Abstract
Low-dose sub-anesthetic ketamine infusion treatment has led to a long-term reduction of treatment-resistant depression and posttraumatic stress disorder (PTSD) symptom severity, as well as reduction of chronic pain states, including migraine headaches. Ketamine also is known to change oscillatory electric brain activity. One commonality between migraine headaches, depression, PTSD, Parkinson’s disease (PD) and L-DOPA-induced dyskinesias (LID) is hypersynchrony of electric activity in the brain, including the basal ganglia. Therefore, we investigated the use of low-dose sub-anesthetic ketamine in the treatment of LID. In a preclinical rodent model of LID, ketamine (5 – 20 mg/kg) led to long-term dose-dependent reduction of abnormal involuntary movements, only when low-dose ketamine was given for 10 hours continuously (5 x i.p. injections two hours apart) and not after a single acute low-dose ketamine i.p. injection. Pharmacokinetic analysis of plasma levels showed ketamine and its major metabolites were not detectable any more at time points when a lasting anti-dyskinetic effect was seen, indicating a plastic change in the brain. This novel use of low-dose sub-anesthetic ketamine infusion could lead to fast clinical translation, and since depression and comorbid pain states are critical problems for many PD patients could open up the road to a new dual therapy for patients with LID.
Keywords: Parkinson’s disease, preclinical rodent model, hypersynchrony, NMDA receptors, opioid receptors
Introduction
Parkinson’s disease (PD) is the 2nd most common progressive neurodegenerative disease with the cardinal motor symptoms of tremor, rigidity, postural instability and bradykinesia [1]. These motor symptoms correspond to the loss of dopaminergic neurons with cell bodies located in the substantia nigra and axonal projections to the striatum leading to reduced dopamine (DA) levels. The most common treatment for PD consists of DA replacement therapy utilizing the DA precursor L-DOPA (L-3,4-dihydroxyphenylalanine). This becomes unsatisfactory as the disease progresses due to short-term and long-term side effects that occur with dose escalation, including LID, the most common and debilitating side effect. An effective treatment of LID to extend the useful lifetime of L-DOPA treatment is a critical unmet need in PD therapy [1,2].
Low-dose ketamine is used to treat various chronic pain syndromes, especially those that have a neuropathic component, and studies on the effect of prolonged infusion (4 – 14 days) show long-term analgesic effects up to 3 months following infusion [3]. Recent publications also showed that low-dose ketamine infusion paradigms are safe and well tolerated in clinical trials for treatment-resistant depression [4–7] and PTSD [8]. Low-dose ketamine has led to a reduction of treatment-resistant depression; it also reduced PTSD symptom severity and comorbid depression. And a NIMH-sponsored multi-institutional clinical trial is currently evaluating the dose-response for ketamine treatment for depression in more detail. The pathophysiology of LID and motor fluctuations is uncertain, although hypotheses include an imbalance between the direct and indirect striatofugal pathways within the basal ganglia (BG), due to repeated daily administration of L-DOPA, the production of “denervation super sensitivity” of DA receptors, and as a result also hypersynchrony of electric activity and maladaptive plastic changes in the brain, including in the BG, which is a commonality between LID, depression and PTSD. Both glutamatergic and opioid dysregulation may also contribute to LID, and anti-dyskinetic effects of NMDA antagonist have been reported. Ketamine is a multifunctional ligand and is a NMDA receptor antagonist and opioid agonist. Based on this information we hypothesized that use of sub-anesthetic ketamine infusions might improve treatment of LID, and investigated this in the standard preclinical LID model.
Material and methods
Animals
Male Sprague-Dawley rats (250 g; Harlan Laboratories, Indianapolis, IN) were used and housed in a temperature and humidity controlled room with 12 hr reversed light/dark cycles with food and water available ad libitum. All animals were treated as approved by the Institutional Animal Care and Use Committee, University of Arizona and in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Both the number of animals used and their suffering were minimized.
Unilateral 6-hydroxydopamine (6-OHDA)-lesion PD model
6-OHDA hydrochloride (5.0 μg/μl in 0.9% sterile saline with 0.02% ascorbic acid; Sigma–Aldrich, St. Louis, MO, USA) was injected into the medial forebrain bundle, as published [9], into 2 locations (10 μg/site) at coordinates: AP −2.8, ML −1.8, DV −8.0 and AP −4.7, ML −1.5, DV −7.9. Rate of injection: 0.5 μl/min using a Stoelting microinjector (Stoelting Co., Wood Dale, IL). The Hamilton syringe was left in place for 5 additional minutes to prevent backflow of solution. Rats were pretreated (30 min prior to 6-OHDA) with 12.5 mg/kg desipramine (Sigma-Aldrich) given intraperitoneally (i.p.) to prevent damage to noradrenergic neurons.
Induction of LID in unilaterally-lesioned rats
1) Amphetamine-induced ipsiversive rotations were recorded, as published [10], to select animals. Mean ipsiversive rotations/min ± SEM: cohort #1 = 5.5 ± 0.7 (n = 5); cohort #2 = 6.9 ± 1.1 (n = 10). 2) Rats were treated daily for 3 weeks with L-DOPA + 14 mg/kg benserazide; both i.p.; Sigma–Aldrich): cohort #1 (severe dyskinesia): 7 mg/kg L-DOPA; cohort #2 (moderate dyskinesia): 5 mg/kg L-DOPA.
Behavioral analysis of LID rats
Following the procedure originally reported in [11], L-DOPA-induced abnormal involuntary movements (AIMs) were scored by an experimentally blinded investigator on a scale from 0 – 4, as published [9]. Limb, axial and orolingual (LAO) AIMs were scored separately from the locomotor AIMs (contralateral rotations). At the end of the experiment the rats were euthanized.
Measurement of dopamine content
Coronal brain slices were collected and 2 mm steel biopsy punches were used to sample striatal tissue. Samples from left and right hemispheres were collected and immediately flash frozen on an aluminum pan at −70 °C, as published [9]. Samples massed at 2.5 ± 0.5 mg and were then homogenized in dilute perchloric acid. High performance liquid chromatography with electrochemical detection (HPLC-EC) was used to quantify DA.
Western analysis of tyrosine hydroxylase (TH) content
After the tissue punch the remaining striata from both hemispheres were immediately flash frozen and stored at −70 °C. Total protein was prepared and semi-quantitative western analysis was conducted as described [9,12] with 3 modifications: 30 μg of protein/sample was loaded; secondary antibody dilution for β-actin: 1:10,000; Images analysis with the G:Box XR5 Chemi system (Syngene, Frederick, MD) using GeneSys v.1.4.0.0.
Measurement of ketamine (K), norketamine (NK) and dehydronorketamine (DHNK)
Tail-vain whole blood samples (approximately 0.5 ml) were taken and frozen at −80 °C. Whole blood was thawed, centrifuged, 300 μL of plasma was removed and placed in a borosilicate culture tube (VWR, Radnor, PA). Samples were basified with 100 μL of 10 M NaOH (Mallinckrodt) in nanopure water (Millipore, Bedford, MA), spiked with 500 ng Nortilidine (N) internal standard (Cayman chemical), and then extracted with a 250 μL mixture of 80:20 DCM:EtAc, (EMD Millipore, Billerica, MA) by sonicating 10 minutes. The organic fraction was recovered and samples were speed-vacuumed to dryness and reconstituted in 200 μL of an acetonitrile (EMD Millipore), nanopure water, formic acid mixture (JT Baker, Center Valley, PA) 50:50:0.1 ratio. Standards for K, NK and DHNK were supplied by Cayman chemical, Ann Arbor, MI. Measurement was performed on an Applied Biosystems Qstar Elite with TurboIon Electrospray ionization (ESI) source. Quantitation of direct flow injections was accomplished by summing analyte fragments in MS/MS mode. For example a molecular ion selection of 240.1 m/z corresponding to K yielded fragments at 165.0 and 181.0 m/z which were integrated and divided by the internal standard signal to yield a relative value for subsequent quantitation via a linear calibration of the instrument. The calibration was made by spiking standards into blank plasma prior to the extraction described above.
Data Analysis
Statistical analysis was performed using GraphPad Prism 5.1 software (GraphPad Software, La Jolla, CA), Origin 9.0 (OriginLab Corporation, Northampton, MA) and Microsoft Excel 2013. The null hypothesis was rejected when p < 0.05. Based on protocols established by Cenci’s group [13] in the repeated drug treatment study, comparisons of AIM scores that had been recorded repeatedly were performed using repeated-measures ANOVA, where treatment (L-DOPA, ketamine or post-ketamine time points) and time (testing session) were entered as independent variables. Post hoc comparisons were performed where appropriate using the Tukey test. To compare two time points we used two-tailed Mann Whitney or two-tailed t tests.
Results
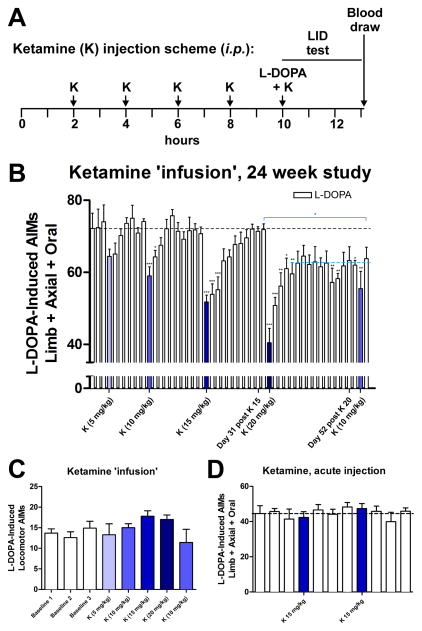
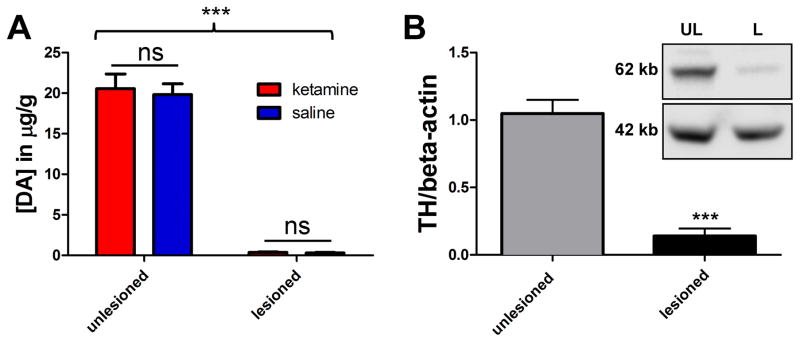
The rats in cohort #1 were primed with 7 mg/kg L-DOPA and showed severe LID. We demonstrated a dose-dependent long-term anti-dyskinetic effect of low-dose ketamine when given for a 10 hour period (Figure 1A, B); ketamine was injected 5 x i.p. two hours apart (referred to in the text as ‘infusion’), 7 mg/kg L-DOPA was co-injected at the 5th injection, and then the AIMs scores were evaluated. Prior work indicated that within two hours of injection ketamine blood level in rats drops by > 80% [14]. We used this approach to achieve 10 hour continuous ketamine exposure, since it is not possible to use a regular infusion in a behaving rat and given the long-term nature of the experiment clogging of surgically inserted mini-pumps was a concern. After the 10 mg/kg ketamine ‘infusion’ the AIMs score was significantly reduced for 3 days and it took 10 days before the baseline AIMs score (black dashed line) was reached again. After the 15 mg/kg ketamine ‘infusion’, there was a significant reduction lasting for 7 days and it took 31 days before the baseline AIMs score level was reached again. Most impressive, however, was the effect of 20 mg/kg ketamine ‘infusion’, which resulted in a persistent significant reduction of AIMs scores that lasted for at least 55 days post-injection, and what appeared to be a new lower stable AIMs level was established (blue dashed line). A 10 mg/kg ketamine ‘infusion’ was given after these treatments to test for sensitization resulting from ketamine treatment. No change in the already-reduced AIMs score was observed following this dosage, suggesting that reduced AIMs scores following the 20 mg/kg injection was not due to sensitization. At all the tested doses ketamine did not significantly change L-DOPA-induced contralateral locomotor behavior (Figure 1C), and specifically did not acutely induce the ipsiversive turning behavior that is indicative of a worsening of the Parkinsonism in this model, as it has been shown by the non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist MK-801. At doses showing anti-dyskinetic activity MK-801 is also pro-Parkinsonian [9,15]. To investigate if the longer exposure to ketamine is necessary we then tested a separate cohort #2 of rats with moderate LID to see if an acute single ketamine injection would also be anti-dyskinetic. Since the 7 mg/kg L-DOPA priming in our hands led to a very severe dyskinetic phenotype (AIMs scores of > 70) we used a lower L-DOPA dose for cohort #2 in expectation that a single acute ketamine challenge might have a reduced effect size, and using more moderately dyskinetic animals was therefore deemed appropriate. The rats were primed and tested with 5 mg/kg L-DOPA, which in our hands led to a more moderate dyskinesia (AIMs scores around 40) similar to publications by Dr. Cenci [13]. Acute single i.p. injections of 15 mg/kg ketamine (fourteen days apart) did not lead to any change in the AIMs scores (Figure 1D), while the 15 mg/kg 10 hour ketamine ‘infusion’ had an anti-dyskinetic effect that remained significant for 7 days (Figure 1B). To study pharmacokinetics we measured serum plasma levels of ketamine, as well as the major metabolites, NK and DHNK, 3 hours after an acute single dose injection of 15 mg/kg ketamine, and 3 hours, 3, 7 and 10 days after a 10 hour ketamine ‘infusion’ (15 mg/kg). There was no significant difference between the ketamine plasma levels 3 hours after injection in either the acute or the 10 hour ‘infusion’, which was expected based on the known fast metabolism [14], while the levels of NK and DHNK were elevated after the 10 hour ‘infusion’ (Table 1). Most importantly, at days 3, 7 and 10 post 10 hour ketamine ‘infusion’ no detectable levels of either ketamine or the metabolites remain in the plasma, indicating that the persistent anti-dyskinetic effect after the 10 hour treatment is indeed not due to remaining levels of ketamine or its metabolites, but rather a plastic change in the brain. Verification for the unilateral lesion at the end of the experiment was done in both cohorts. Striatal DA levels (Figure 2A) and TH-positive striatal dopaminergic fibers (Figure 2B) were reduced ~90% on the lesioned side. We also evaluated a possible acute effect of ketamine on striatal DA levels, since long-term treatment with ketamine or MK-801 can modulate DA levels in the BG and midbrain [16,17]. We injected animals with 20 mg/kg ketamine or saline 30 minutes before they were euthanized. Analysis of the striatal DA levels indicated that there was no effect of ketamine on DA levels in this short-term timeframe (Figure 2A).
Figure 1. Long-term dose-dependent reduction of LID after 10 hour low-dose-ketamine ‘infusion’.
(A) Schematic of the 10 hour experimental protocol to mimic a ketamine infusion. (B) Severely dyskinetic LID rats (cohort #1) were tested with L-DOPA (7 mg/kg) every 3 – 4 days (white bars). The blue bars depict the days of 10 hour ketamine ’infusions’ (K). The graph depicts mean limb, axial and orolingual (LAO)-AIMs ± SEM; n = 5; repeated measures ANOVAs vs. preceding baseline testing sessions; Tukey post-hoc tests; *p < 0.05; **p < 0.01; ***p < 0.005). When comparing the pre-K (20 mg/kg) and the last testing session (blue line), a significantly reduced LAO-AIMs score remained, indicating a new stable lower level of LAO AIMs (**p < 0.01; two-tailed Mann Whitney test). (C) The 10 hour ketamine ‘infusion’ protocol did not have a significant effect on L-DOPA-induced locomotor AIMs (contralateral rotations) in cohort #1. (D) A separate cohort #2 of moderately dyskinetic LID rats was challenged with L-DOPA (5 mg/kg) every 3 – 4 days and AIMs were evaluated. The blue bars depict the days of acute single ketamine (K; i.p.) challenges (mean LAO-AIMs ± SEM; n = 9; repeated measures ANOVAs vs. preceding baseline testing sessions; ns).
TABLE 1.
Plasma levels of ketamine (15 mg/kg; i.p.) and its metabolites
| 7 days pre-K BL (μM) | 3 days pre-K BL (μM) | 3 hours post-K acute (μM) | 3 hours post-K 10 hr (μM) | 3 days post-K 10 hr (μM) | 7 days post-K 10 hr (μM) | 10 days post-K 10 hr (μM) | |
|---|---|---|---|---|---|---|---|
| K | BLD | BLD | 7.6 ± 1.4 | 10.0 ± 3.9 | BLD | BLD | BLD |
| NK | BLD | BLD | 38.0 ± 4.0 | 105 ± 23 | BLD | BLD | BLD |
| DHNK | BLD | BLD | 7.1 ± 1.0 | 28 ± 5 | BLD | BLD | BLD |
Mean ± SEM; n = 7 – 9; BLD: below limit of detection
Figure 2. Verification of unilateral 6-OHDA lesion and evaluation of striatal DA levels after ketamine.
(A) Electrochemical detection of striatal DA content. The DA content (mean ± SEM) is reduced by >95% in the lesioned side, and [DA] was unchanged by an acute i.p. injection of 20 mg/kg ketamine (n = 7) vs. saline (n = 5) 30 min before rats were euthanized, showing that there is no acute effect on overall striatal DA levels by ketamine in these animal with multiple prior exposures to ketamine (2 way ANOVA, Bonferroni post-hoc test). (B) Semi-quantitative western analysis of striatal tyrosine hydroxylase (TH) expression. TH was normalized to β-actin as internal standard, mean values ± SEM are plotted, and expression is reduced by 87% in the lesioned side (n = 15; two-tailed t test) verifying the severity of the lesion. The Inset shows example blots (UL = unlesioned, L = lesioned). Statistical significant differences are depicted by asterisks (***p < 0.001; ns = non-significant).
Discussion
We examined the anti-dyskinetic properties of sub-anesthetic ketamine treatment in a preclinical rodent LID model. Only when the ketamine ‘infusion’ was used in the preclinical model and ketamine was present for 10 hours, we observed a dose-dependent long-term reduction in dyskinesia, but not when ketamine was given as a single dose. This indicates that it is necessary for the ketamine to be present for an extended time in order to be effective as an anti-dyskinetic agent.
The therapeutic effects seen could be due to low-dose ketamine desynchronizing neural hypersynchronous oscillatory activity involved in LID sufficiently to induce a lasting anti-dyskinetic effect. The reduced capacity to initiate, sustain, and control movement in PD and the dyskinesia that follows chronic L-DOPA treatment has its origin in malfunctioning networks in the BG and cortex. LID and PD are associated with reduced dopaminergic innervation to the striatum and a pronounced increase in θ- (4 – 10 Hz) and β-band (13 – 30 Hz) oscillations in the BG [18,19]. These oscillations are associated with the failure to initiate and control movement. In contrast, striatal activity in the γ-band predicts motor initiation [20]. And treatments that disrupt (DBS) or eliminate (lesions to the STN or pallidotomy) the putative sources of these oscillations improve motor symptoms, in part by desynchronizing β- and γ-band activity in the motor cortex (M1) [21]. Single dose ketamine injection increases γ-band activity in M1 [22] and θ-γ band coupling in hippocampus [23], suggesting that ketamine-induced γ-band oscillations could serve to counteract the motor-inhibitory effects of θ- and β-band activity. Coherence between the cortex and STN in the γ-band often bears an inverse relationship with θ and β power in the STN. Injection of low, sub-anesthetic doses of ketamine (2.5 – 10 mg/kg) in rats increases γ-band activity in cortical and subcortical areas [24]. Dosages ranging from 10 – 50 mg/kg enhance γ-band synchrony between M1 and BG [25,26]. In support of ketamine infusion possibly acting as a ‘chemical DBS’, we observed a clear increase in high-frequency (HFO; 130 – 150 Hz) and γ-band (40 – 60 Hz) activity in the dorsolateral striatum and M1 of naïve freely moving rats treated with the same 10 hour ketamine ‘infusion’ protocol used in the current study [27]. This increase was significantly more sustained after the 4th and 5th vs. the 1st injection, which may explain the lack of anti-dyskinetic effects after a single dose injection. Measures of spectral coherence indicate that HFO- and β-band activity was significantly anti-correlated, with coherence in the HFO-band increasing with successive injections and coherence in the β-band decreasing [manuscript in preparation].
The difference from ketamine’s effects compared to the non-competitive NMDA receptor antagonist MK-801 or amantadine, which is a weak NMDA antagonist, could be explained by the fact that ketamine is not only a NMDA receptor antagonist but also a known opioid receptor agonist [28,29]. There is conflicting evidence on the effect of opioids on LID, both μ-opioid receptor antagonism and agonism have been implicated as possible anti-dyskinetic approaches [30], (https://www.michaeljfox.org/foundation/grant-detail.php?grant_id=1159), and δ-opioid receptor agonism is associated with anti-Parkinsonian activity [31].
Overall the prior clinical reports suggest that low-dose ketamine infusions are well tolerated and can improve pain and depression [4–7], both often comorbidities in PD patients [1,32]. Importantly, depression is possibly worsened by L-DOPA [33]. Bioavailability is low when ketamine is given orally (< 20%), because of extensive first pass metabolism in liver and intestine, and might not be an alternative to i.v. infusion. But recently intranasal ketamine (bioavailability: ~50%) has shown promise in a controlled clinical trial for treatment of depression [6], possibly providing a more practical alternative route of administration.
In conclusion we present preclinical data suggesting that low-dose sub-anesthetic ketamine infusion could be a novel long-term treatment modality to reduce LID and should be further investigated in a properly controlled prospective clinical trial.
Highlights.
Low-dose sub-anesthetic ketamine is tested in a rat L-DOPA-induced dyskinesia model
Ketamine did not reduce abnormal involuntary movements (AIMs) after acute injection
When ketamine was given for 10 hours there was a dose-dependent reduction of AIMs
Long-term AIMs reduction lasted days to weeks, depending on the ketamine dose
Acknowledgments
Support: The Jerry T. and Glenda G. Jackson Fellowship in Parkinson’s Research to the University of Arizona.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology. 2009;72:S1–S136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs. 2007;21:677–692. doi: 10.2165/00023210-200721080-00005. [DOI] [PubMed] [Google Scholar]
- 3.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: Risks and benefits. British Journal of Clinical Pharmacology. 2014;77:357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, et al. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol. 2014;28:536–544. doi: 10.1177/0269881114527361. [DOI] [PubMed] [Google Scholar]
- 5.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biol Psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–8. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 9.Flores AJ, Bartlett MJ, So LY, Laude ND, Parent KL, Heien ML, et al. Differential effects of the NMDA receptor antagonist MK-801 on dopamine receptor D1- and D2-induced abnormal involuntary movements in a preclinical model. Neuroscience Letters. 2014;564:48–52. doi: 10.1016/j.neulet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue X, Falk T, Zuniga LA, Szabò L, Porreca F, Polt R, et al. Effects of the novel glycopeptide opioid agonist MMP-2200 in preclinical models of Parkinson’s disease. Brain Research. 2011;1413:72–83. doi: 10.1016/j.brainres.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Yue X, Hariri DJ, Caballero B, Zhang S, Bartlett MJ, Kaut O, et al. Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson’s disease. Neuroscience. 2014;258:385–400. doi: 10.1016/j.neuroscience.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. European Journal of Neuroscience. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 14.Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2013;52:567–570. [PMC free article] [PubMed] [Google Scholar]
- 15.Paquette MA, Anderson AM, Lewis JR, Meshul CK, Johnson SW, Paul Berger S. MK-801 inhibits L-DOPA-induced abnormal involuntary movements only at doses that worsen parkinsonism. Neuropharmacology. 2010;58:1002–1008. doi: 10.1016/j.neuropharm.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonkers N, Sarre S, Ebinger G, Michotte Y. MK801 influences L-DOPA-induced dopamine release in intact and hemi-parkinson rats. European Journal of Pharmacology. 2000;407:281–291. doi: 10.1016/s0014-2999(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 17.Tan S, Lam WP, Wai MSM, Yu WHA, Yew DT. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One. 2012;7:e43947. doi: 10.1371/journal.pone.0043947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends in Neurosciences. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends in Neurosciences. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Masimore B, Schmitzer-Torbert NC, Kakalios J, Redish AD. Transient striatal gamma local field potentials signal movement initiation in rats. Neuroreport. 2005;16:2021–2024. doi: 10.1097/00001756-200512190-00010. [DOI] [PubMed] [Google Scholar]
- 21.de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nature Neuroscience. 2015;18:779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiyoshi T, Kambe D, Karasawa JI, Chaki S. Differential effects of NMDA receptor antagonists at lower and higher doses on basal gamma band oscillation power in rat cortical electroencephalograms. Neuropharmacology. 2014;85:384–396. doi: 10.1016/j.neuropharm.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Caixeta FV, Cornélio AM, Scheffer-Teixeira R, Ribeiro S, Tort ABL. Ketamine alters oscillatory coupling in the hippocampus. Sci Rep. 2013;3:2348. doi: 10.1038/srep02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Nicolás MJ, López-Azcárate J, Valencia M, Alegre M, Pérez-Alcázar M, Iriarte J, et al. Ketamine-induced oscillations in the motor circuit of the rat basal ganglia. PLoS One. 2011;6:e21814. doi: 10.1371/journal.pone.0021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye T, Bartlett MJ, Wiegand J-P, Schmitt M, Sherman SJ, Falk T, et al. Modulation of high-frequency oscillations and beta coherence in striato-cortico-limbic circuits following repeated sub-anesthetic ketamine exposure. Society for Neuroscience Abstracts. 2015:479.09. [Google Scholar]
- 28.Pacheco DDF, Romero TRL, Duarte IDG. Central antinociception induced by ketamine is mediated by endogenous opioids and mu- And delta-opioid receptors. Brain Research. 2014;1562:69–75. doi: 10.1016/j.brainres.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, et al. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26:1253–1260. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- 30.Koprich JB, Fox SH, Johnston TH, Goodman A, Le Bourdonnec B, Dolle RE, et al. The selective mu-opioid receptor antagonist ADL5510 reduces levodopa-induced dyskinesia without affecting antiparkinsonian action in MPTP-lesioned macaque model of Parkinson’s disease. Mov Disord. 2011;26:1225–1233. doi: 10.1002/mds.23631. [DOI] [PubMed] [Google Scholar]
- 31.Hille CJ, Fox SH, Maneuf YP, Crossman AR, Brotchie JM. Antiparkinsonian action of a delta opioid agonist in rodent and primate models of Parkinson’s disease. Experimental Neurology. 2001;172:189–198. doi: 10.1006/exnr.2001.7763. [DOI] [PubMed] [Google Scholar]
- 32.Marsh L. Depression and Parkinson’s disease: current knowledge. Curr Neurol Neurosci Rep. 2013;13:409. doi: 10.1007/s11910-013-0409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskow Jaunarajs KL, Angoa-Perez M, Kuhn DM, Bishop C. Potential mechanisms underlying anxiety and depression in Parkinson’s disease: Consequences of l-DOPA treatment. Neuroscience and Biobehavioral Reviews. 2011;35:556–564. doi: 10.1016/j.neubiorev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]