Abstract
Objective
Ex vivo lung perfusion (EVLP) has been successful in the assessment of marginal donor lungs, including donation after cardiac death (DCD) donor lungs. EVLP also represents a unique platform for targeted drug delivery. We sought to determine if ischemia-reperfusion injury (IRI) would be decreased after transplantation of DCD donor lungs subjected to prolonged cold preservation and treated with an adenosine A2A receptor (A2AR) agonist during EVLP.
Methods
Porcine DCD donor lungs were preserved at 4°C for 12 hours and underwent EVLP for 4 hours. Left lungs were then transplanted and reperfused for 4 hours. Three groups (n=4/group) were randomized according to treatment with the A2AR agonist ATL-1223 or the dimethyl sulfoxide (DMSO) vehicle: DMSO (infusion of DMSO during EVLP and reperfusion), ATL-E (infusion of ATL-1223 during EVLP and DMSO during reperfusion), and ATL-E/R (infusion of ATL-1223 during EVLP and reperfusion). Final PaO2/FiO2 ratios were determined from samples obtained from the left superior and inferior pulmonary veins.
Results
Final PaO2/FiO2 ratios in the ATL-E/R group (430.1 ± 26.4 mmHg) were similar to final PaO2/FiO2 ratios in the ATL-E group (413.6 ± 18.8 mmHg), but both treated groups had significantly higher final PaO2/FiO2 ratios compared to the DMSO group (84.8 ± 17.7 mmHg). Low PO2 gradients during EVLP did not preclude superior postoperative oxygenation capacity.
Conclusions
Following prolonged cold preservation, treatment of DCD donor lungs with an A2AR agonist during EVLP enabled PaO2/FiO2 ratios above 400 mmHg after transplantation in a preclinical porcine model. Pulmonary function during EVLP was not predictive of outcomes after transplantation.
The number of patients on the waiting list continues to exceed the number of patients who undergo lung transplantation each year, though recent advances in donor selection and lung preservation have the potential to close this gap [1]. Lungs are usually selected from donation after brain death (DBD) donors, yet only 15–20% of these lungs are deemed suitable and procured for transplantation after conservative evaluation. Marginal donor lungs, including those from donation after cardiac death (DCD) donors, represent an additional source for transplantation, but use of these lungs has been linked to increased mortality and primary graft dysfunction (PGD) in some studies [2], [3]. PGD is mediated by ischemia-reperfusion injury (IRI) and is associated with significant early and late mortality [4], [5] as well as progression to bronchiolitis obliterans syndrome and delayed graft failure [6], [7].
Cold preservation of donor lungs reduces metabolic demand during ischemic storage but inhibits cellular regeneration that may be crucial before marginal donor lungs are transplanted. Normothermic ex vivo lung perfusion (EVLP) was initially developed to assess function of lungs from DCD donors prior to transplantation [8]. EVLP was subsequently modified to allow prolonged preservation of marginal donor lungs [9], and a pivotal clinical trial established non-inferior outcomes after transplantation of marginal donor lungs passively rehabilitated with EVLP compared to conventional donor lungs [10]. EVLP also offers a unique platform for targeted drug delivery to actively treat marginal donor lungs. We have previously demonstrated that the adenosine A2A receptor (A2AR) agonist ATL-1223 exerts anti-inflammatory effects and reduces IRI when administered to DCD donor lungs during EVLP in a porcine model of lung transplantation [11].
The purpose of this study was to determine if administration of ATL-1223 during EVLP would decrease IRI after transplantation of DCD donor lungs subjected to prolonged 12-hour cold preservation, and if further administration during reperfusion would augment this protection in a preclinical porcine model, with important implications to expand the donor lung pool by including DCD donors and allowing cross-country retrieval. We hypothesized that IRI would be reduced after transplantation of donor lungs treated with ATL-1223 during EVLP compared to lungs that received dimethyl sulfoxide (DMSO) vehicle, and that IRI would be further attenuated by continued administration of ATL-1223 to the recipient during reperfusion.
Materials and Methods
Animals
This study was approved by the Institutional Animal Care and Use Committee at the University of Virginia. Animals received humane treatment in accordance with the “Guide for Care and Use of Laboratory Animals” from the National Institutes of Health.
Study Design
Mature domestic swine (19–35 kg) of both genders were randomized to 3 different groups (n=4/group) based on treatment with the A2AR agonist ATL-1223. Donor swine underwent hypoxic arrest followed by 15 minutes of warm ischemia. Lungs were procured and preserved at 4°C for 12 hours before undergoing normothermic EVLP for 4 hours. Left lungs were then transplanted into size-matched recipients and reperfused for 4 hours. The DMSO group received an infusion of DMSO (vehicle) during EVLP and reperfusion. The ATL-E group received an infusion of ATL-1223 during EVLP and an infusion of DMSO during reperfusion, and the ATL-E/R group received an infusion of ATL-1223 during EVLP and reperfusion.
Donor Lung Procurement
Donor animals were anesthetized, weighed, and prepped for a median sternotomy. Following induction, animals were intubated and maintained under general anesthesia with isoflurane. Animals were ventilated with 100% oxygen before initial donor arterial blood gas (ABG) analysis. Heparin was not administered to donor animals. The endotracheal tube (ETT) was clamped and donor animals underwent hypoxic arrest. Death was confirmed by cessation of electrocardiographic activity and absence of heart sounds. Animals were not touched for a 15-minute period of warm ischemia. Lungs were ventilated throughout the last 5 minutes of this time. A median sternotomy was performed. The main pulmonary artery (PA) was cannulated near the bifurcation with a cardioplegia cannula and 500 mcg prostaglandin E1 (Pfizer Inc) was administered. The main PA was clamped proximally, the left atrial appendage (LAA) was vented, and lungs were flushed with 1.5 L of 4°C Perfadex (XVIVO Perfusion, Englewood, CO). Topical cold slush was applied. At the completion of the flush, the heart and lungs were removed en bloc. A funnel-shaped cannula with a pressure port (XVIVO Perfusion, Englewood, CO) was sewn to the left atrial (LA) cuff with a running 4-0 prolene suture, a cannula with a pressure port (XVIVO Perfusion, Englewood, CO) was tied within the main PA, and a 7.0 mm ETT with the balloon removed was tied within the trachea. Lungs were inflated with gentle positive end expiratory pressure (PEEP) and the ETT was clamped. A 500 ml retrograde flush with 4°C Perfadex was then performed. The lungs were submerged in 4°C Perfadex, surrounded by a double layer of cold slush, and stored at 4°C for 12 hours. Lungs were flushed antegrade with 500 ml 4°C Perfadex immediately prior to EVLP.
Ex Vivo Lung Perfusion
The EVLP circuit was primed with 2 L Steen solution (XVIVO Perfusion, Englewood, CO) supplemented with 10,000 units heparin, 500 mg methylprednisolone, and 500 mg cefazolin. Perfusate drained from the venous (LA) cannula into the venous reservoir and was warmed to 37°C, deoxygenated with a mixture of 86% nitrogen, 8% carbon dioxide, and 6% oxygen gas, and pumped into the arterial (PA) cannula (Medtronic BioMedicus centrifugal pump, Eden Prairie, MN). Lungs were placed within the EVLP chamber (XVIVO Perfusion, Englewood, CO), de-aired, and low flow (0.15 ml/min) through the lungs was established to initiate EVLP. The height of the venous reservoir was adjusted to maintain LA pressures between 0–5 mmHg. PA pressures were dependent on perfusate flow. Throughout the first 30 minutes of EVLP, flow was titrated up to 40% of estimated cardiac output (100 ml/kg/min) and perfusate was warmed to 37°C. After 30 minutes of EVLP, lungs were ventilated on room air (TV 10 ml/kg, RR 8 bpm, PEEP 5 cm H2O). EVLP was performed for 4 hours. Oxygenation was assessed every hour by increasing the FiO2 to 100% for 15 minutes prior to PO2 analysis. Samples taken from the venous and arterial arms of the circuit allowed calculation of the oxygenation gradient across the pulmonary vasculature. Other parameters recorded every hour included LA pressure, PA pressure, peak airway pressure, mean airway pressure, and plateau pressure. The A2AR agonist ATL-1223 (Lewis and Clark Pharmaceuticals, Charlottesville, VA) was administered as an infusion (3.0 ng/kg/min) throughout all 4 hours of EVLP. The same weight-based volume of DMSO was administered as an infusion throughout EVLP. At the conclusion of EVLP, donor lungs were flushed antegrade with 500 ml of 4°C Perfadex. Lungs were separated on the back table and the donor left lung was cooled with topical cold slush for 15 minutes prior to transplantation.
Left Lung Transplantation
Recipient animals were anesthetized, weighed, and prepped for a left thoracotomy. Animals were intubated and maintained under general anesthesia with isoflurane. A central venous line with a PA catheter, arterial line, and oxygenation saturation probe were placed. Initial recipient ABG analysis was performed on 100% oxygen and baseline vitals were recorded, including heart rate, blood pressure, PA pressure, and oxygenation saturation. A left thoracotomy was performed and 50 mg lidocaine were administered prior to dissection of the left hilum. Before clamping the left PA and ligating the left superior and inferior pulmonary veins, 5,000 units of heparin were given and allowed to circulate for 5 minutes. The left mainstem bronchus was clamped, the pneumonectomy was completed, and the donor lung was placed in the chest. The bronchial anastomosis was completed with a running 4-0 prolene suture and the PA anastomosis was then completed with a running 5-0 prolene suture. The recipient LAA was clamped, opened, and sewn to the donor LA cuff with a running 5-0 prolene suture. Clamps were removed to establish reperfusion and ventilation. ABG analysis was performed and vitals were recorded every 15–20 minutes during all 4 hours of reperfusion. Animals were ventilated with 100% oxygen throughout reperfusion. Intermittent gentle PEEP was applied to reduce atelectasis. Acid-base status was optimized by adjusting respiratory rate at a constant tidal volume of 10 ml/kg. Intravenous fluids were given judiciously, and epinephrine was administered as needed. The A2AR agonist ATL-1223 was administered at 3.0 ng/kg/min or an equivalent weight-based volume of DMSO was given as an infusion throughout reperfusion. ABG samples were obtained from the left superior and inferior pulmonary veins to determine isolated oxygenation capacity of the transplanted left lung at the conclusion of reperfusion. Mean values were reported as final PaO2/FiO2 ratios. A final systemic ABG analysis was performed, the recipient was euthanized with Euthasol (Virbac AH, Inc), and the left lung was explanted.
Cytokine Expression
Lung tissue samples were obtained from the left lower lobe (LLL), frozen at −80°C, and homogenized using a gentleMACS tissue dissociator (Miltenyi Biotec, Auburn, CA) in a solution containing PBS, 0.5% BSA, and 2mM EDTA. Total protein concentration was determined using a BCA protein assay (Pierce, Rockford, IL). ELISAs were performed to determine expression of interferon-γ (IFN-γ) (RayBiotech, Inc., Norcross, GA) and interleukin 1β (IL-1β), interleukin 6 (IL-6), and interleukin 8 (IL-8) (Abcam, Cambridge, MA). One sample in the ATL-E/R group had insufficient volume for the latter analyses. Cytokine concentrations were reported per 50 μg total protein in each sample.
Pulmonary Edema
A bronchoalveolar lavage (BAL) of the left upper lobe (LUL) was performed with 50 ml normal saline. Total protein concentration was determined in BAL samples using a BCA protein assay as a measure of pulmonary edema. Lung tissue samples from the LUL (n=2/animal) and LLL (n=3/animal) were weighed immediately and again after a stable weight was achieved through desiccation in a vacuum oven. Wet-to-dry weight ratios were determined as an additional measure of pulmonary edema. The wet-to-dry weight ratio for each lung was calculated as the mean of the ratios from all 5 lung tissue samples.
Histology
Lungs were inflation-fixed by filling the airways with formalin and the lung was submerged in formalin overnight. Lung tissue samples from the LUL (n=2/animal) and LLL (n=3/animal) were placed in 70% ethanol, embedded in paraffin, and stained with hematoxylin and eosin. The lung injury severity score was determined by a pathologist blinded to treatment group. The score ranged from 0–9 and was a composite sum of 3 individual components, including number of neutrophils per high-powered field (score of 0, <5; 1, 6 to 10; 2, 11 to 20; and score of 3, >20), interstitial infiltration (score of 0, none; 1, minimal; 2, moderate; and 3, severe), and alveolar edema (score of 0, < 5%; 1, 6%–25%; 2, 26%–50%; and 3, > 50%). The lung injury severity score for each lung was calculated as the mean of the scores from all 5 lung tissue samples.
Statistical Analysis
Experimental methods were designed to test the null hypothesis that no significant differences in lung IRI would be observed despite treatment with ATL-1223. Differences among groups were calculated using one-way ANOVA, and pairwise comparisons were made using Tukey’s multiple comparisons test (GraphPad Prism version 6.0e). An unpaired t test was used to calculate differences between time points. Data is expressed as the mean ± standard error of the mean. A p value less than 0.05 was considered statistically significant.
Results
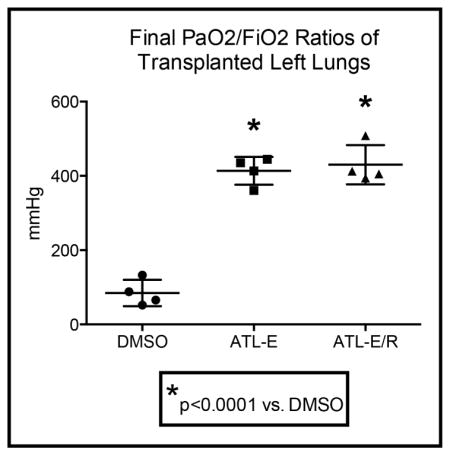
Donor lung oxygenation during reperfusion
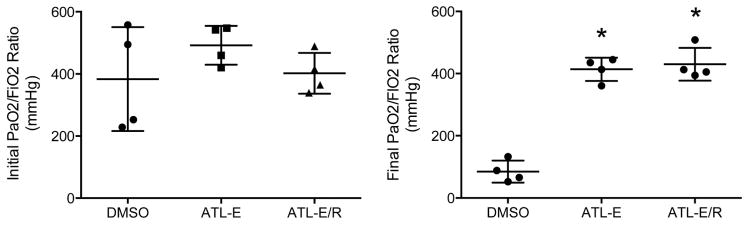
There was no difference in mean initial PaO2/FiO2 ratios of donor animals among groups (Figure 1). However, after 4 hours of reperfusion, final PaO2/FiO2 ratios of donor lungs in both groups treated with ATL-1223 (ATL-E: 413.6 ± 18.8 mmHg; ATL-E/R: 430.1 ± 26.4 mmHg) were significantly higher than final PaO2/FiO2 ratios of control donor lungs (DMSO: 84.8 ± 17.7 mmHg) (p<0.0001). Further administration of ATL-1223 during reperfusion did not significantly increase final PaO2/FiO2 ratios in the ATL-E/R group compared to the ATL-E group (p=0.85) (Figure 1).
Figure 1.
Comparison of initial and final PaO2/FiO2 ratios among groups. Initial donor PaO2/FiO2 ratios were obtained before procurement. Final PaO2/FiO2 ratios of donor lungs were determined from samples obtained from the left superior and inferior pulmonary veins after 4 hours of reperfusion. *p < 0.0001 vs. DMSO.
Mean PaO2/FiO2 ratios of control donor lungs significantly worsened from baseline measurement in the donor animal to final assessment in the recipient. However, there was no significant difference between initial and final mean PaO2/FiO2 ratios of donor lungs in both groups treated with ATL-1223.
Donor lung oxygenation during EVLP
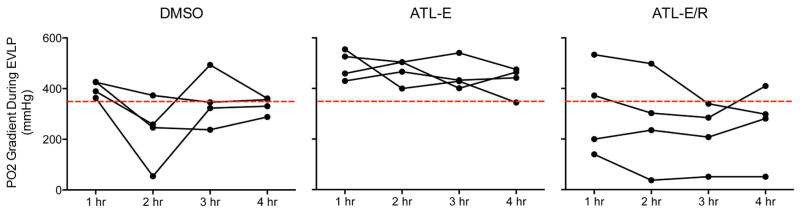
Overall, mean PO2 gradients decreased from the first hour to the fourth hour of EVLP in all groups (Figure 2). Trends in PO2 gradients of individual lungs were highly variable throughout EVLP and were frequently below the 350 mmHg threshold considered acceptable for clinical lung transplantation [10]. Mean final values decreased significantly in the DMSO group from 334.2 ± 16.7 mmHg after EVLP to 84.8 ± 17.7 mmHg after reperfusion (p<0.0001) and decreased only slightly in the ATL-E group from 431.7 ± 29.8 mmHg after EVLP to 413.6 ± 18.8 mmHg after reperfusion. However, mean final values increased in the ATL-E/R group from 260.0 ± 75.3 mmHg after EVLP to 430.1 ± 26.4 mmHg after reperfusion (p=0.08).
Figure 2.
Trends in PO2 gradients throughout 4 hours of EVLP in DMSO, ATL-E, and ATL-E/R groups. The red line represents the 350 mmHg threshold for clinical lung transplantation.
Physiologic parameters during EVLP
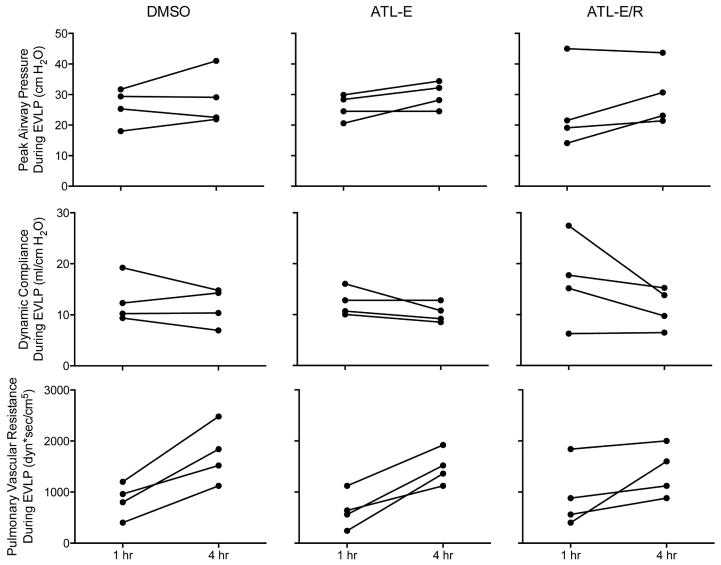
Overall, mean values of peak airway pressure, dynamic compliance, and pulmonary vascular resistance (PVR) worsened throughout EVLP in all groups (Figure 3). Although certain individual parameters remained relatively stable or at times improved during EVLP, at least one of these three parameters in all donor lungs in all groups worsened by more than 15% between the 1st hour and 4th hour of EVLP (Figure 4), the threshold at which lungs would be deemed unsuitable for clinical lung transplantation [10].
Figure 3.
Changes in physiologic parameters throughout 4 hours of EVLP. Peak airway pressure, dynamic compliance, and pulmonary vascular resistance in the DMSO, ATL-E, and ATL-E/R groups.
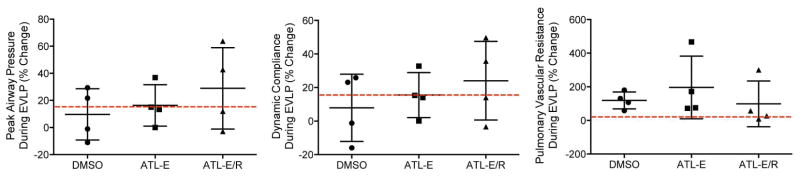
Figure 4.
Percent change in peak airway pressure, dynamic compliance, and pulmonary vascular resistance between the 1st hour and the 4th hour of EVLP among groups. The red line represents the 15% threshold for clinical lung transplantation.
There were no differences among groups in mean percent change in peak airway pressure, dynamic compliance, or PVR throughout EVLP (Figure 4). Mean percent change in peak airway pressure and dynamic compliance were lowest in the DMSO group and highest in the ATL-E/R group. Mean percent change in PVR was highest in the ATL-E group.
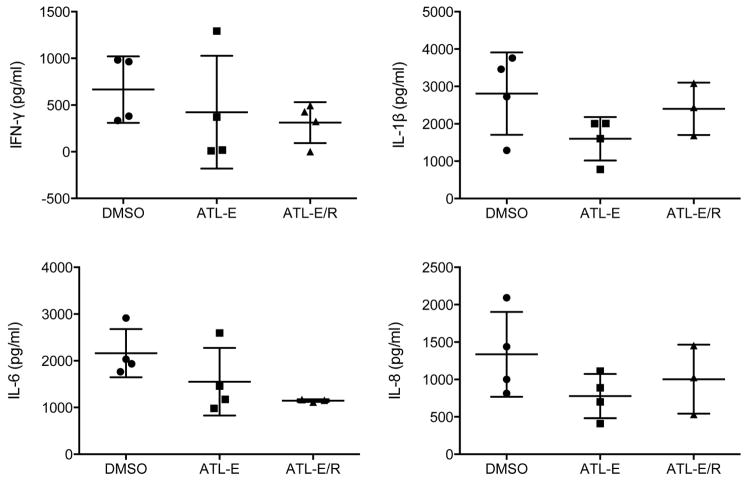
Cytokine Expression
After 4 hours of reperfusion, there was a trend toward decreased expression of proinflammatory cytokines (IFN-γ, IL-1β, IL-6, and IL-8) in the ATL-E and ATL-E/R groups compared to the DMSO group (Figure 5).
Figure 5.
Proinflammatory cytokine levels in lung tissue after 4 hours of reperfusion.
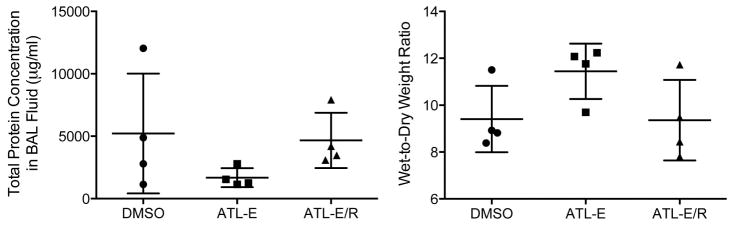
Pulmonary Edema
There was no significant difference in pulmonary edema among groups as measured by total protein concentration in BAL fluid or mean wet-to-dry weight ratios (Figure 6).
Figure 6.
Pulmonary edema after 4 hours of reperfusion as measured by total protein concentration in BAL fluid and lung wet-to-dry weight ratios.
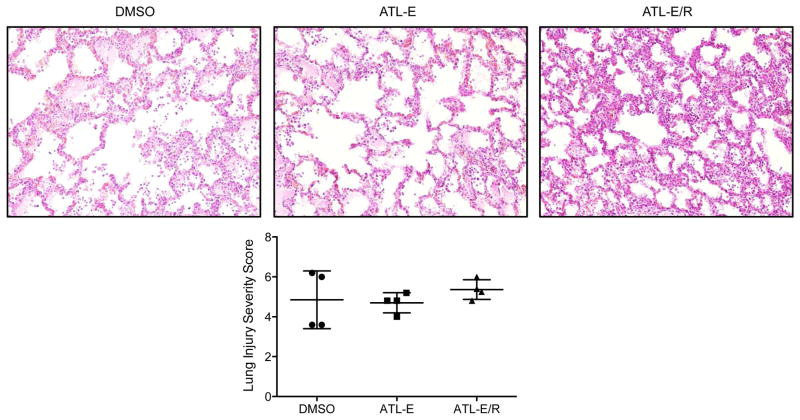
Histology
There was substantial variation in histologic appearance of lung injury within individual lung tissue sections. There was no difference in mean lung injury severity scores among groups (Figure 7), or in values of individual components of the overall score (neutrophil count, interstitial infiltration, and alveolar edema; not shown).
Figure 7.
(Top) Representative lung histology (hematoxylin and eosin stain at 20X magnification) after 4 hours of reperfusion in DMSO, ATL-E, and ATL-E/R groups. (Bottom) Comparison of mean lung injury severity scores among groups as determined from histology.
Discussion
This study demonstrates that PaO2/FiO2 ratios after transplantation of DCD donor lungs subjected to prolonged 12-hour cold preservation are significantly improved following treatment with an A2AR agonist during EVLP. Outcomes highlight the potential of EVLP with targeted A2AR agonist delivery to augment the available donor lung pool by increasing the use of marginal donor lungs, including DCD donor lungs, and by extending the traditionally recognized 6-hour time constraint for cold preservation, thereby decreasing geographical restrictions of donor lung procurement.
IRI manifests as PGD within the first 72 hours after lung transplantation. PGD severity is determined by oxygenation capacity and the radiographic appearance of pulmonary edema within the transplanted lung(s), with grade 0 PGD occurring in the presence of PaO2/FiO2 ratios greater than 300 mmHg and normal lung fields and grades 1–3 PGD occurring in the presence of diffuse pulmonary infiltrates and PaO2/FiO2 ratios greater than 300 mmHg, between 200–300 mmHg, and less than 200 mmHg, respectively [12], [13]. PGD severity correlates with outcomes after lung transplantation. Patients who develop grade 3 PGD have significantly increased operative mortality [14] and higher rates of late mortality and impaired pulmonary function compared to patients with grade 0 or 1 PGD [15]. In this study, final PaO2/FiO2 ratios of the transplanted left lungs from all animals in the ATL-E and ATL-E/R groups were above 300 mmHg, while final PaO2/FiO2 ratios of the transplanted left lungs from all animals in the DMSO group were below 200 mmHg. Although radiographic imaging was not performed in this study, there was no difference in pulmonary edema among groups. Thus, administration of ATL-1223 to donor lungs during EVLP appears to have reduced IRI from grade 3 PGD to grade 1 PGD. These results are similar to prior porcine models of lung transplantation demonstrating a reduction in IRI after A2AR agonist treatment during EVLP [11], [16] or during reperfusion [17], [18].
Further administration of ATL-1223 during reperfusion did not significantly decrease IRI beyond that achieved by administration of ATL-1223 during EVLP alone, since PaO2/FiO2 ratios from the ATL-E/R and ATL-E groups were not different. This finding suggests that the inflammatory cascade mediating IRI is initiated by resident immune cells within the donor lung that are inactivated by ATL-1223 during EVLP, and that this inhibition persists during reperfusion. Previous studies have shown distinct populations of cells to be important at different stages of lung IRI: resident macrophages and invariant natural killer T cells in the donor lung in an early phase and circulating neutrophils in the recipient in a later phase [19], [20], [21]. In prior animal models of lung IRI, administration of an A2AR agonist to the donor animal before lung procurement decreased IRI to a similar extent as administration of the A2AR agonist during reperfusion, suggesting that both donor and recipient populations of immune cells are important in the development of lung IRI [18], [22]. In a rabbit model of lung IRI, pretreatment of the donor with the A2AR agonist resulted in a significant decrease in the number of pulmonary macrophages during reperfusion compared to treatment of the recipient at a later time [22]. Taken together, these studies support a role for early activation of resident immune cells in the donor lung in the initiation of IRI. EVLP provides an ideal platform for isolated drug delivery to inactivate these cells in marginal donor lungs, thereby avoiding side effects associated with systemic therapy in the donor or recipient.
The accuracy of ex vivo evaluation in predicting lung function after transplantation has been shown to depend on physiologic parameters (peak airway pressure, dynamic compliance, and PVR) more than on PO2 gradients during EVLP [23]. In a previous clinical trial, marginal donor lungs rehabilitated with EVLP were transplanted if physiologic parameters did not deteriorate by more than 15% and if PO2 gradients were greater than 350 mmHg during 4 hours of EVLP [10]. No single lung in the present study met these inclusion criteria after 4 hours of EVLP. Physiologic parameters and oxygenation capacity during EVLP were frequently superior in the DMSO group, yet these lungs were associated with severe IRI in the recipient. Conversely, lungs treated with ATL-1223 often had inferior pulmonary function during EVLP, yet had mean PaO2/FiO2 ratios greater than 400 mmHg after 4 hours of reperfusion. Therefore, neither physiologic parameters nor PO2 gradients during EVLP were predictive of lung function during reperfusion in this preclinical porcine model. Future studies will require identification of more reliable markers of lung function during EVLP that correlate with pulmonary function in the recipient before lungs can be safely transplanted clinically.
A related limitation of this study is the lack of data to explain how ATL-1223 administration promoted superior final PaO2/FiO2 ratios in the treated groups. There was a trend toward decreased proinflammatory cytokine expression in these groups, and statistical significance may have been achieved with more animals per group. It is unclear why there was no difference in pulmonary edema or histologic appearance of lung injury among groups. There was considerable variation within individual lung tissue sections, which made calculation of a single representative lung injury severity score somewhat challenging, and this score may not accurately reflect differences in IRI among groups. Instead, the most established and clinically applicable measure of IRI is the PaO2/FiO2 ratio after transplantation, which was significantly increased in recipients of lungs treated with ATL-1223 during EVLP.
Clinically, potential lung donors are evaluated with imaging modalities, including CT and bronchoscopy, before a decision is made regarding donor lung eligibility. Imaging of porcine donor lungs was not performed prior to procurement in this study. Therefore, it was not possible to screen donor lungs for intrinsic pulmonary disease. There were several instances in which it became increasingly difficult to ventilate donor lungs during reperfusion in all groups due to severe baseline pulmonary disease, and these animals were not included in the final analysis. This demonstrates that pulmonary injury within a subset of donor lungs is beyond rehabilitation with targeted ATL-1223 treatment during EVLP. Moreover, severe IRI occurred in all control donor lungs without apparent pulmonary disease. These findings show that EVLP alone without concomitant ATL-1223 treatment is unable to prevent IRI after transplantation of DCD donor lungs subjected to prolonged 12-hour cold preservation. Quantifying a threshold of irreversible donor lung injury will be an important component of clinical translation of animal models of EVLP.
Conclusions
EVLP with targeted A2AR agonist treatment attenuates IRI after transplantation of DCD donor lungs subjected to prolonged 12-hour cold preservation in a preclinical porcine model. Pulmonary function during EVLP was not predictive of oxygenation capacity during reperfusion, as donor lungs that would have been rejected clinically after EVLP had postoperative PaO2/FiO2 ratios above 400 mmHg if treated with an A2AR agonist during EVLP. Translation of this study may significantly expand the donor lung pool.
Acknowledgments
The authors wish to thank Dr. Curtis Tribble and Dr. Benjamin Kozower for their mentorship and Sheila Hammond, Tony Herring, and Cindy Dodson for their technical assistance.
Abbreviations and Acronyms
- A2AR
adenosine A2A receptor
- ABG
arterial blood gas
- ANOVA
analysis of variance
- ATL-E
A2AR agonist administration during EVLP
- ATL-E/R
A2AR agonist administration during EVLP and reperfusion
- BAL
bronchoalveolar lavage
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- CT
computed tomography
- DBD
donation after brain death
- DCD
donation after cardiac death
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- ETT
endotracheal tube
- EVLP
ex vivo lung perfusion
- FiO2
fraction of inspired oxygen
- IFN
interferon
- IL
interleukin
- IRI
ischemia-reperfusion injury
- LA
left atrium
- LAA
left atrial appendage
- LLL
left lower lobe
- LUL
left upper lobe
- mmHg
millimeter of mercury
- PA
pulmonary artery
- PaO2
arterial oxygen partial pressure
- PBS
phosphate buffered saline
- PEEP
positive end expiratory pressure
- PGD
primary graft dysfunction
- PO2
oxygen partial pressure
- PVR
pulmonary vascular resistance
- RR
respiratory rate
- TV
tidal volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cynthia E. Wagner, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: T32 HL007849, R01 HL130053, Conflicts of interest: none.
Nicolas H. Pope, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: T32 HL007849, R01 HL130053, Conflicts of interest: none.
Eric J. Charles, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: T32 HL007849, R01 HL130053, Conflicts of interest: none.
Mary E. Huerter, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: R01 HL130053, Conflicts of interest: none.
Ashish K. Sharma, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: R01 HL130053, Conflicts of interest: none.
Morgan D. Salmon, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: R01 HL130053, Conflicts of interest: none.
Benjamin T. Carter, University of Virginia, School of Medicine, Funding: R01 HL130053, Conflicts of interest: none.
Mark H. Stoler, University of Virginia, Department of Pathology, Funding: none, Conflicts of interest: none.
Christine L. Lau, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: R01 HL130053, Conflicts of interest: none.
Victor E. Laubach, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: R01 HL130053, Conflicts of interest: none.
Irving L. Kron, Email: ilk@virginia.edu, University of Virginia, Department of Surgery, Division of Thoracic and Cardiovascular Surgery, Funding: R01 HL130053, Conflicts of interest: none, PO Box 800679, Charlottesville, VA 22908, Phone: 434-924-2158, Fax: 434-982-3885.
References
- 1.Cypel M, Keshavjee S. Strategies for safe donor expansion: donor management, donations after cardiac death, ex vivo lung perfusion. Curr Opin Organ Transplant. 2013;18:513–17. doi: 10.1097/MOT.0b013e328365191b. [DOI] [PubMed] [Google Scholar]
- 2.Pierre AF, Sekine Y, Hutcheon MA, Waddell TK, Keshavjee SH. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg. 2002;123:421–8. doi: 10.1067/mtc.2002.120345. [DOI] [PubMed] [Google Scholar]
- 3.Botha P, Trivedi D, Weir CJ, Searl CP, Corris PA, Dark JH, Schueler SV. Extended donor criteria in lung transplantation: impact on organ allocation. J Thorac Cardiovasc Surg. 2006;131:1154–60. doi: 10.1016/j.jtcvs.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 4.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, Tribble CG, Kron IL. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681–5. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, Kotloff RM. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–65. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 6.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, Robbins MK, Kron IL. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–8. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 7.Kreisel D, Krupnick AS, Puri V, Guthrie TJ, Trulock EP, Meyers BF, Patterson GA. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–22. doi: 10.1016/j.jtcvs.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–29. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–25. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M, Chow CW, Chaparro C, Hutcheon M, Singer LG, Slutsky AS, Yasufuku K, de Perrot M, Pierre AF, Waddell TK, Keshavjee S. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–40. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 11.Mulloy DP, Stone MS, Crosby IK, LaPar DJ, Sharma AK, Webb DV, Lau CL, Laubach VE, Kron IL. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg. 2012;144:1208–16. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie JD, Raemdonck DV, de Perrot M, Barr M, Keshavjee S, Arcasoy S, Orens J Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on primary lung graft dysfunction Part I: Introduction and Methods. J Heart Lung Transplant. 2005;24:1451–3. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on primary lung graft dysfunction Part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Christie JD, Ahya VN, Sager JS, Pocchetino A, DeMissie E, Zhou L, Kotloff RM. ISHLT PGD grade predicts differential mortality following lung transplantation. J Heart Lung Transplant. 2005;24(suppl):S71–2. [Google Scholar]
- 15.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, Dahlberg PS. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–11. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Emaminia A, LaPar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, Linden J, Kron IL, Lau CL. Adenosine A2A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92:1840–6. doi: 10.1016/j.athoracsur.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reece TB, Ellman PI, Maxey TS, Crosby IK, Warren PS, Chong TW, LeGallo RD, Linden J, Kern JA, Tribble CG, Kron IL. Adenosine A2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1137–43. doi: 10.1016/j.jtcvs.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 18.LaPar DJ, Laubach VE, Emaminia A, Crosby IK, Hajzus VA, Sharma AK, Sumner HM, Webb DV, Lau CL, Kron IL. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–94. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, Kern JA, Kron IL. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121(6):1069–75. doi: 10.1067/mtc.2001.113603. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–26. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–49. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, Ellman PI, Lisle TC, Kron IL. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg. 2008;135:156–65. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 23.Yeung JC, Cypel M, Machuca TN, Koike T, Cook DJ, Bonato R, Chen M, Sato M, Waddell TK, Liu M, Slutsky AS, Keshavjee S. Physiologic assessment of the ex vivo donor lung for transplantation. J Heart Lung Transplant. 2012;31:1120–6. doi: 10.1016/j.healun.2012.08.016. [DOI] [PubMed] [Google Scholar]