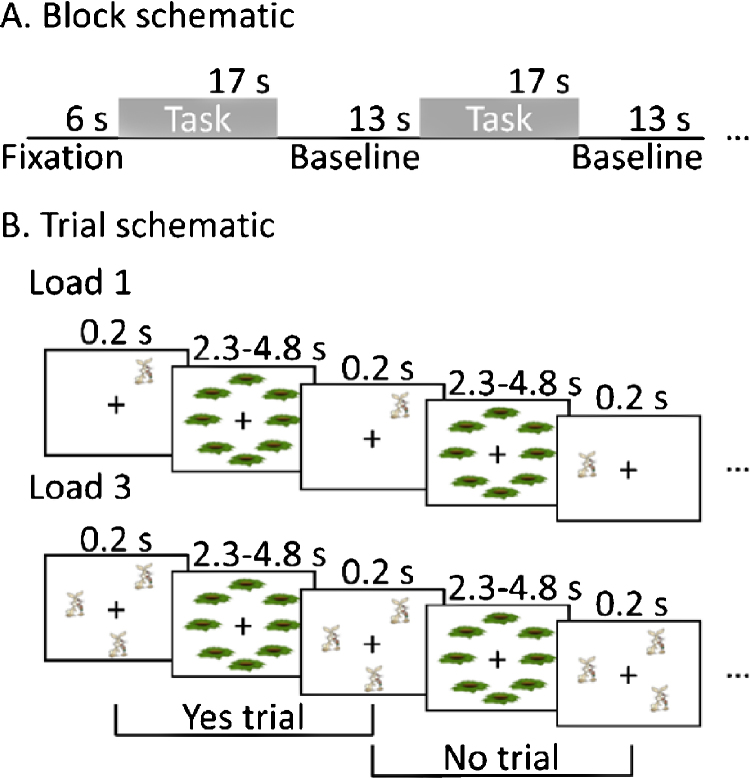
During fMRI, the participants performed a block design 1-back spatial working memory task with two load conditions (Load 1 and Load 3). See Fig. 1 for a schematic of the paradigm. During the Load 1 condition, participants tracked the location of 1 rabbit stimulus and in the Load 3 condition participants tracked the locations of 3 rabbit stimuli.
Fig. 1.
Spatial working memory task paradigm. (A) A schematic of the block sequence of task (Load 1 or Load 3) and fixation periods in a run. (B) A schematic showing a yes and no trial in Load 1 and in Load 3. In each task block, subjects were presented with either 1 (Load 1) or 3 (Load 3) rabbits at different locations and were required to track these locations and make yes/no responses through 5 trials per block. Delay between trials was jittered (2.3, 3.3 or 4.8 s). (Stimuli not to scale).
The two load conditions were presented in blocks of 5 trials. On each trial except the first trial of each block, participants pressed a button to indicate whether any rabbit was in a different location from the previous trial. In the Load 3 condition, only one of the rabbits ever changed location on a trial (the participants were made aware of this). To reduce verbalization, the rabbits were located randomly around an invisible circle with a radius of 5 degrees visual angle. The participants did not report verbalization as a strategy in the post experiment interview, though several reported a shape forming strategy. The rabbits were presented for 200 ms and were an average size of 1° in visual angle. There were 8 possible locations shifted between 5° to 10° from regular clock orientation to prevent verbalization and the locations were masked with 8 rabbit holes (average 1 degree of visual angle) presented during a variable inter-trial interval (ITI) of 2.3, 3.3 or 4.8 s, with an average ITI of 3.2 s. Each task block lasted 17 s and was followed by a 13 s no-task fixation period. Each run started with 6 s of dummy scans and 6 s of fixation and lasted 4 min 12 s. There were 2 runs and 8 task blocks per run, with 4 blocks per load condition per run, for a total of 16 blocks. Participant responses were collected with a button box held by both hands. Participants were instructed to respond with their left and right index fingers (yes and no responses were counterbalanced across participants). Each participant practiced the task for 10 min before scanning.
Behavioral data from the spatial working memory task were analyzed with SPSS (Statistics 22, IBM). For task performance we calculated d′, a measure of discriminability in signal detection theory, from the z-transformed hits and false alarm scores (Peterson et al., 1954). All trials except the first trial of each block contributed to the hits and false alarm calculation. The average reaction time (RT) of each participant was calculated from correct trials after removing outliers more than 3 standard deviations away from the participant's mean reaction time. Paired t-tests were used to examine the significance of task performance differences between the two load conditions. A bivariate correlation matrix was calculated by correlating age (in months), PDS score, d′ and RT to determine potential influence of age and pubertal status on task performance.
2.3. Behavioral measures of executive function at age 3, 6 and 9
At age 3, participants completed several subscales of the Developmental Neuropsychological Assessment (NEPSY) (Korkman et al., 1998). We used the visual attention (VAST) subscale as an index of visuo-spatial ability. This subscale requires the participant to scan an array of figures to locate and mark a target figure (a cat). The VAST subscale was scored by summing the number of correct targets, subtracting the number of incorrect targets and adjusting for speed.
At age 6, participants completed two tasks commonly used to index executive function. The Backward Color Span Task, which requires participants to name colors in reverse order of presentation (Gathercole and Pickering, 2000) and the Trail Making Task (Reitan and Wolfson, 1985), which requires participants to draw a trail through a number of letters and numbers, alternating between the two domains, in alphabetic and numerical order. The time taken to complete this trail was taken as the performance measure, which was reverse scored by reversing the sign to allow for compatible interpretation of the results across tasks.
At age 9, participants completed the standard Backward Digit Span Task, which requires them to remember a list of digits and then recall in reverse order. They also completed the Trail Making Task.
2.4. Image acquisition
Participants lay supine in a 3T Siemens Tim Trio whole-body MRI system (Siemens Medical Solutions, Erlangen, Germany) and viewed visual stimuli projected to a screen through a mirror mounted on the head coil. Whole brain, high resolution structural anatomical images were acquired in the sagittal plane with a T1 weighted MPRAGE scanning sequence (TR = 2400 ms; TE = 3.16 ms; slices = 176; flip angle = 8°; FOV = 256 × 256; matrix = 256 × 256; resolution = 1 × 1 × 1 mm3). A second set of T2-weighted structural images were collected in the axial plane oblique to the AC-PC (TR = 6450; TE = 88; slices = 37, 3.5 mm with 0 mm gap; flip angle = 120°; FOV = 256 × 256; matrix = 256 × 256; resolution = 1 × 1 × 3.5 mm3). Two runs of functional images were acquired in the axial plane oblique to the AC–PC with a T2*-weighted single-shot echo-planar pulse sequence (TR = 2000 ms; TE = 26 ms; slices = 37; flip angle = 90°; FOV = 224 × 224; matrix = 64 × 64; resolution = 3.5 × 3.5 × 3.5 mm3; 126 volumes per run).
Before scanning, all participants went through a mock scanning session to acclimate them to the scanning environment. The mock scanning was done with a MRI simulator (Model number: PST-100355 from Psychological Software Tools, USA). During mock scanning, an audio simulation of scanner noise was played to the participants. Feedback was given to the participants regarding head movement using the Flock of Birds motion tracking system (Ascension Technology Corporation, USA) and the MoTrack motion tracking software (Psychology Software Tools, USA). Motion feedback was only given to the participants during mock scanning. During both mock scanning and scanning sessions, the participants’ heads were stabilized in the head coil using foam pads around the head to minimize motion.
2.5. Image processing and analysis
All images were preprocessed in SPM8 (Welcome Trust Center for Neuroimaging, University College London, http://www.fil.ion.ucl.ac.uk/spm/). The first 3 volumes of each functional run were discarded to allow T2* signal to reach equilibrium. The remaining functional images were corrected for differences in slice acquisition timing and head motion. A mean EPI image was then generated from the realigned images. T2 weighted inplane structural images were co-registered with the mean EPI image, then MPRage images were co-registered to the T2 weighted inplane structural image. Segmentation was applied to the high resolution T1 structural image and segmented images were normalized to the Montreal Neurological Institute (MNI) template. The same transformation parameters were then applied to all functional images. Finally, all functional images were spatially smoothed with a Gaussian kernel of 6 mm at full-width half-maximum and were high-pass filtered with a cutoff of 1/128 Hz.
For each participant, a general linear model (GLM) was constructed that included two conditions (Load 1 and Load 3). Trial onsets for each load condition were entered as regressors in the GLM. Error trials were modeled with a separate regressor, but were not further investigated (14% on average, ranging from 5–30%). Head motion was further corrected by including 6 motion parameters in the x, y, z, roll, yaw and pitch, as well as motion outliers as regressors in the GLM. Motion outliers were defined as volumes with a scan-to-scan motion greater than 0.5 mm in either the x, y or z plane, or 0.01 radians in either the roll, pitch or yaw rotation, using the Artifact Detection Tools (ART) (http://www.nitrc.org/projects/artifact_detect/). On average 17% of scans were identified as outliers per run (ranging from 5% to 31% across subjects). Runs were excluded from analysis if more than 1/3 of volumes were outliers. There was no significant relationship between participants’ motion and age or between motion and performance (−0.2 < r's < 0.2, p's > 0.1). A second level univariate analysis was done to investigate blood–oxygen-level-dependent (BOLD) activation in response to load demand (Load 3 > Load 1) using a t-test.
A multiple regression was performed to examine group level correlations between load-dependent brain activation, age (in months) and task performance (d′) across participants. A similar multiple regression was done with PDS scores and task performance (d′). In each multiple regression model, we entered each participant's Load 3 > Load 1 contrast values into the model with age/PDS scores and performance as covariates. This regression model allowed us to investigate the relationship between performance and BOLD responses to load with developmental measures (age and PDS scores) being controlled for and vice versa. Three more multiple regressions were conducted to test if the results were sensitive to model parameters. Each model used the Load 3 > Load 1 contrast from all participants but included only one covariate either age, PDS scores or performance. The combined models generated similar results to the independent models, so only the results from the combined models are reported here.
Unless otherwise stated, all whole-brain maps at the group level were thresholded at p < 0.001, uncorrected and statistical significance with multiple comparisons corrected at the cluster level at p < 0.05, false discovery rate (FDR). Figures are displayed at p < 0.001, uncorrected with clusters size ≥12.
2.6. ROIs
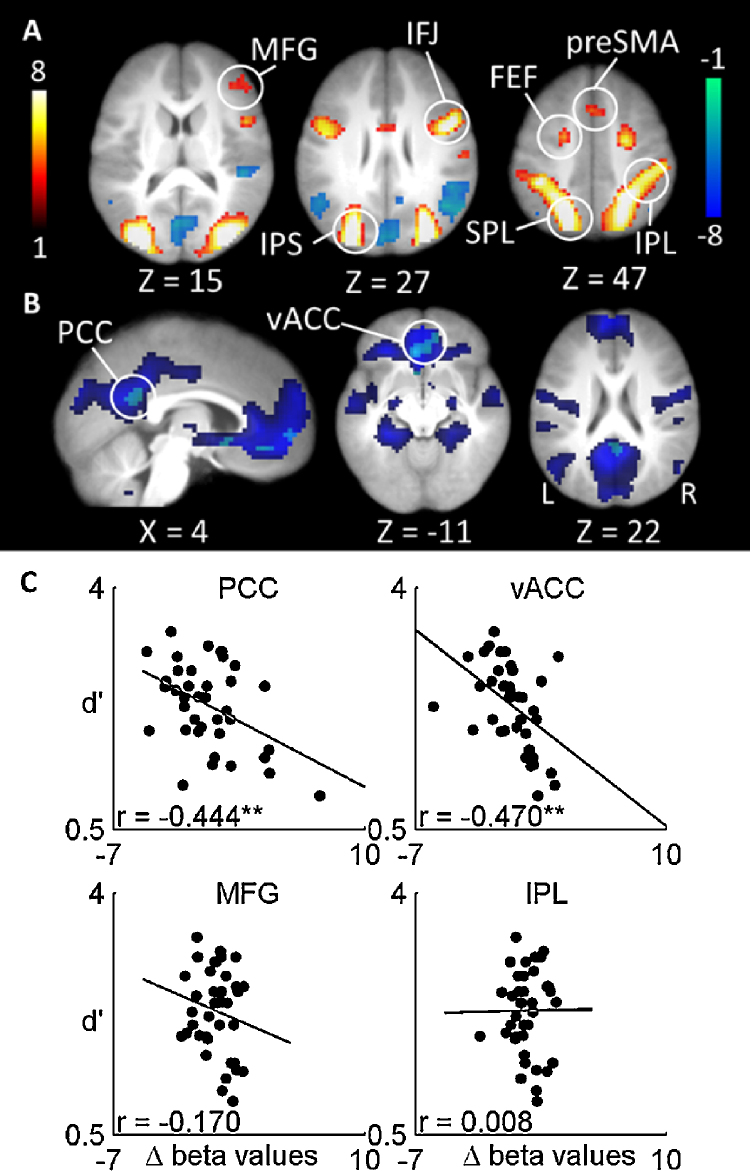
To further examine the relationship between load-dependent BOLD activation and behavioral measures, we defined a set of 4 ROIs (spheres of 8 mm radius) chosen from the literature. A single ROI was constructed from two spheres, one in the left and another in the right middle frontal gyrus (MFG) (−36, 44, 20 and 40, 32, 30 respectively), a second ROI was created with two spheres in the left and right IPL regions (−36, −50, 40 and 40, −48, 38 respectively), all spheres were centered on peak coordinates from Owen et al.’s (2005) meta-analysis of n-back task-related activations. These areas are known to be part of the executive control network. Two more ROIs were created from two default network regions were chosen from Laird et al.’s (2009) meta-analysis on the default network, a vACC ROI (2, 32, −8) (referred to as ACC in Laird et al., 2009) and a PCC ROI (−4, 52, 22). Mean beta values for each subject were extracted for the Load 3 > Load 1 contrast using the MarsBar toolbox (http://marsbar.sourceforge.net/). Pearson's r correlation was calculated between the mean beta values for each ROI with task performance (d′).
We further examined whether the regions that show correlations with behavior (from the multiple regression analyses) are also correlated with visuospatial attention and executive function behavioral measures collected prior to scanning, at ages 3, 6 and 9. A single ROI was created for this analysis by selecting significant clusters from the d′ multiple regression analysis (p < 0.001, uncorrected) (see Fig. 2(B)), including activation in the vACC (−6, 32, −11, cluster size = 77 voxels) and PCC (3, −52, 22, cluster size = 27 voxels).
Fig. 2.
(A) Effects of spatial working memory load. Load-related activations are shown with a contrast of Load 3 > Load 1. Greater increases and decreases in BOLD signal with load demand are shown in hot and cool colors, respectively. (B) Correlation between load-related BOLD signal and task performance (d′). Results are from multiple regression analyses overlaid on the task-related deactivations during both Load 1 and Load 3 (blue). Cyan color indicates regions that show a negative correlation between load-related activity and d′ across subjects. Whole-brain maps were cluster-corrected for multiple comparisons at p < 0.05 and displayed at p < 0.001, uncorrected with clusters size ≥12. (C) Scatter plots showing correlation between load-related BOLD signal (Load 3 > Load 1) in selected ROIs and task performance (d′) across subjects. Bilateral middle frontal gyrus (MFG; −36, 44, 20 and 40, 32, 30) and bilateral inferior parietal lobule (IPL; −36, −50, 40 and 40, −48, 3) ROIs were defined according to Owen et al. (2005) and the ventral anterior cingulate cortex (vACC; 2, 32, −8) posterior cingulate cortex (PCC; −4, −52, 22) ROIs were defined according to Laird et al. (2009). ** significant to p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Three more ROIs in frontal and parietal regions were constructed based on the univariate Load 3 > Load 1 contrast for functional connectivity analyses. These ROIs included the right MFG (sphere of 8 mm at 42, 35, 7), the inferior frontal junction (IFJ) (two spheres of 8 mm at −48, 2, 31 and 48, 5, 28), and the IPL (two spheres of 8 mm at −36, −37, 43 and 48, −31, 43). The vACC and PCC ROIs used for functional connectivity were the same as those used for the correlation with performance.
2.7. Functional connectivity analysis
Seed-based functional connectivity analysis was conducted using data from the entire working memory task period. Basic preprocessing was done in the same manner as the univariate analysis described above. Further processing was conducted on the data to make them suitable for functional connectivity analysis using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012; http://www.nitrc.org/projects/conn). Despiking was first applied to the data, followed by regressing out nuisance variables, including CSF and white matter signal, motion parameters and outlier scans (defined as global motion greater than 0.5). A band pass filter of 0.008–0.09 Hz bandwidth was applied last. Seeds were the ROIs in the right MFG, IFJ, IPL, PCC and vACC described in the last section. Average time series was calculated for each seed region, and then its correlations with the time series of voxels in the rest of the brain were calculated. Group level regression analysis was conducted to determine the correlations between seed-based functional connectivity and working memory task performance (d′) across subjects.
3. Results
3.1. Behavioral results
As expected, participants performed better on the Load 1 compared to Load 3 condition, with higher d′ (Load 1: 2.81 ± 0.62; Load 3: 1.79 ± 0.70; t(38) = 8.44, p < 0.001) and faster response times (Load 1: 1107 ± 272 ms; Load 3: 1210 ± 304 ms; t(38) = −4.29, p < 0.001). There was no main effect of gender or interaction between load and gender (F's < 1) on d′. There was also no effect of gender on RT (F < 1), but there was a trend toward significance in interaction between gender and load (F(1,37) = 3.72, p = 0.062). No significant correlations were found between PDS scores, age, d′ and RT (−0.1 < r's < 0.1, p's > 0.1), though the correlation between age and PDS scores was trending to significance (r = 0.28, p = 0.089).
There were significant positive correlations between spatial working memory performance (d′) at the current age and Trail Making Task (reversed) at age 9 (r = 0.42, p < 0.01) and age 6 (r = 0.50, p < 0.01) and a trend toward significance with NEPSY VAST scores at age 3 (r = 0.31, p = 0.051). Correlations between d′ and Backward Digit Span Task at age 9 (r < 0.3, p > 0.09) and Backward Color Span Task at age 6 (r < 0.2, p > 0.10) did not reach the significance threshold. Taken together, better spatial working memory task performance during the present fMRI study was associated with previous measures of executive function at younger ages.
3.2. Working memory load-dependent activation and deactivation
We observed stronger activation in the prefrontal and parietal cortices during the Load 3 condition relative to the Load 1 condition of the current spatial working memory task in these participants. Specifically, regions that showed a significant increase in activation with load included the pre-SMA, right MFG, bilateral FEF, IFJ, SPL, IPL and temporo-occipital regions (p < 0.05, FDR corrected clusters). See Fig. 2(A) for the group activation maps and Table 1 for the peak co-ordinates.
Table 1.
Suprathreshold activations in the Load 3 > Load 1 contrast, and correlations between load related activity and d′. Significance threshold is at p <0.05 FDR cluster corrected. Abbreviations: frontal eye fields (FEF), middle frontal gyrus (MFG), inferior frontal junction (IFJ), medial superior frontal gyrus (mSFG), supplementary motor area (SMA), pre-supplementary motor area (pre-SMA), superior parietal lobule (SPL), inferior parietal lobule (IPL), intraparietal sulcus (IPS), lateral occipital gyrus (LOG), inferior temporal gyrus (ITG), superior temporal gyrus (STG), sensorimotor cortex (SMC), supramarginal gyrus (SMG), ventromedial prefrontal cortex (vACC) and posterior cingulate cortex (PCC). L, left hemisphere; R, right hemisphere.
| Region | t value | Cluster size | MNI Coordinates (mm) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Load 3 > Load 1: Task Positive Activity | |||||
| Frontal | |||||
| L FEF | 6.15 | 139 | −24 | −1 | 58 |
| L IFJ | 7.63 | 190 | −48 | 2 | 31 |
| R FEF | 7.87 | 169 | 27 | −7 | 52 |
| R MFG | 5.38 | 80 | 42 | 35 | 7 |
| R IFJ | 10.20 | 224 | 48 | 5 | 28 |
| mSFG (pre-SMA) | 4.91 | 49 | −3 | 20 | 43 |
| Parietal | |||||
| L SPL | 11.22 | 2437 | −18 | −64 | 52 |
| L IPL | 9.54 | −36 | −37 | 43 | |
| L IPS | 10.36 | −24 | −73 | 31 | |
| R SPL | 12.87 | 2714 | 21 | −61 | 58 |
| R IPL | 8.49 | 48 | −31 | 43 | |
| R IPS | 11.07 | 30 | −82 | 31 | |
| Temporo-occipital | |||||
| L LOG/ITG b | 11.33 | −36 | −82 | 13 | |
| R LOG/ITG c | 12.72 | 48 | −64 | −8 | |
| Load 3 > Load 1: Task Negative Activity | |||||
| Frontal | |||||
| SMA | 4.43 | 108 | −3 | −22 | 46 |
| Parietal | |||||
| L SMG | 6.42 | 238 | −57 | −52 | 43 |
| R SMG | 5.71 | 321 | 48 | −58 | 28 |
| Precuneus/Cuneus | 5.61 | 345 | 0 | −82 | 31 |
| PCC | 5.56 | 71 | 12 | −55 | 31 |
| R SMC | 4.38 | 74 | 42 | −19 | 64 |
| Negative correlation between load effect and d′ in load-dependent regions | |||||
| vACC | 5.91 | 77 | −6 | 32 | −11 |
| PCCa | 3.85 | 27 | 3 | −52 | 22 |
Cluster at p < 0.001, uncorrected (FDR = 0.253).
Part of the L SPL cluster.
Part of the R SPL cluster.
Load-dependent decreases in activation were also observed, with stronger deactivation in the supplementary motor area (SMA), precuneus/cuneus, bilateral supramarginal gyrus (SMG), right sensorimotor cortex (SMC), and PCC in Load 3 compared to Load 1 (p < 0.05, FDR corrected clusters) (see Fig. 2(A) and Table 1).
3.3. Correlation between current spatial working memory performance and activation in fronto-parietal regions
To examine whether individual differences in load-dependent activation varied in association with their working memory performance (d′), we applied whole-brain multiple regression analyses. We did not find any suprathreshold correlations between load-related responses in frontal or parietal regions and performance at p < 0.001 (see Fig. 2(B) and (C)). As fronto-parietal activity has previously been associated with better performance, we examined whether such correlations exist at more lenient thresholds. Only a very lenient threshold (p < 0.05, uncorrected) revealed a positive correlation between performance (d′) and load-related responses in the right FEF and left IPL (data not shown). Using ROIs from the literature (Owen et al., 2005), we also found no significant correlations between task performance and load-related responses in MFG and IPL (r's < 0.2, p's > 0.1).
Functional connectivity analysis, however, revealed that better working memory performance across subjects is correlated with stronger coupling between the IFJ seed and bilateral SPL and IPL (p < 0.05, FDR corrected clusters) (see Fig. 3). Similar effects were not observed for the right MFG or IPL seeds.
Fig. 3.
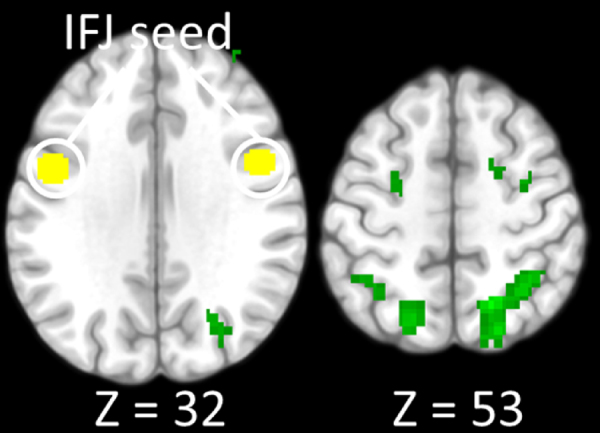
Functional connectivity of prefrontal areas in association with task performance. Bilateral inferior frontal junction seed (IFJ; −48, 2, 31 and 48, 5, 28) are presented in yellow. Green shows IFJ functional connectivity with posterior parietal areas that increases with higher performance scores (d′). Whole-brain maps were cluster-corrected for multiple comparisons at p = 0.05 and displayed at p < 0.001, uncorrected with clusters size ≥12. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Correlation between current spatial working memory performance and deactivation in default network regions
Whole-brain multiple regression analyses revealed suprathreshold negative across-participant correlations between task performance and load-dependent decreases in activation (Load 3 > Load 1) in the vACC (p < 0.05, FDR corrected clusters) and the PCC (p < 0.001, uncorrected) (see Fig. 2(B) and Table 1). These results showed that greater activation differences in the vACC and PCC areas for the high load relative to the low load condition (i.e., less deactivation at higher load) is associated with poorer working memory performance for this group of participants. Similar results were confirmed using ROIs from the literature (see Fig. 2(C)). However, seed regions in the default network did not show functional connectivity patterns that were significantly associated with task performance across participants.
3.5. Correlation between behavioral measures at age 3, 6 and 9 and current load-related responses
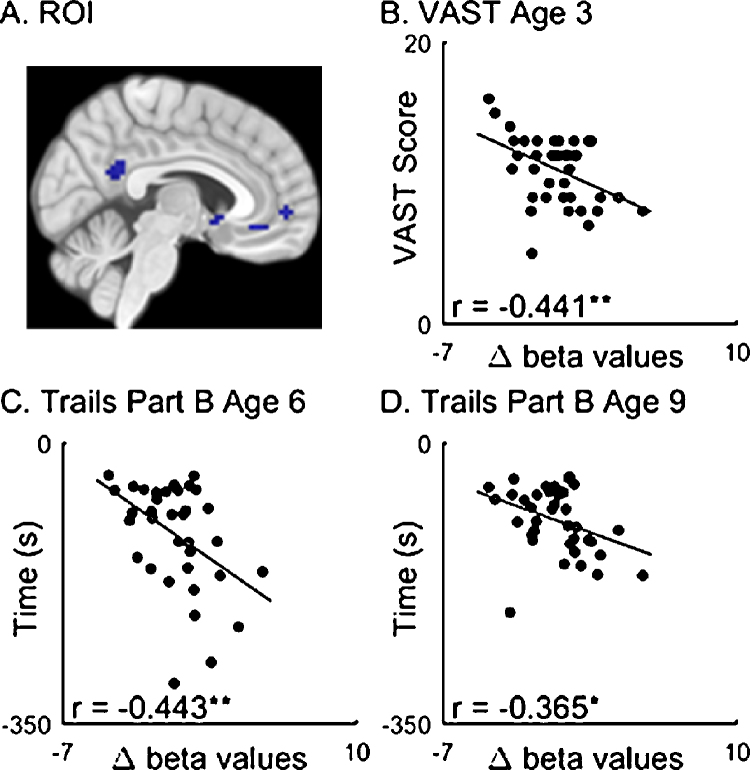
We further examined whether the parts of PCC and vACC that showed load-related responses in association with task performance were also associated with executive function performance measured at earlier ages: 3, 6 and 9. An ROI including the PCC and vACC clusters from the multiple regression between load-dependent activation and task performance was used in this analysis. As shown in Fig. 4, greater load-dependent deactivations in the ROI were correlated with better performance on the Trail Making Task at age 9 (r = −0.365, p < 0.05, which did not survive Bonferroni correction) and age 6 (r = −0.443, p < 0.01), as well as NEPSY VAST at age 3 (r = −0.441, p < 0.01). The correlation between load-dependent deactivation and Backward Digit Span Task at age 9 was significant (r = −0.406, p < 0.01), but not the Backward Color Span Task at age 6 (r = 0.007, p > 0.1).
Fig. 4.
Correlation between load-related deactivations (Load 3 > Load 1) and cognitive performance at earlier ages. (A) The ROI, comprised of vACC (−6, 32, −11) and PCC (3, −52, 22) was defined from clusters found in the whole brain multiple regression analysis. (B) NESPY visual attention (VAST) scores at age 3. (C and D). Trail Making Part B time scores (reverse scored with positive values for better performance) at ages 6 (C) and 9 (D). * significant to p < 0.05, ** significant to p < 0.01.
For comparison purposes, we also examined the associations between these attention/executive function measures from earlier time points and the load-related responses of the prefrontal and posterior parietal regions observed at the current age. There was a correlation between the right MFG ROI and backward digit span at age 9 (r = −0.32, p < 0.05, which did not survive Bonferroni correction), but no significant correlations were found between the IPL ROI and behavioral measures from earlier ages (−0.3 < r's < 0.3, p > 0.1). Taken together, these results suggest that load responses of the default network regions (vACC and PCC) in this group of participants at ages 9–12 are more closely related to their executive function at earlier time points.
4. Discussion
In this study, we investigated the relationship between fronto-parietal and default network regions and spatial working memory performance in a sample of youth in late childhood or early adolescence. We also investigated whether previous executive function abilities at younger ages were associated with current brain activation during working memory. We found load-dependent increases in BOLD signal in prefrontal and posterior parietal regions and load-dependent decreases in default network regions. Across subjects, better working memory performance was significantly associated with greater load-dependent deactivation of default network regions but not with activation of frontal or parietal regions. However, functional connectivity analysis revealed that stronger coupling between prefrontal (IFJ) and posterior parietal areas was significantly associated with better task performance across subjects. Intriguingly, we found that executive function measures taken at ages 3, 6, and 9 were also positively correlated with load-dependent deactivation of the default network at time of scanning (aged 9–12).
The increased activation in frontal and parietal regions and decreased activation in lateral and medial parietal regions with increasing memory load suggest that similar brain systems are involved during spatial working memory in 9–12 year old youth as in adults. These findings are consistent with the literature on spatial working memory in adults (Courtney et al., 1997, Hampson et al., 2006, Leung et al., 2002, McKiernan et al., 2003) and youth at this age (Thomason et al., 2008, Thomason et al., 2009). Increased prefrontal activation in response to a spatial working memory task compared to rest has been found in a near infrared spectroscopy study with preadolescent children between ages 3–7 (Perlman et al., 2015), suggesting that the fronto-parietal architecture for working memory is likely in place early in development, though changes to this system continue through adolescence (Geier et al., 2009, Klingberg, 2006, Klingberg et al., 2002, Luna and Sweeney, 2001, Scherf et al., 2006, Velanova et al., 2008).
Frontal and parietal activation have previously been associated with individual differences in spatial working memory performance in youth aged 9–18 (Klingberg et al., 2002, Olesen et al., 2007, Spencer-Smith et al., 2013). In our sample of 9–12 year olds, spatial working memory performance was not significantly correlated with load-dependent brain activation in fronto-parietal regions, even though robust load-dependent responses in these regions were evident. A possible reason for the different findings is differences in participant age ranges across studies. Previous studies that found fronto-parietal activation predicting performance used a wider age range including older adolescents (Klingberg et al., 2002, Mürner-Lavanchy et al., 2014, Nagel et al., 2005, Spencer-Smith et al., 2013) and young adults (Olesen et al., 2007) in their samples. When restricted to a younger group at age 13, Olesen et al. (2007) found no significant associations between activation in fronto-parietal regions and spatial working memory performance. Spencer-Smith and colleagues (2013) found that a neuropsychological measure of working memory performance using a shape location task with a progressively increasing span was positively correlated with posterior parietal activation during a spatial delayed match-to-sample task in older participants (10–12 years) but not in younger participants (7–9 years). Taken together, the magnitude of fronto-parietal activation and its relationship to working memory performance may be age dependent. The lack of a significant association between frontal load-dependent BOLD responses with performance at younger ages may be a result of the protracted development of the prefrontal cortex (Casey et al., 2005, Casey et al., 1997, Crone et al., 2006, Geier et al., 2009, Giedd and Rapoport, 2010, Scherf et al., 2006). As our study and others (Perlman et al., 2015) indicate that the prefrontal regions of older children and early adolescents are active during working memory, it is possible that these regions are involved in supporting working memory functions between ages 9 and 12 or younger, but in a somewhat different manner compared to older individuals. Indeed, our data showed that there was a significant positive association between the strength of fronto-parietal functional coupling during spatial working memory and performance differences across subjects. This suggests that there is a potentially greater role of network activation rather than regional activation in supporting good working memory performance at this stage of development, though further studies would be needed to determine if this network activation during both task and resting states reflect working memory performance.
In contrast to frontal and parietal regions, our data showed that better spatial working memory performance is significantly associated with greater load-related regional deactivation in vACC and PCC. This finding is consistent with the emerging body of literature showing that behavioral performance is associated with the degree of deactivation in the default network during non-spatial working memory tasks in both adults and youth (age 8–22) (Anticevic et al., 2012, Anticevic et al., 2010, Prakash et al., 2012, Sambataro et al., 2010, Satterthwaite et al., 2013). Our study shows that this correlation between task induced deactivation in default network regions and performance exists in spatial working memory as well as in a younger sample with a restricted age range. The exact function of vACC and PCC deactivation during working memory is a subject of intense debate in the adult literature. While many studies suggested that the medial default network regions play a general role in cognition (Anticevic et al., 2012, Spreng and Grady, 2010, Spreng et al., 2010), functional heterogeneity has also been found (Laird et al., 2009). Studies showing decreased activation in response to greater cognitive load (McKiernan et al., 2003, Thomason et al., 2008) have been interpreted as being a result of attention being allocated away from cognitive functions performed by default network regions (Binder, 2012) or reduction of internal or spontaneous thoughts (see Andrews-Hanna, 2012, Buckner et al., 2008 for review). A study of selective encoding of visual information showed that stronger functional connectivity between default network regions and visual association areas is associated with the ability to ignore task-irrelevant information (Chadick and Gazzaley, 2011). Therefore, it is possible that successfully shifting focus away from distraction and reduction of default network activation contribute to the success of working memory performance by focusing on task relevant information (which seemingly involves frontal and parietal activations [Corbetta and Shulman, 2002, Gazzaley and Nobre, 2012]). Indeed, it has been shown that deactivation of the default network predicted performance on a trial-by-trial basis in adults (Anticevic et al., 2010, Li et al., 2007).
Coupling between vACC and PPC both at rest (during the rest baseline of a working memory task and during resting state fMRI) and during a working memory task have previously been shown to be associated with non-spatial working memory performance in adults (Hampson et al., 2006, Sambataro et al., 2010) though we did not find that to be the case in our sample of youth. Some studies also suggested that the fronto-parietal and default network are negatively correlated (Barber et al., 2013, Chai et al., 2012, Fox et al., 2009) and that the degree of this anticorrelation during resting state predict behavioral performance in a non-spatial working memory task in adults (Hampson et al., 2010), as well as neuropsychological measures of working memory (Keller et al., 2015). In developmental populations (ages 8–24), it has been shown that the fronto-parietal network and default network anticorrelation during resting state scans seem to increase with age (Chai et al., 2014). It is possible that the relationship previously found between default network coupling and performance emerges from the interactions between the fronto-parietal network and default network, and that this cross-network architecture is not yet fully developed at the age of our sample (9–12). The coupling between default network and fronto-parietal network nodes may also differ as a function of different working memory processes. A recent study with a spatial delayed match-to-sample task found that the beta series correlation between PCC and DLPFC was only significant during the maintenance phase of the task, while the correlation within the fronto-parietal network was significant throughout the trial (Piccoli et al., 2015), suggesting that the default network interaction with the fronto-parietal network may vary across different stages of working memory. Further studies should examine whether they interact differently during spatial working memory in comparison to resting state (Kelly et al., 2008, Uddin et al., 2009).
The trajectory of maturation in brain systems and behavioral performance is highly variable between individuals (Giedd and Rapoport, 2010). Recent findings support this view by showing that individuals with more mature patterns of brain activation and structure perform better on non-spatial working memory tasks (Erus et al., 2014, Satterthwaite et al., 2013). Our findings further suggest that within individuals, executive function measures obtained from earlier in childhood (age 3, 6 and 9) seemed to predict current spatial working memory performance and vACC and PCC responses to working memory load. This suggests that up to age 12, developmental trajectories within the default network may be less variable within individuals, though this would need to be further tested. Previous longitudinal studies have shown that structure and function in fronto-parietal regions predict cognition at a later age (Darki and Klingberg, 2014, Dumontheil and Klingberg, 2012, Ullman et al., 2014). A study found that working memory dependent activity in the PPC during a spatial working memory task in a group of youth aged 6–16 years predicted arithmetic performance two years later, and that there was an effect of task dependent activity even after accounting for age (Dumontheil and Klingberg, 2012). In another study by the same group, they showed that BOLD responses during spatial working memory and fractional anisotropy in and around the basal ganglia and anterior thalamus seem to predict working memory capacity in a group of 6–20 year olds two years after scanning (Ullman et al., 2014). In contrast, our retrospective analysis revealed that the cognitive performance of participants at ages 3, 6 or 9 significantly predicted load-dependent brain activity in vACC and PCC rather than fronto-parietal regions. It is possible that the vACC and PCC regions contribute more to cognitive performance in young children while the fronto-parietal areas contribute more in older children. Further longitudinal studies would be needed to distinguish the roles of default network and fronto-parietal network in supporting working memory across age.
5. Conclusion
In summary, our study suggests that activation in both the default network and fronto-parietal network during spatial working memory contributes to individual differences in behavioral performance even at a young age. In a group of youth 9–12 years of age, across participant differences in load-dependent deactivation in vACC and PCC significantly predicted better task performance while load-dependent activation in prefrontal and posterior parietal areas did not. In contrast, stronger coupling between prefrontal (IFJ) and PPC predicted better working memory performance across individuals, though coupling between default network regions did not. Furthermore, measures of executive function as early as age 3 were predictive of better spatial working memory performance and level of load-dependent deactivation in default network regions at the current age of 9–12 years. This suggests that there is a need to investigate the relationship between performance and default network deactivation at earlier ages. Our results have implications for psychopathologies with onsets in adolescence and early adulthood, such as schizophrenia, which is marked by spatial working memory deficits (Lett et al., 2014) and dysfunction in the default network (Whitfield-Gabrieli and Ford, 2012).
Conflict of interests
All authors declare that we have no direct or indirect conflict of interest with regards to the work present in this article.
Acknowledgements
We thank all participants and their parents who took part in this study. We also thank Laura Klein for project management and participant recruitment, and Anna Small, Ellen Kessel, Brandon Goldstein, Alyssa Lopez, Mayuri Ravi, Staci Weiss, Sean Ahluwalia, Irene Vogiatzis, and Colin O’Neil for their assistance when running participants. This work was funded by RO1 MH069942 (Klein) from the National Institutes of Health and the Stony Brook Research Foundation (Leung).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.10.007.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Andrews-Hanna J.R. The Brain's default network and its adaptive role in internal mentation. Neuroscience. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.-J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Shulman G.L., Barch D.M. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R. Task-induced deactivation and the “resting” state. Neuroimage. 2012;62:1086–1091. doi: 10.1016/j.neuroimage.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Galvan A., Hare T.A. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Trainor R.J., Orendi J.L., Schubert A.B., Nystrom L.E., Giedd J.N., Castellanos F.X., Haxby J.V., Noll D.C., Cohen J.D., Forman S.D., Dahl R.E., Rapoport J.L. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J. Cogn. Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chadick J.Z., Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat. Neurosci. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Castañón A.N., Ongür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D.E., Whitfield-Gabrieli S. Development of deactivation of the default-mode network during episodic memory formation. Neuroimage. 2014;84:932–938. doi: 10.1016/j.neuroimage.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtney S., Ungerleider L., Keil K., Haxby J. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F., Klingberg T. The role of fronto-parietal and fronto-striatal networks in the development of working memory: a longitudinal study. Cereb. Cortex. 2014:1–9. doi: 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb. Cortex. 2012;22:1078–1085. doi: 10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Erus G., Battapady H., Satterthwaite T.D., Hakonarson H., Gur R.E., Davatzikos C., Gur R.C. Imaging patterns of brain development and their relationship to cognition. Cereb. Cortex. 2014:1–9. doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell Pagulayan K., Busch R.M., Medina K.L., Bartok J.A., Krikorian R. Developmental normative data for the Corsi Block-tapping task. J. Clin. Exp. Neuropsychol. 2006;28:1043–1052. doi: 10.1080/13803390500350977. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S.E., Pickering S.J. Working memory deficits in children with low achievements in the national curriculum at 7 years of age. Br. J. Educ. Psychol. 2000;70(Pt 2):177–194. doi: 10.1348/000709900158047. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Pickering S.J., Ambridge B., Wearing H. The structure of working memory from 4 to 15 years of age. Dev. Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Nobre A.C. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Garver K., Terwilliger R., Luna B. Development of working memory maintenance. J. Neurophysiol. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Hampson M., Driesen N.R., Roth J.K., Gore J.C., Constable R.T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N.R., Skudlarski P., Gore J.C., Constable R.T. Brain connectivity related to working memory performance. J. Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J.B., Hedden T., Thompson T.W., Anteraper S.A., Gabrieli J.D.E., Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex. 2015;64:271–280. doi: 10.1016/j.cortex.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Korkman M., Kirk U., Kemp S. Psychological Corporation; 1998. NEPSY: A Developmental Neuropsychological Assessment. [Google Scholar]
- Laird A.R., Eickhoff S.B., Li K., Robin D.A., Glahn D.C., Fox P.T. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett T.T.A., Voineskos A.A.N., Kennedy J.J.L., Levine B., Daskalakis Z.J. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol. Psychiatry. 2014;75:361–370. doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Leung H.-C., Gore J.C., Goldman-Rakic P.S. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J. Cogn. Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Leung H.-C., Seelig D., Gore J.C. The effect of memory load on cortical activity in the spatial working memory circuit. Cogn. Affect. Behav. Neurosci. 2004;4:553–563. doi: 10.3758/cabn.4.4.553. [DOI] [PubMed] [Google Scholar]
- Li C.S.R., Yan P., Bergquist K.L., Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie R.H. Psychology Press; New York, NY: 1995. Visuo-Spatial Working Memory. [Google Scholar]
- Luna B., Sweeney J.A. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr. Bull. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- McKiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mürner-Lavanchy I., Ritter B.C., Spencer-Smith M.M., Perrig W.J., Schroth G., Steinlin M., Everts R. Visuospatial working memory in very preterm and term born children – impact of age and performance. Dev. Cogn. Neurosci. 2014;9C:106–116. doi: 10.1016/j.dcn.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel B.J., Barlett V.C., Schweinsburg A.D., Tapert S.F. Neuropsychological predictors of BOLD response during a spatial working memory task in adolescents: what can performance tell us about fMRI response patterns? J. Clin. Exp. Neuropsychol. 2005;27:823–839. doi: 10.1080/13803390490919038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Monk C.S., Lin J., Carver L.J., Thomas K.M., Truwit C.L. Functional neuroanatomy of spatial working memory in children. Dev. Psychol. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Macoveanu J., Tegnér J., Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb. Cortex. 2007;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Huppert T.J., Luna B. Functional near-infrared spectroscopy evidence for development of prefrontal engagement in working memory in early through middle childhood. Cereb. Cortex. 2015:1–10. doi: 10.1093/cercor/bhv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Peterson W.W., Birdsall T.G., Fox W. The theory of signal detectability. Trans. IRE Prof. Group Inf. Theory. 1954;4:171–212. [Google Scholar]
- Piccoli T., Valente G., Linden D.E.J., Re M., Esposito F., Sack A.T., Salle F.Di. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PLOS ONE. 2015;10:e0123354. doi: 10.1371/journal.pone.0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R.S., Heo S., Voss M.W., Patterson B., Kramer A.F. Age-related differences in cortical recruitment and suppression: implications for cognitive performance. Behav. Brain Res. 2012;230:192–200. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Raichle M., MacLeod A. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M., Wolfson D. vol. 4. Reitan Neuropsychology; 1985. (The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation). [Google Scholar]
- Sambataro F., Murty V., Callicott J., Tan H. Age-related alterations in default mode network: impact on working memory performance. Neurobiol. Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Erus G., Ruparel K., Elliott M.A., Gennatas E.D., Hopson R., Jackson C., Prabhakaran K., Bilker W.B., Calkins M.E., Loughead J., Smith A., Roalf D.R., Hakonarson H., Verma R., Davatzikos C., Gur R.C., Gur R.E. Functional maturation of the executive system during adolescence. J. Neurosci. 2013;33:16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Shulman G.L., Corbetta M., Buckner R.L., Fiez J.A., Miezin F.M., Raichle M.E., Petersen S.E. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J. Cogn. Neurosci. 1997;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M., Ritter B.C., Mürner-Lavanchy I., El-Koussy M., Steinlin M., Everts R. Age, sex, and performance influence the visuospatial working memory network in childhood. Dev. Neuropsychol. 2013;38:236–255. doi: 10.1080/87565641.2013.784321. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., King S.W., Franzen P.L., Welsh T.F., Berkowitz A.L., Noll D.C., Birmaher V., Casey B.J. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Chang C.E., Glover G.H., Gabrieli J.D.E., Greicius M.D., Gotlib I.H. Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage. 2008;41:1493–1503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Race E., Burrows B., Whitfield-Gabrieli S., Glover G.H., Gabrieli J.D.E. Development of spatial and verbal working memory capacity in the human brain. J. Cogn. Neurosci. 2009;21:316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman H., Almeida R., Klingberg T. Structural maturation and brain activity predict future working memory capacity during childhood development. J. Neurosci. 2014;34:1592–1598. doi: 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M.J. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.