Abstract
Many drugs in common use possess pleiotropic properties that make them capable of interfering with carcinogenesis mechanisms. We discuss here the ability of pharmacological agents to mitigate the pulmonary carcinogenicity of mainstream cigarette smoke. The evaluated agents included antiinflammatory drugs (budesonide, celecoxib, aspirin, naproxen, licofelone), antidiabetic drugs (metformin, pioglitazone), antineoplastic agents (lapatinib, bexarotene, vorinostat), and other drugs and supplements (phenethyl isothiocyanate, myo-inositol, N-acetylcysteine, ascorbic acid, berry extracts). The drugs have been evaluated in mouse models mimicking interventions either in current smokers or in ex-smokers or a prenatal chemoprevention. They displayed a broad spectrum of activities by attenuating either smoke-induced preneoplastic lesions or benign tumors and/or malignant tumors. Together with epidemiological data, these findings provide useful information to predict the potential effects of pharmacological agents in smokers.
Keywords: lung cancer, cigarette smoke, pharmacological prevention, antiinflammatory drugs, antidiabetic drugs, antineoplastic drugs
Carcinogenicity of Cigarette Smoke in Humans and Animal Models
Tobacco smoke, and in particular cigarette smoke (CS), is a dominant risk factor in the epidemiology of human cancer and of several other chronic degenerative diseases worldwide. Mainstream CS (MCS) is generated at 1200–1600°C and is inhaled as an undiluted complex mixture by active smokers, whereas environmental CS (ECS), or second-hand smoke, is a mixture of that portion of MCS that is exhaled by active smokers and of sidestream CS (SCS) generated at 900°C at the tip of a lit cigarette and is inhaled by involuntary (or passive) smokers. Both MCS and ECS are categorized as Group 1 carcinogens by the International Agency for Research on Cancer (IARC) [1]. In particular, exposure to MCS is associated with cancers affecting a number of anatomical sites. Due to obvious first-pass effects, the main target is the respiratory system, including the nasal cavity and paranasal sinuses, nasopharynx, oropharynx and hypopharynx, larynx, and above all the lung. In addition, MCS causes cancers in the urinary tract (kidney pelvis, ureter, and bladder), digestive system (oral cavity, esophagus, stomach, colon-rectum, liver, and pancreas), reproductive tract (ovary and uterine cervix), and hematopoietic system (myeloid leukemia) [1]. Furthermore, smoking is associated with a variety of chronic degenerative diseases, such as chronic obstructive pulmonary diseases (COPD), cardiovascular diseases, and cerebrovascular diseases, as well as reproductive effects. Overall, CS-related diseases result in a 10-year loss of life expectancy and are responsible for 443000 deaths in the USA and 650000 deaths in the EU [2]. The large majority of the smokers live nowadays in low- and middle-income countries, which in the future is expected to produce large disparities in cancer-related mortality rates between the developed and less developed countries of the world [3].
The overwhelming epidemiological evidence supporting the major role of CS in human cancer epidemiology are mechanistically strengthened by the fact that both MCS and ECS are positive in virtually all in vitro and in vivo short-term genotoxicity tests in which they have been tested. For instance, we demonstrated evident alterations of a variety of intermediate biomarkers in the lung and other organs of either MCS-exposed or ECS-exposed rodents, such as chromosome aberrations, bulky adducts to either nuclear DNA or mitochondrial DNA, hemoglobin adducts, oxidative DNA damage, microRNA, transcriptome and proteome alterations, apoptosis and proliferation of bronchial epithelial cells [4,5]. Combustion of tobacco leaves generates more than 8000 identified chemical compounds, including molecules that virtually belong to any chemical family, 73 of which have been evaluated by IARC to be carcinogenic in humans and/or experimental animals [6]. The prototypes of carcinogenic CS compounds are polycyclic aromatic hydrocarbons, such as benzo(a)pyrene [B(a)P], tobacco-specific nitrosamines, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N′-nitrosonornicotine (NNN), and reactive oxygen species (ROS) and in general free radicals [6].
It is very difficult to reproduce the carcinogenicity of CS in animal models, which limits the studies on CS and evaluation of protective effects. This drawback depends on the general difficulty to test complex mixtures rather than individual compounds in experimental settings as well as on various problems inherent to exposure by inhalation of rodents, such as the different anatomy of the upper respiratory tract and the fact that rodents are obligate nose-only breathers. As a consequence, most carcinogenicity studies in a variety of animal species showed that inhaled CS is either negative or just weakly positive [7–10]. Our attempts to use transgenic mice, such as p53 mutant mice [11], failed to enhance the carcinogenic response.
Physiologically, nucleotide and transcriptional alterations occur in the mouse lung at birth [12]. This finding prompted us to start exposure of mice soon after birth, when the respiratory tract is particularly stressed, for a period corresponding to weaning, adolescence, and young adulthood in mice. In humans, that period would cover the post-natal time, followed by puberty, adolescence, and adulthood. Under these conditions, MCS becomes a potent carcinogen [13], especially when compared with exposure during adulthood [14].
Prevention of Smoking-Related Cancers
The most obvious strategy to prevent smoking-related cancers and other diseases is to minimize exposures to both MCS and ECS. Avoiding exposure to MCS can be achieved either by refraining from smoking (never-smokers) or by quitting smoking (ex-smokers), whereas diseases associated with exposure to ECS can be prevented by suitable regulations that prohibit smoking in public areas and indoor environments. Epidemiological studies have demonstrated, on a large scale, that a decrease in the consumption of cigarettes is successful in attenuating the epidemic of lung cancer either in selected groups of population or in the whole male population of several countries [15].
As a complementary strategy, it is possible to interfere with the mechanisms of the carcinogenesis process, at any stage [16], and to render the host organism more resistant during the long latency time (generally 2–3 decades) elapsing between the first exposure to CS and the clinical onset of CS-related cancers. This strategy, referred to as cancer chemoprevention, uses dietary and pharmacological agents. In the case of CS-related cancers, it is particularly targeted to (i) addicted active smokers who are unable to quit smoking, (ii) ex-smokers, who are at a substantially increased risk of developing lung cancer compared with lifetime never smokers [17], and (iii) passive smokers, including transplacentally exposed individuals.
The major limitations in evaluating the efficacy of chemopreventive agents against CS–related cancers are represented by the problems encountered in clinical trials, which have often produced disappointing results to date [17–19]. In general, animal models provide a valuable tool to predict the potential efficacy of dietary and pharmacological agents. This is also the case for typical CS components, such as B(a)P and NNK, which have extensively been investigated in chemoprevention research [20,21]. However, due to the above discussed difficulties in inducing lung tumors in rodents exposed to CS as a complex mixture, it is problematic to evaluate its modulation by pharmacological agents and dietary interventions.
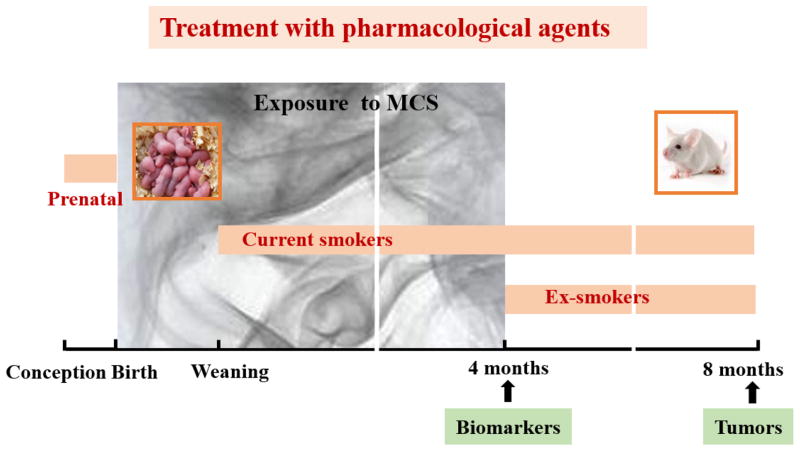
We review here the findings of epidemiological investigations, clinical trials, and preclinical studies concerning the effect of drugs in pulmonary carcinogenesis. Moreover, we report the results of a series of studies that evaluated, under comparable conditions, both safety and efficacy of 15 drugs or combinations thereof towards the carcinogenicity of MCS in the murine model developed in our laboratory [13]. As shown in Figure 1, in the chemoprevention studies in mice the pharmacological agents were administered per os according to 3 different protocols: (i) administration to the dams throughout pregnancy (prenatal intervention); (ii) administration to the litters after weaning (4–5 weeks) until the end of the experiment (intervention in current smokers); and (iii) administration starting after discontinuation of exposure to MCS (4 months) and continuing until the end of the experiment (intervention in ex-smokers).
Figure 1.
Outline of the Murine Model Used for Detecting the Carcinogenicity of MCS. The mice were exposed whole-body during the first 4 months of life. The pharmacological agents were administered with the diet either during pregnancy (prenatal treatment) or after weaning (intervention in current smokers) or after discontinuation of exposure do MCS (intervention in ex-smokers).
Some of the investigated pharmacological agents are among the most extensively prescribed drugs worldwide and are often used for long periods of time. Therefore, it is of interest to know how their use can potentially interfere with the pulmonary carcinogenesis process in current smokers and ex-smokers.
Yield of Tumors and Other Histopathological Lesions in the Murine Model
In each study, neonatal mice were randomized and divided into experimental groups, including untreated mice kept in filtered air for 7–8 months (sham-exposed mice), mice exposed to MCS, starting within 12 h after birth and continuing daily during the first 4 months of life, followed by an additional 3–4 months in filtered air (MCS-exposed mice), and MCS-exposed mice treated with the investigated pharmacological agent(s) according to varying protocols (see Figure 1). Each experimental group was composed of 60 to 80 mice of both genders. Exposure to MCS resulted in a number of histopathological alterations, whose morphological appearance has been exemplified in previous papers [13,14,22]. In particular, compared with sham-exposed mice, MCS induced a significant increase in the incidence of several lung alterations, including emphysema, alveolar epithelial hyperplasia, blood vessel proliferation and hemangiomas, microadenomas, adenomas, and malignant tumors. In addition, exposure to MCS often caused liver parenchymal degeneration, kidney tubular epithelial hyperplasias and adenomas as well as epithelial hyperplasia and papillomas of the urinary bladder, whose increase was statistically significant in some studies.
For the sake of simplicity, we mainly focus here on evaluation of lung microadenomas, adenomas, and malignant tumors, examples of which are given on the top of Figure 2, while we refer to the individual papers for other lesions in the lung and other organs. MCS induced a strong tumorigenic response in mouse lung, with a sharp difference in tumor incidence between sham-exposed and MCS-exposed mice, which leaves a broad margin for appreciating possible protective effects of test agents. In fact, using a total of 1,025 mice either as negative controls (448 sham-exposed mice) or as positive controls (577 MCS-exposed mice) in various studies, the overall incidence of microadenomas was the 38.3% in MCS-exposed mice vs. the 0.9% in sham-exposed mice, and the incidence of adenomas was the 25.5% vs. the 2.0%. Malignant tumors were totally absent in sham-exposed mice, whereas their incidence was the 11.3% in MCS-exposed mice. Microadenomas are preneoplastic lesions that are probably related to MCS-related chronic inflammation. At variance with adenomas, they are only detectable at the microscopic level and may regress spontaneously [23]. Adenomas are benign tumors that mostly originate from type II pneumocytes [24]. On the whole, by pooling the results of 5 studies [13,14,25–27] and irrespective of the administration of pharmacological agents, we detected a total of 165 foci of malignant tumors, which were classified as follows: 41 foci of bronchiolo-alveolar carcinoma (24.8%); 11 of squamous cellular carcinoma in situ (6.7%); 22 of squamous cellular carcinoma (13.3%); 3 of small cell carcinoma (1.8%); 9 of adenocarcinoma (5.5%); and 79 of adenosquamous carcinoma (47.9%). The incidence of individual malignant lesions was too low to discriminate differential protective effects of drugs towards specific histopathological types.
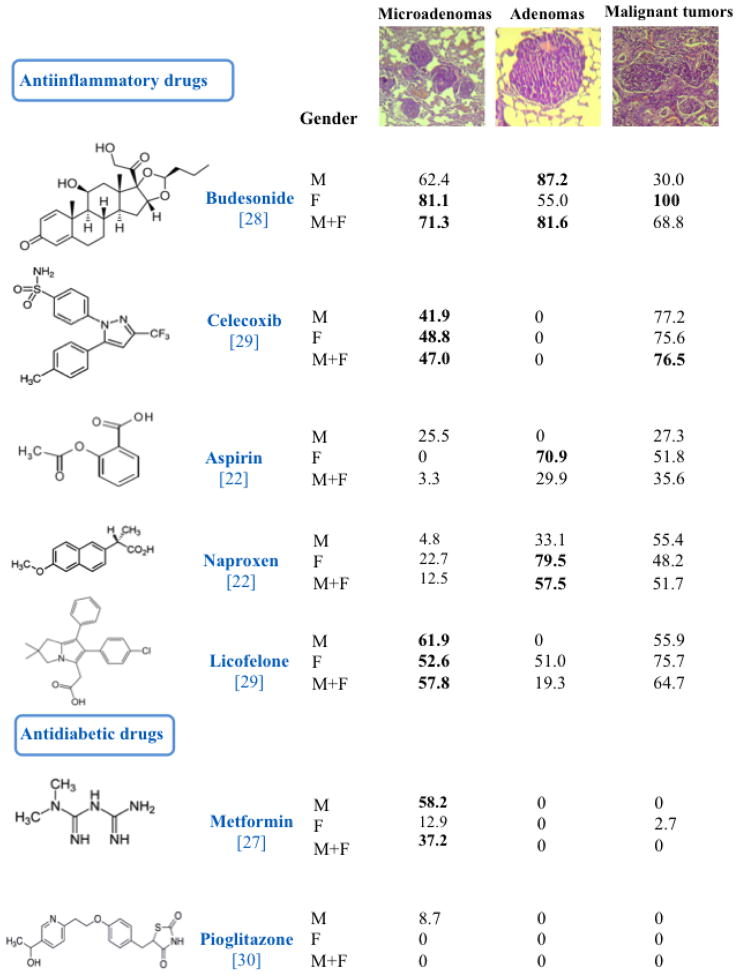
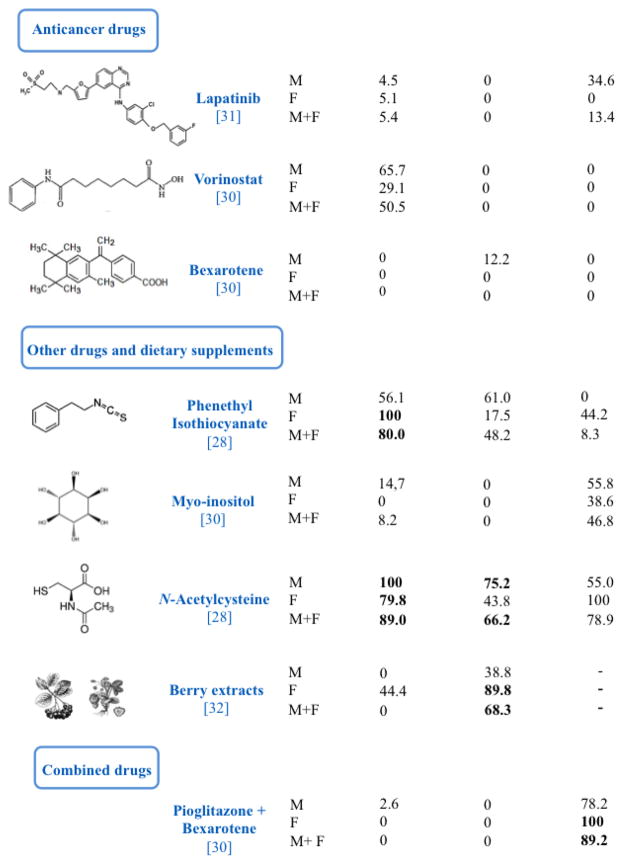
Figure 2.
Inhibition of the Incidence of Microadenomas, Adenomas, and Malignant Tumors in Mouse Lung. The mice were exposed to MCS during the first 4 months of life and subjected to 15 chemopreventive regimens, administered under conditions mimicking an intervention in current smokers. See text for the calculation of inhibition of tumor incidence and for other details. Values in bold indicate that the difference between the incidence of tumors in MCS-exposed mice in the absence of drugs and the incidence of tumors in MCS-exposed mice in the presence of a given drug was statistically significant. The values in brackets under the name of test drugs are the reference numbers.
Figure 2 summarizes the effects of 15 chemopreventive regimens (CHEM), administered under conditions mimicking an intervention in current smokers, on the incidence of microadenomas, adenomas, and malignant tumor in mouse lung. Inhibition of tumor incidence in each study was calculated as follows:
Thus, 0 means no inhibition, while 100 means a total inhibition. Values in bold indicate that the difference between the incidence of tumors in MCS-exposed mice in the absence of drugs and the incidence of tumors in MCS-exposed mice in the presence of a given drug was statistically significant. We refer to the original papers for details.
Overview of Experimental and Epidemiological Studies Evaluating Pharmacological Agents
Anti-Inflammatory Drugs
Anti-inflammatory agents provide a promising approach to the prevention of smoking-associated cancers, owing to the fact that chronic inflammation plays a key role at different stages of the carcinogenesis process [33,34] and is crucial in tobacco smoke carcinogenesis [35].
We investigated a glucocorticoid and four nonsteroidal anti-inflammatory drugs (NSAIDs) for the ability to inhibit lung tumors in MCS-exposed mice. Glucocorticoids are potent inhibitors of flogosis by inhibiting inflammation-associated molecules. Their anti-inflammatory effects are mediated either by direct binding of theglucocorticoid/glucocorticoid receptor complex to glucocorticoid responsive elements in the promoter region of genes, or by an interaction of this complex with other transcription factors, in particular activating protein-1 (AP-1) and nuclear factor-kappa B (NF-κB) [36]. NSAIDs exert their anti-inflammatory activity mainly by interfering with the metabolism of arachidonic acid, a ω-6 essential fatty acid that is the substrate for various enzyme systems, including cyclooxygenases (COX), lipoxygenases (LOX), and cytochromes P450 (CYP). Of the two COX enzymes, COX-1 is the housekeeping isoform, and prostaglandins derived from COX-1 are involved in the homeostatic maintenance of the gastric mucosa. In contrast, COX-2 is the inducible isoform having a pro-inflammatory function, which is expressed in response to certain stimuli such as mitogens, cytokines and growth factors [37]. Arachidonic acid is also metabolized by 5-LOX to leukotrienes, one of which has been shown to be involved in the development of gastrointestinal ulcers [38]. In addition, NSAIDs may reduce the “inflammogenesis of cancer” not only by interfering with the arachidonic acid cascade but also by lowering the levels of inflammation mediators, such as cytokines, chemokines, and adhesion molecules [33,39,40].
Budesonide
Budesonide is an anti-inflammatory drug that is extensively used for the treatment of asthma and COPD. Epidemiological studies and clinical trials using this synthetic glucocorticoid did not clearly indicate protective effects in decreasing the risk of lung cancer [17], whereas animal studies have shown the ability of budesonide to inhibit the induction of lung tumors by CS components. In particular, in one study budesonide, administered by aerosol to avoid potential side-effects, inhibited the formation of B(a)P-induced pulmonary adenomas in female A/J mice, an effect that was further potentiated by its combination with myo-inositol [41]. In another study, budesonide attenuated the multiplicity of B(a)P-induced lung tumors and their size in A/J mice. In addition, budesonide inhibited the B(a)P-induced hyperplasia of the bronchial epithelium in both wildtype and FHIT+/− B6/129 F1 mice and hyperplasia of alveolar walls in FHIT+/− B6/129 F1 mice [42].
As shown in Figure 2, the dietary administration of budesonide to Swiss H mice after weaning, mimicking an intervention in current smokers, was quite effective in inhibiting induction of microadenomas, adenomas, and malignant tumors in mice exposed to MCS since birth. Interestingly, these striking protective effects were unchanged when this glucocorticoid was administered after discontinuation of exposure to MCS [28], thus mimicking an intervention in ex-smokers. Being a potent anti-inflammatory agent, budesonide is expected to work also in advanced carcinogenesis stages, as it was previously demonstrated in A/J mice treated with B(a)P, in which this glucocorticoid inhibited all stages of tumor progression, from hyperplasia to cancer [43]. Unfortunately, under all experimental conditions, administration of budesonide to MCS-exposed mice was found to increase the incidence of parenchymatous degeneration of the liver [28].
Celecoxib
The fact that the prostaglandins derived from COX-1 are involved in the homeostatic maintenance of the gastric mucosa prompted the development of selective COX-2 inhibitors, denominated coxibs. One of them is celecoxib (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzene-1-sulfonamide), which seems to be safer with regard to gastric damage compared to nonselective NSAIDs [44]. Coxibs have been tested in animal models of cancer chemoprevention [45], and celecoxib administration attenuated oxidative stress in mice exposed to cigarette smoke [46]. However, celecoxib failed to inhibit lung tumors in mice treated either with urethane or subjected to a two-stage carcinogenesis protocol in which 3-methylcholanthrene administration was followed by chronic butylated hydroxytoluene treatment [47]. In humans, a case-control study showed that use of selective COX-2 inhibitors, among which celecoxib, reduced the lung cancer risk, thus demonstrating that selective COX-2 blocking agents have strong potential for the chemoprevention of human lung cancer [48]. A trial of celecoxib in former smokers decreased the bronchial Ki-67 labeling index and reduced lung nodules on computed tomography, which supports the continued investigation of celecoxib for lung cancer chemoprevention in former smokers at a low risk of cardiovascular disease [49]. In fact, high dose celecoxib may involve cardiovascular risks, such as myocardial infarction, stroke, or heart failure, due to shunt of arachidonic acid into the LOX pathway, which plays a role in cardiovascular disease [50].
Our results, obtained in mice exposed to MCS since birth, showed that celecoxib, administered after weaning at the dose of 1600 mg/kg diet, is able to inhibit the formation of microadenomas, which reflects the anti-inflammatory properties of this NSAID. Celecoxib failed to significantly affect the incidence of MCS-induced adenomas, but there was a slight decrease in their multiplicity, which reached the statistical significance threshold in male mice. In addition, celecoxib significantly interfered with the progression of lung tumors to malignancy (Figure 2). The protective ability of celecoxib was slightly lowered when this NSAID was administered starting after discontinuation of exposure to MCS, at 4 months of life. In addition, under both experimental conditions, celecoxib exhibited protective effects in the urinary tract of MCS-exposed mice [37]. Unfortunately, the selected dose of celecoxib, which did not produce any adverse effects in smoke-free mice treated for 6 weeks, became rather toxic when administered to MCS-exposed mice for longer periods of time, as shown by alterations of survival and body weight data after 4 months of life and by an increase of liver degeneration in celecoxib-exposed mice treated with celecoxib [37]. Celecoxib is known to be primarily metabolized by CYP2C9, which is involved in the metabolism of tobacco smoke polycyclic aromatic hydrocarbons [51]. Accordingly, it has been cautioned that increased clinical vigilance is required during the co-administration of celecoxib and other substrates or inhibitors of CYP2C9 [52], as it could be the case for smokers.
Aspirin and Naproxen
The salicylate derivative aspirin, or acetylsalicylic acid, and the propionic acid derivative naproxen are nonselective inhibitors of both COX-1 and COX-2. Aspirin has emerged as the most likely NSAID for use in cancer chemoprevention, possibly in combination with a gastroprotective agent, because of its known cardiovascular benefits and available safety and efficacy data [53]. In animal models of pulmonary carcinogenesis, aspirin inhibited the lung tumors induced either by NNK in A/J mice [54] or by 7,12-dimethylbenz(a)anthracene in Balb/c mice [55]. In humans, the protective role of aspirin and other NSAIDs is well established for colorectal cancer, whereas there are uncertainties regarding other types of cancer, including lung cancer [56]. A recent pooled analysis, evaluating the effect of allocation of aspirin on the 20-year risk of death, convincingly showed a significant attenuation of deaths due to lung cancer, particularly to adenocarcinoma [57]. As to naproxen, this NSAID has been shown to exert protective effects in some rodent models but failed to inhibit NNK-induced lung tumors in mice [54].
Based on subchronic toxicity studies, we used a dose of 1600 mg/kg diet for aspirin and 320 mg/kg diet for naproxen. After four months of exposure of Swiss H mice to MCS, starting at birth, and 3 months of treatment with the two NSAIDs, naproxen attenuated the MCS-induced systemic genotoxic damage but only in female mice [22]. After recovery in filtered air for an additional 3.5 months, MCS induced a variety of histopathological alterations in the lung. As shown in Figure 2, aspirin and naproxen failed to affect the incidence of microadenomas but exhibited a remarkable protective effect by decreasing the incidence of lung adenomas, an effect that was statistically significant in female mice. The incidence of malignant tumors was lower in MCS-exposed mice treated with either NSAID, but the difference was not statistically significant compared with MCS-exposed mice in the absence of chemopreventive agents [22]. Interestingly, similar findings were generated in a separate study evaluating the protective effects of aspirin and naproxen, at the same doses, in A/J mice exposed to ECS. In fact, after exposure to ECS for 4 months followed by 5 months in filtered air, ECS induced a significant increase in the yield of surface lung tumors, the 43.7% of which were adenomas and the 56.3% were adenocarcinomas, some of which bore Oct-4 (octamer-binding transcription factor 4), a marker of cell stemness. Both aspirin and naproxen attenuated the yield of lung tumors. Again, prevention of ECS-induced lung adenomas was statistically significant only in female mice treated with aspirin [58].
As noted in the study on berry extracts (see below), these results lend further support to the view that estrogens contribute to the CS pulmonary carcinogenicity. In fact, studies in A/J mice demonstrated the presence of 17β-estradiol in the lung and showed modulation of CYP1B1 and other estrogen metabolism genes by tobacco smoke [59]. Moreover, studies in heterozygous 129/SvJ Cyp1b1-KO mice suggested that tobacco smoke accelerates the production, within the lung, of estrogen metabolites that could potentially contribute to lung tumor development [60]. Estrogens are functional in the normal lung and tumor cell lines and can directly stimulate the transcription of estrogen-responsive genes in the nucleus of lung cells, thereby transactivating the epidermal growth factor (EGF) pathway [61]. In addition, it appears that NSAIDs have antiestrogenic properties, which result from their ability to inhibit COX-2. In fact, prostaglandin E2 upregulates the expression of the aromatase gene (aromatase CYP), the product of CYP19, which catalyzes the final step in estrogen biosynthesis [62]. It is noteworthy that the results obtained in our murine model were able to discriminate intergender differences in the antitumor effects of NSAIDs, whereas, in the analysis of individual patient data from randomized trials [57], “the protective effects of aspirin on lung cancer in humans were rather small, and it was not possible to point out any intergender differences” (P. Rothwell, personal communication). In a recent meta-analysis of randomized controlled trials, aspirin showed intergender differences in the primary prevention of cardiovascular events [63].
Licofelone
Licofelone (2-[6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1H-pyrrolizin-5yl]acetic acid), an inhibitor of both COX-1 and COX-2 and additionally of 5-LOX, has been proposed for the treatment of osteoarthritis [64]. It was found to have protective effects in carcinogenesis models, including pulmonary adenomatosis induced by B(a)P in A/J mice treated with licofelone via oropharyngeal aspiration [65].
We tested licofelone at the dose of 960 mg/kg diet, in the same study that evaluated safety and efficacy of celecoxib [29]. Like celecoxib, administration since weaning of licofelone to mice exposed to MCS during the first 4 months of life significantly reduced the incidence of microadenomas, whereas the reduction in the yield of both adenomas and malignant tumors was biologically appreciable, especially in female mice, but it did not reach the statistical significance threshold (Figure 2). In addition, licofelone significantly attenuated the incidences of both tubular epithelial hyperplasias in the kidney and of papillary epithelial hyperplasias in the urinary bladder of MCS-exposed mice [29]. In general, the protective effects of licofelone in both lung and kidney of MCS-exposed mice were more pronounced compared to celecoxib, which strengthens the assumption that dual inhibitors that block both COX and 5-LOX may provide synergistic antiinflammatory effects compared with COX inhibitors [66]. Unfortunately, like celecoxib, licofelone became rather toxic when administered to MCS-exposed mice after discontinuation of exposure to MCS, when survival was worsened in mice treated with either NSAID, which failed to improve their body weights until the end of the experiment [29].
Antidiabetic Drugs
Both cancer and diabetes have an outstanding health impact all over the world. These diseases are often diagnosed within the same individual, and epidemiological studies suggest that diabetic patients have an increased risk of developing several types of cancer. Cancer and type 2 diabetes share many risk factors, both unmodifiable and modifiable, but the mechanisms underlying the links between these diseases are not completely understood [78]. In particular, although there is no epidemiological evidence that lung cancer is associated with an increased risk in diabetes [78], it appears that smoking is an independent risk factor not only for lung cancer and cancers at other sites [1] but also for the development of diabetes [79]. For these reasons, it is important to evaluate whether administration of antidiabetic drugs may be associated with a decreased risk of developing cancer. We investigated the two antidiabetic drugs metformin and pioglitazone for the ability to modulate the induction of lung tumors in MCS-exposed mice.
Metformin
Metformin (N,N-dimethylimidodicarbonimidic diamide) is at present the most widely prescribed drug in the world for the treatment of type 2 diabetes [67]. This biguanide represses hepatic gluconeogenesis, lowers circulating insulin and decreases insulin resistance by activating AMP-activated protein kinase (AMPK), which is the primary downstream kinase regulated by the tumor suppressor gene liver kinase B1 (LKB1). This mechanism results in an inhibition of the mammalian target of rapamycin (mTOR) [68]. The results of epidemiological studies evaluating the cancer protective properties of metformin are rather controversial [69–78]. With specific reference to the relationships between metformin and pulmonary carcinogenesis, an observational cohort study [76] and a case–control study [77] led to the conclusion that metformin does not alter the risk of lung cancer in diabetic patients. However, a recent nationwide study in Taiwan suggested that metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner [78]. In animal models, metformin was able to reduce the multiplicity of surface lung tumors when given both orally and intraperitoneally to A/J mice treated parenterally with NNK [79], whereas it slightly but significantly inhibited the incidence of total tumors but not that of solid or trabecular lung adenomas in 129/Sv mice treated parenterally with urethane [80].
In our laboratory, the oral administration of metformin to mice after weaning, at the dose of 800 mg/kg diet, did not affect the systemic clastogenic damage induced by MCS during the first 4 months of life [27]. After an additional 3.5 months in filtered air, metformin did not significantly affect the yield of MCS-induced lung adenomas and malignant tumor, but it caused a significant decrease in both incidence and multiplicity of microadenomas, especially in males (Figure 2). In addition, the MCS-induced tubular epithelial hyperplasia in kidney was totally suppressed by metformin in males [27]. In the same study, it was shown that, at 10 weeks of life, metformin considerably decreased DNA adduct levels and oxidative DNA damage, and normalized the expression of several microRNAs in the lung of MCS-exposed mice [27]. Thus, there was a contrast between the ability of metformin to modulate early biomarkers in the lung and preneoplastic lesions both in lung and kidney and its failure to protect towards induction by MCS of systemic clastogenic damage at 4 months and of lung tumors, either benign or malignant, at 7.5 months.
Regarding the greater susceptibility of male mice to the protective effects of metformin in lung and kidney, it is noteworthy that the cumulative urinary excretion and renal tissue-to-plasma concentration ratio of metformin in male rats were found to be markedly higher compared to that of female rats due to gender-related differences in the expression of organic cation transporter 2 (rOCT2) [81]. It has also been shown that exposure of neonatal 129/Sv mice to metformin slows down aging and prolongs lifespan in males but not in females [82].
Pioglitazone
Pioglitazone is a synthetic ligand of peroxisome proliferator-activated receptor γ (PPARγ) [83] and is approved for the therapy of type 2 diabetes. It has been proposed that PPARγ may be a target for cancer chemopreventive agents [84]. However, the clinical relevance of in vitro findings is unclear [67], and rodent studies even indicate that PPAR agonists can potentiate tumorigenesis, to such an extent that they have been considered to be multi-species carcinogens [85]. For instance, pioglitazone promoted lung cancer progression and metastasis in immunocompetent mice [86].
We tested oral pioglitazone, at the dose of 120 mg/kg diet, in mice exposed to MCS since birth. After 4 months, this thiazolidinedione failed to affect the systemic clastogenic damage produced by MCS and, after 7 months, it failed to inhibit the MCS-induced formation of microadenomas, adenomas, and malignant tumors (Figure 2). On the contrary, pioglitazone significantly enhanced the yield of pulmonary adenomas in MCS-exposed mice [30] and, in parallel, it enhanced the incidence of kidney lesions in the same mice, compared with mice exposed to MCS only [87].
It is noteworthy that a recent meta-analysis of 17 studies showed a modest excess risk of bladder cancer in type 2 diabetes patients treated with pioglitazone [88] and that PPAR agonists have been reported to be tumorigenic in multiple sites of rodents and in the urothelial epithelium of monkeys [89,90].
Antineoplastic Drugs
In recent years, the armamentarium available for the therapy of cancer has been enriched with targeted therapies that specifically modulate molecular mechanisms involved in the growth, progression, and spread of cancer cells. The same pharmacological agents would be expected to find useful applications, often with personalized approaches, by hampering the progression of a benign lesion into a malignant tumor (secondary prevention) and/or by avoiding local relapses and invasion and metastasis of cancer cells (tertiary prevention). With these premises, we assayed three anticancer drugs among the agents evaluated for the prevention of smoking-related tumors. They included a drug approved for breast cancer therapy (lapatinib) and two drugs proposed for the therapy of cutaneous cell lymphoma (bexarotene and vorinostat).
Lapatinib
Lapatinib, or N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2methylsulfonylethylamino)methyl]-2-furyl]quinazolin-4-amine, is a dual tyrosine kinase inhibitor (TKI) targeting both the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2, c-erbB2, c-neu). Based both on preclinical studies and clinical trials, lapatinib is indicated for the treatment of patients with advanced breast cancer or metastatic breast cancer whose tumors overexpress HER-2 [91]. Its use was approved by the US FDA (Food and Drug Administration) in 2007 and by the EMA (European Medicines Agency) in 2008, with a conditional marketing authorization that has been renewed every year. Since a variety of malignancies are associated with the mutation or increased expression of members of the EGFR or ErbB family, drugs targeting ErbB-related pathways could find applications in the treatment of other cancers as well. With specific reference to lung cancer, both EGFR [92] and HER-2 [93] have been shown to play a role in smoke-related lung carcinogenesis. In vitro, lapatinib was found to reduce cell proliferation, DNA synthesis and colony formation capacity in A549 human bronchoalveolar carcinoma cells. Moreover, A549 tumor-bearing mice treated with lapatinib had significantly less tumors compared to untreated mice [94]. However, a randomized Phase II study in chemotherapy-naive patients with non-small cell lung cancer (NSCLC), which was later amended to target patients with bronchioloalveolar carcinoma or no smoking history, showed that lapatinib monotherapy did not induce a significant number of tumor regressions in NSCLC [95].
We tested lapatinib at the dose of 1600 mg/kg diet in mice exposed to MCS since birth. After 10 weeks, lapatinib significantly attenuated the MCS-related nucleotide changes (bulky DNA adducts and oxidative DNA damage) and upregulated several low-intensity microRNAs in lung. At the end of the period of exposure to MCS (4 months), a decrease in the frequency of MN PCNE in MCS-exposed mice treated with lapatinib after weaning did not reach the statistical significance threshold [31]. After 7.5 months, lapatinib had poor effects on MCS-induced histopathological alterations. When given under conditions simulating an intervention either in current smokers or in ex-smokers, this quinazoline derivative did not affect tumor incidence (Figure 2) but lowered the multiplicity of microadenomas, an effect that became statistically significant when combining the two genders. In addition, under both conditions, lapatinib inhibited the MCS-induced tubular epithelial hyperplasia of kidney, especially in male mice. However, this drug was hepatotoxic to MCS-exposed female mice. Thus, on the whole, lapatinib appears to have a low impact in the smoke-related lung carcinogenesis models used, especially in terms of modulation of the tumorigenic response [31].
Vorinostat
Vorinostat, or suberoylanilide hydroxamic acid (SAHA, Zolinza®), is an inhibitor of histone deacetylases (HDACs), including all 11 known human class I and class II HDACs [96]. Vorinostat has been shown to upregulate gap junctional intercellular communications in nonmalignant human peritoneal mesothelial cells via acetylation of histones H3 and H4 in the chromatin fragments associated with the connexin 43 (Cx43) gene locus [97].
Treatment of EGFR-dependent human lung cancer cell lines with a HDAC inhibitor induced apoptosis through Hsp90 acetylation, and combination with a TKI resulted in a synergistic tumor cell death [98]. A prospective, non-randomized, multicenter, Phase I/II trial evaluated the activity of vorinostat plus the TKI erlotinib in EGFR-mutated NSCLC patients. The results showed that full dose of continuous erlotinib with vorinostat 400 mg p.o. on alternate weeks can be safely administered. However, the combination had no meaningful activity in EGFR-mutated NSCLC patients after TKI progression, and accordingly further studies of vorinostat and erlotinib in advanced NSCLC patients were not recommended [99]. In experimental animals, preliminary imaging studies with a lung cancer model in A/J mice suggested that positron emission tomography (PET) using 6([(18)F]fluoroacetamido)-1-hexanoicanilide [(18)F-FAHA] may target HDAC in lung cancer for early diagnosis of NNK-induced pulmonary tumorigenesis [100]. In the same mouse strain, administration of vorinostat 26 weeks after NNK initiation caused a significant decrease in total and large tumors, which was more pronounced by its combination with either budesonide or atorvastatin [101]. Moreover, the combination of SAHA with a triterpenoid was effective in decreasing the lung tumor yield in A/J mice treated with vinyl carbamate [102].
In our laboratory, vorinostat downregulated the expression of HDAC in the lung of Swiss H mice. The most remarkably upregulated protein was P25, a P53-dependent regulator of cell cycle, which is targeted by two miRNAs modulated by vorinostat, i.e. miR-106 and miR-302 [30]. However, treatment with the drug at 1000 mg/kg diet did not attenuate the systemic genotoxic damage induced in after 4 months of exposure of mice to MCS. As shown in Figure 2, after 7 months SAHA moderately attenuated the MCS-related increase of incidence of microadenomas, but did not attenuate at all the incidences of both adenomas and malignant tumors. Therefore, in spite of the fact that epigenetic alterations have been implicated in the aggressive behavior of lung cancer, treatment of mice with SAHA failed to mitigate the carcinogenic effects of MCS [30].
Bexarotene
Bexarotene, or 9cUAB30 (Targretin®), is a rexinoid, i.e., a selective ligand for the retinoid X receptor (RXR). Preclinical studies suggest that receptor-selective retinoids are promising agents for the prevention of breast cancer and that they may be particularly useful in preventing estrogen receptor–negative breast cancer [103]. Moreover, bexarotene has shown some clinical efficacy in the treatment of cutaneous T cell lymphoma [104] and, based both on experimental data and clinical studies, this drug holds promises also in the prevention of lung cancer [105]. Bexarotene was evaluated in animal models of lung carcinogenesis by testing CS components. In female A/J mice, this retinoid attenuated the multiplicity of lung tumors induced either by vinyl carbamate or NNK [106]. Another study used genetically engineered B5 (A/J × Trp53F2-10/F2-10;Rb1F19/F19) mice, in which Rb1 and p53 alleles were conditionally inactivated in the lung epithelium by using adenovirus-mediated somatic gene transfer of Cre recombinase. This genetic alteration results in the spontaneous development of aggressive lung tumors that have morphologic and immunophenotypic similarities to small cell lung carcinoma. Bexarotene, given by oral gavage at the dose of 180 mg/kg body weight to mice two weeks before intratracheal administration of adeno-Cre virus, significantly reduced tumor incidence [105].
In our laboratory, bexarotene was administered at the dose of 240 mg/kg diet to post-weaning Swiss H mice exposed to MCS since birth. This rexinoid failed to attenuate the systemic clastogenic damage induced in the peripheral blood of mice after 4 months of exposure to MCS. As shown in Figure 2, after 7 months bexarotene did not produce any significant effects on the incidence either of microadenomas or of adenomas or malignant tumors in MCS-exposed mice. Therefore, administration of bexarotene alone did not exert any protection in the murine model used [30].
However, combination of bexarotene with pioglitazone was very effective in attenuating the MCS-induced cytogenetic damage in male mice and in inhibiting formation of malignant tumors in the lung, which were almost totally prevented (Figure 2). Moreover, the previously discussed adverse effects of pioglitazone, both in the respiratory tract [30] and in the urinary tract [87] of MCS-exposed mice, were lost when this antidiabetic drug was combined with bexarotene. From a mechanistic point of view, it should be taken into account that both RXRs and PPARs belong to the nuclear hormone superfamily and contribute to control the expression of multiple target genes. Interestingly, RXR is an obligate heterodymeric partner for PPARs [108,109], which may explain the finding that not only tumorigenicity of pioglitazone is suppressed when PPARγ is bound to RXR but even that this combination is successful in preventing the formation of MCS-induced malignant tumors in the lung.
Other Drugs and Dietary Supplements
We include in this category a variety of compounds, some of which are of natural origin or are even constituents of the body where they play physiological functions. These molecules, which are either derived from the human diet or synthetized as over-the-counter drugs, possess an array of properties that render them suitable candidates for applications both in curative medicine and preventive medicine. They are also investigated for the ability to modulate several mechanisms involved in the carcinogenesis process.
Phenethyl Isothiocyanate
Phenethyl isothiocyanate (PEITC) is a naturally occurring compound contained in watercress (Nasturtium officinale), a plant belonging to the family of brassica vegetables (Brassicaceae). In epidemiological studies, consumption of isothiocyanate-rich vegetables has been associated with a decreased lung cancer risk in relationships with glutathione S-transferase polymorphisms [110]. The main mechanism of PEITC and other isothiocyanates is that these agents modify the metabolism of carcinogens both by inhibiting Phase I cytochrome P450 (CYP) activities involved in the activation of procarcinogens and by inducing Phase II detoxifying enzymes [110]. In addition, PEITC affects multigene expression in the lung of smoke–exposed rats [111] and is also an inducer of apoptosis [112]. PEITC significantly reduced both lung tumor multiplicity and incidence in mice treated with NNK but failed to affect the weak tumorigenicity of ECS in A/J mice [113].
When given to MCS-exposed mice at the dose of 1000 mg/kg diet, under conditions mimicking an intervention in current smokers, PEITC totally prevented the formation of microadenomas in female mice and had a borderline protective effect towards the induction of adenomas in males (Figure 2). PEITC lost part of its protective activity when administered under conditions mimicking an intervention in ex-smokers [28], which is consistent with the notion that this agent interferes with the metabolism of carcinogens occurring during the initial steps of the carcinogenesis process.
Myo-Inositol
Myo-inositol, or cis-1,2,3,5-trans-4,6-cyclohexanehexol (formerly incorrectly classified as vitamin B8), is a constituent of a variety of foods and is also available in some dietary supplements and energy drinks as an essential nutrient. Dietary myo-inositol was successful in inhibiting the formation of pulmonary adenomas in mice treated with either B(a)P or NNK [114,115] but failed to inhibit lung tumors in vinyl carbamate-treated mice [16]. Dietary myo-inositol was also effective in combination with either budesonide or beclomethasone dipropionate, also when these glucocorticoids were administered by aerosol [41]. Moreover, combination of myo-inositol with dexamethasone was the only chemopreventive regimen that attenuated the weak carcinogenicity of unfractionated ECS [7].
In our laboratory, myo-inositol was administered to MCS-exposed mice at the dose of 8000 mg/kg diet, according to the protocol mimicking an intervention in current smokers. At four months of age, at the end of the period of exposure to MCS, myo-inositol did not significantly affect the MCS-related increase in the frequency of MN NCE. After seven months, myo-inositol failed to attenuate the increased incidence of alveolar epithelial hyperplasia and bronchial epithelial hyperplasia in the lung of MCS-exposed mice [30]. Likewise, as shown in Figure 2, no significant protective effect was observed on induction by MCS of lung microadenomas and adenomas. However, myo-inositol decreased both incidence and multiplicity of malignant tumors, an effect that became close to statistical significance only for multiplicity in combined genders [30].
N-acetyl-L-cysteine
The aminothiol N-acetyl-L-cysteine (NAC) is a drug used as a mucolytic agent and as an antitoxic agent towards paracetamol overdosage. Moreover, it is used as a dietary supplement. NAC works in the extracellular environment and is a precursor of intracellular L-cysteine, which is the rate-limiting amino acid in the synthesis of reduced glutathione (GSH). A number of studies performed during the past 30 years have provided evidence for the potential ability of NAC to prevent cancer and other mutation-related diseases, thanks to a broad array of mechanisms. Its primary mechanisms depend on its nucleophilicity and ability to scavenge free radicals and reactive oxygen species. Secondary mechanisms and effects include modulation of metabolism, effects in mitochondria, modulation of DNA repair, inhibition of genotoxicity and cell transformation, modulation of signal transduction pathways, regulation of cell survival and apoptosis, antiinflammatory activity, antiangiogenetic activity, immunological effects, influence on cell cycle progression, and inhibition of invasion and metastasis [117].
By focusing on smoke-related pulmonary carcinogenesis, treatment with NAC had no benefit in a multicenter intervention study, called EUROSCAN, in 2592 patients affected by head and neck cancer or by lung cancer, most of whom were former or currents smokers. No significant effect was observed in the arm of patients treated with NAC (600 mg/day), either alone or in combination with retinyl palmitate, relatively to local/regional recurrences, second primary tumors, distant metastases, or death [118]. By contrast, NAC exerted protective effects in a randomized double blind phase II chemoprevention trial in apparently healthy smoking volunteers. In fact, while in the placebo group there was no significant variation in any of the investigated biomarkers, after 6 months of treatment with NAC (2 x 600 mg/day) a significant decrease occurred in some of the investigated biomarkers, including the levels of bulky DNA adducts and 8-hydroxy-2′-deoxyguanosine in bronchoalveolar lavage cells as well as the frequency of micronuclei in mouth floor and soft palate cells [119]. Moreover, a time-course evaluation of the urinary excretion of mutagens in smokers receiving oral NAC (600–800 mg/day) showed a significant decrease of urinary mutagenicity in six out of 10 subjects, starting on the first day of NAC administration until withdrawal of treatment with the drug [120]. Several studies evaluated the ability of NAC to exert protective effects in animal models of lung tumorigenesis caused by individual CS components. Oral NAC significantly attenuated lung tumor incidence and/or multiplicity in mice treated with urethane (ethyl carbamate) [113,121–123]. Two studies are available in rats and mice treated with the tobacco-specific nitrosamine NNK. In Fischer rats receiving subcutaneous injections of NNK, NAC decreased the incidence of nasal cavity tumors in a dose-dependent way but failed to affect the incidence of lung tumors. In that study, however, lung tumor multiplicity had not been evaluated [124]. In NNK-treated A/J mice, NAC significantly retarded malignant progression in the lung [125]. Based on studies in NNK- and B(a)P-treated A/J mice, the combination of NAC with isothiocyanates was also proposed [124]. Moreover, NAC decreased the size of B(a)P-induced lung tumors in A/J mice and inhibited the B(a)P-induced hyperplasia of the bronchial epithelium in both wildtype and FHIT+/− B6/129 F1 mice [42]. NAC failed to affect the lung tumor yield in A/J mice exposed to unfractionated ECS [113] but, intriguingly, it was able to inhibit the tumorigenicity of the ECS gas phase [126], which is responsible for the carcinogenicity of the whole mixture [127].
As shown in Figure 2, administration of NAC with the drinking water after weaning, at the calculated concentration of 1000 mg/kg body weight, proved to be quite effective in inhibiting the yield of lung microadenomas and adenomas in mice exposed to MCS since birth. A considerable decrease of malignant tumors was also observed, with an effect that was borderline to statistical significance in female mice, in which no malignant tumor was detected [28]. These findings reflect the broad array of protective mechanisms of this aminothiol, which cover all stages of the carcinogenesis process.
In addition, NAC was also assayed in the same mouse model after transplacental treatment (see Figure 1). NAC was added to the drinking water of pregnant mice at a concentration accounting for a calculated daily intake of 1000 mg/kg body weight. The offspring was exposed to MCS during the first 4 months of life. After an additional 3 months in filtered air, the 61.1% of MCS-exposed mice born from untreated dams had lung tumors, with a multiplicity of 3.24 ± 0.53 tumors/mouse (mean ± SE). Treatment with NAC during pregnancy exerted a striking protective effect on the yield of MCS-induced lung tumors after birth. In fact, only the 17.0% of these mice developed lung tumors, with an overall multiplicity of 0.65 ± 0.21 tumors/mouse. Prenatal NAC also protected the mice from induction by MCS of emphysema and alveolar epithelial hyperplasia and of urinary bladder epithelial hyperplasia as well [25]. These results were confirmed, although with less sharp effects, in a further study using the same protocol [26]. In that study, a significant decrease of adenomas occurred in the lung of MCS-exposed mice whose dams had been treated with NAC during pregnancy. Interestingly, exposure to MCS of mice born from untreated dams also resulted in a significant increase of blood vessel proliferation in the lung, which was inhibited by prenatal NAC [26]. This conclusion is consistent with the findings that exposure of mice to ECS downregulates microRNAs involved in regulation of angiogenesis, such as let-7a, let-7b, let-7f, and miR-345, in mouse lung [128], and that NAC shows antiangiogenetic properties both in vitro [129] and in vivo [130]. The fact that NAC was able to inhibit the carcinogenicity of MCS in neonatal mice also when it was administered only during pregnancy correlates with the conclusion that prenatal NAC inhibits genomic and postgenomic alterations occurring spontaneously in mouse lung as a consequence of birth-related oxidative stress [12].
Ascorbic Acid
Ascorbic acid, or vitamin C, is a major exogenous antioxidant, which possesses a variety of properties, the most important of which is its reducing ability as an electron donor [131]. In preclinical models, ascorbic acid protected A/J mice from the induction of lung adenomas induced by a subscapular injection of B(a)P within 24 hours after birth [132]. However, in spite of the quite extensive use of this vitamin for a variety of preventive purposes, from randomized controlled clinical trials there appears to be no evidence for recommending supplements of vitamin C for decreasing lung cancer mortality in healthy people [133].
In the above described model of transplacental chemoprevention in mice, administration of ascorbic acid with the drinking water throughout pregnancy, at a concentration accounting for a calculated daily intake of 1000 mg/kg body weight, protected the MCS-exposed offspring from the induction of lung blood vessel proliferation and microadenomas and from the occurrence of papillary epithelial hyperplasia of the urinary bladder. In addition, ascorbic acid decreased the incidence of pulmonary malignant tumors, but this effect was borderline to statistical significance in female mice only [26].
Berry Extracts
Berries have been reported to possess a broad array of health protective properties, such as antioxidative, antimutagenic, anticancer, cardioprotective, hepatoprotective, gastroprotective, antidiabetic, antiinflammatory, antibacterial, antiviral, radioprotective, and immunomodulatory activities [134]. These activities have been related to a multitude of bioactive phytochemical contained in these colorful fruits, including phenolic molecules such as anthocyanins, flavonols, flavanols, proanthocyanidins, ellagitannins and phenolic acids; α-carotene, β-carotene and lutein; phytosterols such as β-sitosterol and stigmasterol; triterpene esters; and vitamins A, C and E and folic acid; calcium and selenium [135]. Although many studies showed that berry extracts can inhibit the formation of neoplastic and preneoplastic lesions, either “spontaneous” in transgenic mice or induced by individual carcinogens, using a variety of experimental rodent models [32], freeze-dried strawberries failed to affect the yield of tumors in the lung of A/J mice treated with either NNK or B(a)P [136].
We evaluated the ability of berry extracts to inhibit the formation of lung tumors in mice exposed to MCS by testing aqueous extracts either of Aronia melanocarpa (black chokeberry or BCB) or Fragaria ananassa (strawberry or SB). These extracts, containing high concentrations of polyphenols and in particular of anthocyanins, were given after weaning to mice exposed to MCS since birth as the only source of drinking water. At 4 months of life, immediately after discontinuation of exposure to MCS, both BCB and SB extracts produced a significant protective effect towards MCS-induced cytogenetical damage, but only in female mice [32]. At 8 months of life, MCS produced a variety of histopathological alterations in the lung and other organs of mice. However, no malignant tumors were observed in this study, presumably because it was the only experiment in which we used Swiss ICR mice rather than Swiss H mice. Both BCB and SB extracts attenuated MCS-induced emphysema as well as the yield of lung adenomas. An example of the results obtained with a 17.5% BCB extract is shown in Figure 2. It is noteworthy that the protective effect was more pronounced in female mice. In addition, both berries inhibited the MCS-induced liver parenchymal degeneration, and again this effect was more pronounced in female mice [32].
These gender differences strengthen the view that (a) estrogens play a role in pulmonary carcinogenesis and (b) berries are able to modulate enzymes involved in estrogen metabolism. β-Estradiol and estrogen receptors have been shown to be co-localized in the murine airway epithelium, and CS induces early changes in gene expression that highlight the importance of estrogens in the lung tissue [59,137]. Black raspberry and ellagic acid, a major component of berries, interfere in estrogen-induced mammary tumorigenesis by reducing the expression of CYP1A1, CYP1B1, and other phase I enzymes metabolizing β-estradiol [138]. In addition, anthocyanins and ellagic acid have been found to display estrogen receptor modulating activity [139,140].
Considerations about Modulation of Smoke-Related Lung Carcinogenesis in Smokers
Epidemiological and experimental data demonstrate that some drugs, which are in common use for other therapeutic or preventive purposes, are also able to interfere with different mechanisms of the pulmonary carcinogenesis process. This circumstance depends on the fact that some drugs possess pleiotropic properties that render them applicable to cure or to prevent different diseases. In addition, in spite of their clinical diversities, chronic degenerative diseases may share common risk factors (also including CS), common protective factors as well as common pathogenetic mechanisms [141]. Thus, certain pharmacological agents can be repurposed for cancer control, and exploring established non-cancer drugs for anticancer activities provides an opportunity rapidly to advance strategies into clinical trials [142].
In interpreting our data, it should be kept in mind that the investigated drugs had not to counteract the adverse effects of a single carcinogen but rather those of a complex mixture that contains a broad variety of carcinogens. Therefore, in principle, an optimal protective agent would be expected to be able to mitigate at the same time the carcinogenicity of several CS components belonging to different chemical classes and working with different mechanisms of action. Of the compounds tested, the ligands of the nuclear hormone superfamily bexarotene and pioglitazone were the only agents that, when tested individually, had no apparent effect on the MCS-related induction of preneoplastic and neoplastic lesions. Lapatinib, vorinostat, and metformin only affected the yield of MCS-induced microadenomas, which are inflammation-related, reversible preneoplastic lesions. Likewise, PEITC and the NSAIDs celecoxib (selective COX-2 inhibitor) and licofelone (inhibitor of COX-1, COX-2 and 5-LOX) mainly attenuated induction by MCS of microadenomas and exhibited marginal effects on formation of adenomas. Some of these drugs, such as lapatinib, metformin and licofelone, inhibited the formation of MCS-induced preneoplastic lesions not only in the lung but also in the urinary tract. These protective effects are important because targeting premalignant lesions is one of the prerequisites for the application of chemopreventive agents in clinical trials [143]. However, anthocyanin-rich berry extracts and the COX-1 and COX-2 inhibitors aspirin and naproxen had no effect on microadenomas but inhibited MCS-induced adenomas in females, which suggests that, at least in the model used, the antiproliferative properties of these agents prevailed on antiinflammatory mechanisms. Other agents specifically attenuated more advanced stages of the MCS-related carcinogenesis. This was the case for myo-inositol, with borderline effects, and especially of the combination of pioglitazone plus bexarotene, whose targets (PPAR and RXR) are obligate heterodymeric partners, which almost totally prevented the formation of malignant tumors. Finally, budesonide and NAC were found to affect all stages of MCS-related carcinogenesis, which underlies the multiple mechanisms of action of these agents.
Interestingly, when administered during pregnancy, the antioxidants NAC and ascorbic acid were found to inhibit the induction of lung tumors and other lesions induced by exposure to MCS after birth. This finding highlights the crucial role played by oxidative stress in the lung of newborns, both “physiological” and CS-induced. It underscores the feasibility of a possible strategy of transplacental chemoprevention, aimed at protecting not only the newborn from oxidative stress but also the adult from CS-related diseases appearing later in life. In fact, according to the “Barker hypothesis”, early life exposure to toxic agents is an important risk factor for the development of late-life diseases [144], and perinatal exposure to carcinogens is suspected to contribute to both childhood cancers and cancers appearing later in life [145].
The murine model used proved to be suitable not only for evaluating the ability of drugs to affect MCS-related carcinogenesis but also for detecting possible adverse effects. In fact, although all agents had preliminarily been checked for the lack of apparent toxicity in subchronic studies using MCS-free mice, some of them displayed adverse effects when administered for longer periods of time to MCS-exposed mice. Thus, celecoxib and licofelone were found to affect survival and/or body weight gain, as compared with MCS-exposed mice in the absence of chemopreventive agents. In some cases, histopathological signs of liver toxicity were observed, as it was the case with budesonide, lapatinib, and celecoxib. As previously commented, toxicity of celecoxib may be ascribed to interactions with CS components that affect pathways metabolizing this COX-2 inhibitor. The worse adverse effects were observed with pioglitazone, which even increased the incidence of both lung adenomas and kidney lesions in MCS-exposed mice. Our finding that this drug, which is extensively used for the treatment of diabetes, may potentially increase the tumorigenicity in the lung of smokers deserves particular attention. As a general comment, no side effects can be tolerated when chemopreventive agents are administered to healthy individuals, in primary prevention settings, but some risk-benefit compromises can be accepted when the drugs are administered in more advanced stages of the carcinogenesis process or when the benefits resulting from the therapy with a drug outweigh the risks of side effects.
In evaluating the translatability to humans of the results obtained, it should be kept in mind that the murine model used has several advantages but also suffers from some limitations. A practical limitation is that this bioassay is complex and time-consuming, especially for the long time needed to execute a huge number of histopathological examinations of tissue sections. Moreover, there are obvious differences in the anatomy, physiology, and biochemistry of the respiratory tract in rodents and humans [24]. Furthermore, a common drawback of animal models is represented by the high doses both of the carcinogen and of the putative protective agents that it is necessary to use in order to detect significant results within a relatively short period of time. About the dose of smoke, it should be taken into account that the mice were exposed whole-body to MCS, while humans directly inhale concentrated MCS into their respiratory airways, which probably results in an even higher intake of CS carcinogens. As to the doses of drugs, they were selected based on subchronic toxicity studies that covered a wide range of doses, including doses that are realistic for clinical uses. Since in most cases there was no adverse effect either on survival and body weight gain or on general appearance and behavior during the 6 weeks of the toxicity study, we selected subtoxic doses that were something higher than those used in humans. In comparing human and mouse doses, however, it should be noted that, due to metabolic reasons, mice eat as often as they can. Accordingly, by incorporating the drugs in the diet, their oral intake in mice is highly fractionated during the day, to an extent that is not feasible in humans even by taking several pills per day.
However, use of the animal model has the advantage that exposure to both CS and putative protective agents occurs under well controlled conditions, which were always the same in different experiments and yielded reproducible data across all the studies performed. This murine model is unique in that it convincingly reproduces the carcinogenicity of CS in experimental animals and accordingly it is suitable to evaluate its modulation by chemopreventive agents. The results obtained, together with the findings of parallel molecular studies, allowed us to make inferences regarding the mechanisms of action of the investigated drugs and their intervention stage throughout the lung carcinogenesis process. By modifying the period of treatment with test agents with respect to exposure to MCS, which always covered the first 4 months of life, it was possible to mimic different types of intervention.
In addition, the method used was effective in pointing out gender differences in response to pharmacological agents, which can hardly be detected in epidemiological studies. In particular, female mice were more responsive than male mice regarding inhibition of MCS-induced lung adenomas by the COX-1/COX-2 inhibitors aspirin and naproxen and by anthocyanin-rich berry extracts. As previously discussed, these differences may be ascribed to the ability of these agents to regulate pathways involved in the metabolism of estrogens in pulmonary carcinogenesis. Indeed, targeting the estrogen pathway has been proposed for the treatment and prevention of lung cancer [146]. On the other hand, male mice were more responsive than female mice to metformin in the modulation of early biomarkers in the lung and of preneoplastic lesions both in lung and kidney.
Finally, a further advantage of experimental studies over clinical trials is that it is more feasible to assess the outcome of combinations of drugs. The assessment of the efficacy and safety of individual chemopreventive agents is quite complex, but even more difficult is to evaluate combinations of different agents. Yet, like therapy with protocols combining different drugs is the optimal strategy to cure cancer and many other diseases, combined chemoprevention is expected to be the preferable approach to prevent cancer. The association between pioglitazone and bexarotene, which was designed in the light of mechanistic expectations, provides a convincing example of how drugs that are individually inactive may become highly effective in preventing the progression towards malignancy of smoke-induced lung tumors when they are combined.
Concluding Remarks
Dietary and pharmacological interventions provide well established strategies for the prevention of cardiovascular diseases, especially in high-risk individuals. Potentially, a large number of pharmacological agents would be expected to be able to interfere in the carcinogenesis process thereby mitigating the risk of developing cancer. Such a type of strategy would be of paramount importance for lung cancer prevention, due to the high proportion of addicted current smokers, ex-smokers, and involuntary smokers in the population. However, there are still numerous questions that must be addressed (see Outstanding Questions). One of the critical points is that chemoprevention trials in humans are quite difficult and time-consuming, and they are often either negative or inconclusive. Animal studies are quite useful to evaluate the ability of pharmacological and dietary agents to modulate carcinogenesis mechanisms under well standardized experimental conditions and to predict, at least potentially, their efficacy in humans. The strong interindividual variability in response to drugs further complicates the assessment of protective properties in humans. Indeed, we have simple methods, such as measurement of arterial pressure or of blood cholesterol, which can be used to evaluate the efficacy of drugs for the prevention of cardiovascular diseases. We are still in search of suitable biomarkers that may be used to predict the efficacy of pharmacological agents and of combinations thereof in the prevention of lung cancer, possibly via pharmacogenomics approaches in the single individual.
Outstanding Questions.
To more fully predict the ability of pharmacological agents to modulate pulmonary carcinogenesis in cigarette smokers, the following outstanding questions must be addressed.
In analogy with established strategies applied to the prevention of cardiovascular diseases, can we propose the use of pharmacological agents for lung cancer prevention in individuals at risk?
Will methodologies, both experimental and epidemiological, be optimized in order to predict accurately the modulation of smoking-related cancers in addicted smokers, ex-smokers, and involuntary smokers?
Are the mechanisms of action of drugs thoroughly understood in order to envisage their potential ability to interfere in the carcinogenesis process and to design rational combinations of chemopreventive agents?
Will we be able to develop and validate biomarkers aimed at predicting the efficacy and safety of drugs used for preventative purposes?
Can we evaluate the interindividual variability in response to pharmacological agents in order to choose the optimal drugs for lung cancer prevention tailored on an individual base?
Trends.
Many drugs possess pleiotropic properties that, potentially, would be expected to interfere with carcinogenesis mechanisms.
Assessment of protective effects in humans is exceedingly challenging, and there are difficulties in reproducing smoke carcinogenicity in experimental animals.
A murine model can be used to evaluate pharmacological agents by simulating interventions either in current smokers or in ex-smokers or even mimicking a transplacental chemoprevention.
Reviewed agents include anti-inflammatory drugs, antidiabetic drugs, antineoplastic agents, and other drugs and supplements.
These drugs displayed a broad spectrum of activities by attenuating smoke-induced preneoplastic lesions or benign tumors (adenomas) and/or malignant tumors in mouse lungs.
Both experimental and epidemiological data contribute to predict possible effects of pharmacological agents in smokers.
Acknowledgments
The reported studies were supported by grants from NIH (National Cancer Institute, contracts HHSN-261200533001C, HHSN-261200433000C, and HHSN-2612012000151) and by the Bulgarian Ministry of Education, Youth and Science (National Research Fund).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans. part E. Vol. 100. IARC; Lyon: 2012. A review of human carcinogens: personal habits and indoor combustions. [PMC free article] [PubMed] [Google Scholar]
- 2.De Flora S, et al. Rationale and approaches to the prevention of smoking-related diseases: Overviews of recent studies on chemoprevention of smoke-induced tumors in animal models. J Env Sci Health, Part C, Env Carcin Ecotox Rev. 2014;32:105–120. doi: 10.1080/10590501.2014.907459. [DOI] [PubMed] [Google Scholar]
- 3.Kanavos P. The raising burden of cancer in the developing world. Ann Oncol. 2006;17(Suppl 8):viii15–viii23. doi: 10.1093/annonc/mdl983. [DOI] [PubMed] [Google Scholar]
- 4.De Flora S, et al. Modulation of cigarette smoke–related end–points in mutagenesis and carcinogenesis. Mutat Res. 2003;523–524:237–252. doi: 10.1016/s0027-5107(02)00340-8. [DOI] [PubMed] [Google Scholar]
- 5.De Flora S, et al. Smoke-induced microRNA and proteome alterations and their modulation by chemopreventive agents. Int J Cancer. 2012;131:2763–2773. doi: 10.1002/ijc.27814. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witschi H. A/J mouse as a model for lung tumorigenesis caused by tobacco smoke: strengths and weaknesses. Exp Lung Res. 2005;31:3–18. doi: 10.1080/01902140490494959. [DOI] [PubMed] [Google Scholar]
- 8.Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis. 2005;26:1488–1492. doi: 10.1093/carcin/bgi148. [DOI] [PubMed] [Google Scholar]
- 9.Hutt JA, et al. Life-span inhalation exposure to mainstream cigarette smoke induces lung cancer in B6C3F1 mice through genetic and epigenetic pathways. Carcinogenesis. 2005;26:1999–2009. doi: 10.1093/carcin/bgi150. [DOI] [PubMed] [Google Scholar]
- 10.Stinn W, et al. Towards the validation of a lung tumorigenesis model with mainstream cigarette smoke inhalation using the A/J mouse. Toxicology. 2013;305:49–64. doi: 10.1016/j.tox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 11.De Flora S, et al. Molecular alterations and lung tumors in p53 mutant mice exposed to cigarette smoke. Cancer Res. 2003;63:793–800. [PubMed] [Google Scholar]
- 12.Izzotti A, et al. Birth related genomic and transcriptional changes in mouse lung. Modulation by transplacental N–acetylcysteine. Mutat Res Rev. 2003;544:441–449. doi: 10.1016/j.mrrev.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Balansky R, et al. Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis. 2007;28:2236–2243. doi: 10.1093/carcin/bgm122. [DOI] [PubMed] [Google Scholar]
- 14.Balansky R, et al. Differential carcinogenicity of cigarette smoke in mice exposed either transplacentally, early in life or in adulthood. Int J Cancer. 2012;130:1001–1010. doi: 10.1002/ijc.26103. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS, Szabo E. Fifty years of tobacco carcinogenesis research: from mechanisms to early detection and prevention of lung cancer. Cancer Prev Res (Phila) 2014;7:1–8. doi: 10.1158/1940-6207.CAPR-13-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Szabo E, et al. Chemoprevention of lung cancer. Chest. 2013;143(5 Suppl):e40S–e60S. doi: 10.1378/chest.12-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dragnev K, et al. Lung cancer chemoprevention: difficulties, promise and potential agents? Expert Opin Investig Drugs. 2013;22:35–47. doi: 10.1517/13543784.2013.731392. [DOI] [PubMed] [Google Scholar]
- 19.Sheth SH, et al. Chemoprevention targets for tobacco-related head and neck cancer: past lessons and future directions. Oral Oncol. 2015;51:557–564. doi: 10.1016/j.oraloncology.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 20.Hecht SS, et al. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasala ER, et al. Benzo(a)pyrene-induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol Rep. 2015;67:996–1009. doi: 10.1016/j.pharep.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Balansky R, et al. Selective inhibition by aspirin and naproxen of mainstream cigarette smoke-induced genotoxicity and lung tumors in female mice. Arch Toxicol. 2015 Jun 24; doi: 10.1007/s00204-015-1550-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell EP, et al. Quantitative analysis of early chemically induced pulmonary lesions in mice of varying susceptibilities to lung tumorigenesis. Cancer Lett. 2006;241:197–202. doi: 10.1016/j.canlet.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Nikitin AY, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 64:2307–2316. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 25.Balansky R, et al. Prenatal N-acetylcysteine prevents cigarette smoke-induced lung cancer in neonatal mice. Carcinogenesis. 2009;30:1398–1401. doi: 10.1093/carcin/bgp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balansky R, et al. Transplacental antioxidants inhibit lung tumors in mice exposed to cigarette smoke after birth: a novel preventative strategy? Curr Cancer Drug Targets. 2012;12:164–169. doi: 10.2174/156800912799095153. [DOI] [PubMed] [Google Scholar]
- 27.Izzotti A, et al. Modulation by metformin of molecular and histopathological alterations in the lung of cigarette smoke-exposed mice. Cancer Med. 2014;3:719–730. doi: 10.1002/cam4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balansky R, et al. Prevention of cigarette smoke-induced lung tumors in mice by budesonide, phenethyl isothiocyanate, and N-acetylcysteine. Int J Cancer. 2010;126:1047–1054. doi: 10.1002/ijc.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balansky R, et al. Modulation by licofelone and celecoxib of experimentally induced cancer and preneoplastic lesions in mice exposed to cigarette smoke. Curr Cancer Drug Targets. 2015;15:188–195. doi: 10.2174/1568009615666150216170008. [DOI] [PubMed] [Google Scholar]
- 30.Izzotti A, et al. Relationships between pulmonary microRNA and proteome profiles, systemic cytogenetic damage, and lung tumors in cigarette smoke-exposed mice treated with chemopreventive agents. Carcinogenesis. 2013;34:2322–2329. doi: 10.1093/carcin/bgt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balansky R, et al. Assay of lapatinib in murine models of cigarette smoke carcinogenesis. Carcinogenesis. 2014;35:2300–2307. doi: 10.1093/carcin/bgu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balansky R, et al. Inhibition of lung tumor development by berry extracts in mice exposed to cigarette smoke. Int J Cancer. 2012;131:1991–1997. doi: 10.1002/ijc.27486. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal BB, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi H, et al. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta-and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Velden VH. Glucocorticoids: mechanisms of action and anti-inflammatory potential in asthma. Mediators Inflamm. 1998;7:229–237. doi: 10.1080/09629359890910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazhar D, et al. COX and cancer. QJM. 2005;98:711–718. doi: 10.1093/qjmed/hci119. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, et al. Gastroprotective therapy and risk of gastrointestinal ulcers: risk reduction by COX-2 therapy. J Rheumatol. 2002;29:467–473. [PubMed] [Google Scholar]
- 39.Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 40.Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013;2013:791231. doi: 10.1155/2013/791231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wattenberg LW, et al. Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis. 2000;21:179–82. doi: 10.1093/carcin/21.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Balansky R, et al. Influence of FHIT on benzo[a]pyrene-induced tumors and alopecia in mice: chemoprevention by budesonide and N-acetylcysteine. Proc Natl Acad Sci USA. 2006;103:7823–7828. doi: 10.1073/pnas.0601412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estensen RD, et al. Effect of chemopreventive agents on separate stages of progression of benzo[alpha]pyrene induced lung tumors in A/J mice. Carcinogenesis. 2004;25:197–201. doi: 10.1093/carcin/bgg196. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe F, et al. Gastroprotective therapy and risk of gastrointestinal ulcers: risk reduction by COX-2 therapy. J Rheumatol. 2002;29:467–473. [PubMed] [Google Scholar]
- 45.Fischer SM, et al. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1728–1735. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koul A, Arora N. Celecoxib mitigates cigarette smoke induced oxidative stress in mice. Indian J Biochem Biophys. 2010;47:285–291. [PubMed] [Google Scholar]
- 47.Kisley LR, et al. Celecoxib reduces pulmonary inflammation but not lung tumorigenesis in mice. Carcinogenesis. 2002;23:1653–1660. doi: 10.1093/carcin/23.10.1653. [DOI] [PubMed] [Google Scholar]
- 48.Harris RE, et al. Reduced risk of human lung cancer by selective cyclooxygenase 2 (COX-2) blockade: results of a case control study. Int J Biol Sci. 2007;3:328–334. doi: 10.7150/ijbs.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao JT, et al. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev Res (Phila) 2011;4:984–993. doi: 10.1158/1940-6207.CAPR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffield-Lillico AJ, et al. Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res (Phila) 2009;2:322–329. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tranah GJ, et al. Epoxide hydrolase and CYP2C9 polymorphisms, cigarette smoking, and risk of colorectal carcinoma in the Nurses’ Health Study and the Physicians’ Health Study. Mol Carcinog. 2005;44:21–30. doi: 10.1002/mc.20112. [DOI] [PubMed] [Google Scholar]
- 52.Davies NM, et al. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]
- 53.Cuzick J, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 54.Jalbert G, Castonguay A. Effects of NSAIDs on NNK-induced pulmonary and gastric tumorigenesis in A/J mice. Cancer Lett. 1992;66:21–28. doi: 10.1016/0304-3835(92)90275-z. [DOI] [PubMed] [Google Scholar]
- 55.Saini RK, Sanyal SN. Chemopreventive effect of nonsteroidal anti-inflammatory drugs on 9,10-dimethylbenz[a]anthracene induced lung carcinogenesis in mice. Oncol Res. 2009;17:505–518. doi: 10.3727/096504009789745520. [DOI] [PubMed] [Google Scholar]
- 56.Bosetti C, et al. Aspirin and cancer risk: a summary review to 2007. Recent Results Cancer Res. 2009;181:231–251. doi: 10.1007/978-3-540-69297-3_22. [DOI] [PubMed] [Google Scholar]
- 57.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 58.La Maestra S, et al. Modulation by aspirin and naproxen of nucleotide alterations and tumors in the lung of mice exposed to environmental cigarette smoke since birth. Carcinogenesis. 2015 Oct 13; doi: 10.1093/carcin/bgv149. pii: bgv149. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meireles SI, et al. Early changes in gene expression induced by tobacco smoke: evidence for the importance of estrogen within lung tissue. Cancer Prev Res. 2010;3:707–717. doi: 10.1158/1940-6207.CAPR-09-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng J, et al. Estrogen metabolism within the lung and its modulation by tobacco smoke. Carcinogenesis. 2013;34:909–915. doi: 10.1093/carcin/bgs402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stabile LP, Siegfried JM. Estrogen receptor pathways in lung cancer. Curr Oncol Rep. 2004;6:259–267. doi: 10.1007/s11912-004-0033-2. [DOI] [PubMed] [Google Scholar]
- 62.Terry MB, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291:2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 63.Xie M, et al. Aspirin for primary prevention of cardiovascular events: meta-analysis of randomized controlled trials and subgroup analysis by sex and diabetes status. PLoS One. 2014;9:e90286. doi: 10.1371/journal.pone.0090286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvaro-Gracia JM. Licofelone-clinical update on a novel LOX/COX inhibitor for the treatment of osteoarthritis. Rheumatol (Oxford) 2004;43:i21–i25. doi: 10.1093/rheumatology/keh105. [DOI] [PubMed] [Google Scholar]
- 65.Sharma S, et al. Chemopreventive efficacy and mechanism of licofelone in a mouse lung tumor model via aspiration. Cancer Prev Res (Phila) 2011;4:1233–1242. doi: 10.1158/1940-6207.CAPR-10-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiorucci S, et al. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001;62:1433–1438. doi: 10.1016/s0006-2952(01)00747-x. [DOI] [PubMed] [Google Scholar]
- 67.Giovannucci E, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willi C, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 69.Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol. 2013;705:96–108. doi: 10.1016/j.ejphar.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 70.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decensi A, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 72.Noto H, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevens RJ, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593–2603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- 74.Soranna D, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang ZJ, et al. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. Am J Epidemiol. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 76.Libby G, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bodmer M, et al. Metformin does not alter the risk of lung cancer: a case–control analysis. Lung Cancer. 2012;78:133–137. doi: 10.1016/j.lungcan.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Tsai MJ, et al. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer. 2014;86:137–143. doi: 10.1016/j.lungcan.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 79.Memmott RM, et al. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Popovich IG, et al. Metformin effect on urethane-induced tumorigenesis in mice. Vopr Onkol. 2012;58:549–553. [PubMed] [Google Scholar]
- 81.Ma YR, et al. Gender-related differences in the expression of organic cation transporter 2 and its role in urinary excretion of metformin in rats. Eur J Drug Metab Pharmacokinet. 2015 Apr 17; doi: 10.1007/s13318-015-0278-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Anisimov VN, et al. Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle. 2015;14:46–55. doi: 10.4161/15384101.2014.973308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zieleniak A, et al. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch Immunol Ther Exp (Warsz) 2008;56:331–345. doi: 10.1007/s00005-008-0037-y. [DOI] [PubMed] [Google Scholar]
- 84.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: implications for chemoprevention. Clin Cancer Res. 2009;15:2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]
- 85.Rubenstrunk A, et al. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Li H, et al. Activation of PPARγ in myeloid cells promotes lung cancer progression and metastasis. PLoS One. 2011;6:e28133. doi: 10.1371/journal.pone.0028133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.La Maestra S, et al. DNA damage in exfoliated cells and histopathological alterations in the urinary tract of mice exposed to cigarette smoke and treated with chemopreventive agents. Carcinogenesis. 2013;34:183–189. doi: 10.1093/carcin/bgs314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosetti C, et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18:148–156. doi: 10.1634/theoncologist.2012-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hardisty JF, et al. Histopathology of the urinary bladders of cynomolgus monkeys treated with PPAR agonists. Toxicol Pathol. 2008;36:769–776. doi: 10.1177/0192623308323624. [DOI] [PubMed] [Google Scholar]
- 90.Sato K, et al. Suppressive effects of acid-forming diet against the tumorigenic potential of pioglitazone hydrochloride in the urinary bladder of male rats. Toxicol Appl Pharmacol. 2011;251:234–244. doi: 10.1016/j.taap.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30:1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Filosto S, et al. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther. 2012;11:795–804. doi: 10.1158/1535-7163.MCT-11-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landi L, Cappuzzo F. HER2 and lung cancer. Expert Rev Anticancer Ther. 2013;13:1219–1228. doi: 10.1586/14737140.2013.846830. [DOI] [PubMed] [Google Scholar]
- 94.Diaz R, et al. Antitumor and antiangiogenic effect of the dual EGFR and HER-2 tyrosine kinase inhibitor lapatinib in a lung cancer model. BMC Cancer. 2010;10:188. doi: 10.1186/1471-2407-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ross HJ, et al. Randomized phase II multicenter trial of two schedules of lapatinib as first- or second-line monotherapy in patients with advanced or metastatic non-small cell lung cancer. Clin Cancer Res. 2010;16:1938–1949. doi: 10.1158/1078-0432.CCR-08-3328. [DOI] [PubMed] [Google Scholar]
- 96.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 97.Hayashi T, et al. Suberoylanilide hydroxamic acid enhances gap junctional intercellular communication via acetylation of histone containing connexin 43 gene locus. Cancer Res. 2005;65:9771–9778. doi: 10.1158/0008-5472.CAN-05-0227. [DOI] [PubMed] [Google Scholar]
- 98.Edwards A, et al. Effect of the histone deacetylase inhibitor LBH589 against epidermal growth factor receptor-dependent human lung cancer cells. Mol Cancer Ther. 2007;6:2515–2524. doi: 10.1158/1535-7163.MCT-06-0761. [DOI] [PubMed] [Google Scholar]
- 99.Reguart N, et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer. 2014;84:161–167. doi: 10.1016/j.lungcan.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 100.Tang W, et al. Targeting histone deacetylase in lung cancer for early diagnosis: (18)F-FAHA PET/CT imaging of NNK-treated A/J mice model. Am J Nucl Med Mol Imaging. 2014;4:324–332. eCollection 2014. [PMC free article] [PubMed] [Google Scholar]
- 101.Casto BC, Pereira MA. Prevention of mouse lung tumors by combinations of chemopreventive agents using concurrent and sequential administration. Anticancer Res. 2011;31:3279–3284. [PubMed] [Google Scholar]
- 102.Tran K, et al. The combination of the histone deacetylase inhibitor vorinostat and synthetic triterpenoids reduces tumorigenesis in mouse models of cancer. Carcinogenesis. 2013;34:199–210. doi: 10.1093/carcin/bgs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, et al. Organ-specific expression profiles of rat mammary gland, liver, and lung tissues treated with targretin, 9-cis retinoic acid, and 4-hydroxyphenylretinamide. Mol Cancer Ther. 2006;5:1060–1072. doi: 10.1158/1535-7163.MCT-05-0322. [DOI] [PubMed] [Google Scholar]
- 104.Budgin JB, et al. Biological effects of bexarotene in T cell lymphoma. Arch Dermatol. 2005;141:315–321. doi: 10.1001/archderm.141.3.315. [DOI] [PubMed] [Google Scholar]
- 105.Dragnev KH, et al. Nonclassical retinoids and lung carcinogenesis. Clin Lung Cancer. 2005;6:237–244. doi: 10.3816/CLC.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 106.Pereira MA, et al. Prevention of mouse lung tumors by targretin. Int J Cancer. 2006;118:2359–2362. doi: 10.1002/ijc.21618. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, et al. Preventive effects of bexarotene and budesonide in a genetically engineered mouse model of small cell lung cancer. Cancer Prev Res (Phila) 2009;2:1059–1064. doi: 10.1158/1940-6207.CAPR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582:32–38. doi: 10.1016/j.febslet.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 109.Tachibana K, et al. The role of PPARs in cancer. PPAR Res. 2008;2008:102737. doi: 10.1155/2008/102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 111.Izzotti A, et al. Modulation of multigene expression and proteome profiles by chemopreventive agents. Mutat Res. 2005;591:212–223. doi: 10.1016/j.mrfmmm.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 112.D’Agostini F, et al. Modulation of apoptosis by chemopreventive agents. Mutat Res. 2005;591:173–86. doi: 10.1016/j.mrfmmm.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 113.Witschi H, et al. The effects of phenethyl isothiocyanate, N-acetylcysteine and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis. 1998;19:1789–1794. doi: 10.1093/carcin/19.10.1789. [DOI] [PubMed] [Google Scholar]
- 114.Wattenberg LW. Chemoprevention of pulmonary carcinogenesis by myo-inositol. Anticancer Res. 1999;19:3659–3661. [PubMed] [Google Scholar]
- 115.Hecht SS, et al. Inhibition of lung tumorigenesis in A/J mice by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1455–1461. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- 116.Gunning WT, et al. Chemoprevention of vinyl carbamate-induced lung tumors in strain A mice. Exp Lung Res. 2000;26:757–772. doi: 10.1080/01902140150216800. [DOI] [PubMed] [Google Scholar]
- 117.De Flora S, et al. Mechanisms of N–acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking–related end–points. Carcinogenesis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]
- 118.van Zandwijk N, et al. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 119.Van Schooten FJ, et al. Effects of oral N-acetylcysteine: A multi-biomarker study in smokers. Cancer Epidem Biomarkers Prev. 2002;11:167–175. [PubMed] [Google Scholar]
- 120.De Flora S, et al. Smokers and urinary genotoxins. Implications for selection of cohorts and modulation of endpoints in chemoprevention trials. J Cell Biochem Suppl. 1996;25:92–98. [PubMed] [Google Scholar]
- 121.De Flora S, et al. Inhibition of urethan-induced lung tumors in mice by dietary N-acetylcysteine. Cancer Lett. 1986;32:235–241. doi: 10.1016/0304-3835(86)90175-8. [DOI] [PubMed] [Google Scholar]
- 122.Balansky RM, De Flora S. Chemoprevention by N-acetylcysteine of urethane-induced clastogenicity and lung tumors in mice. Int J Cancer. 1998;77:302–305. doi: 10.1002/(sici)1097-0215(19980717)77:2<302::aid-ijc21>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 123.D’Agostini F, et al. Interactions between N-acetylcysteine and ascorbic acid in modulating mutagenesis and carcinogenesis. Int J Cancer. 2000;88:702–707. doi: 10.1002/1097-0215(20001201)88:5<702::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 124.Chung FL, et al. Chemopreventive efficacy of arylalkyl isothiocyanates and N-acetylcysteine for lung tumorigenesis in Fischer rats. Cancer Res. 1996;56:772–778. [PubMed] [Google Scholar]
- 125.Conaway CC, et al. Chemopreventive potential of fumaric acid, N-acetylcysteine, N-(4-hydroxyphenyl) retinamide and beta-carotene for tobacco-nitrosamine-induced lung tumors in A/J mice. Cancer Lett. 1998;124:85–93. doi: 10.1016/s0304-3835(97)00454-0. [DOI] [PubMed] [Google Scholar]
- 126.Witschi H. Carcinogenic activity of cigarette smoke gas phase and its modulation by beta-carotene and N-acetylcysteine. Toxicol Sci. 2005;84:81–87. doi: 10.1093/toxsci/kfi043. [DOI] [PubMed] [Google Scholar]
- 127.Witschi H, et al. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]
- 128.Izzotti A, et al. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J. 2009;23:3243–3250. doi: 10.1096/fj.09-135251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai T, et al. N–Acetylcysteine inhibits endothelial cell invasion and angiogenesis while protecting from apoptosis. Lab Invest. 1999;79:1151–1159. [PubMed] [Google Scholar]
- 130.Albini A, et al. Inhibition of angiogenesis-driven Kaposi’s sarcoma tumor growth in nude mice by oral N–acetylcysteine. Cancer Res. 2001;61:8171–8178. [PubMed] [Google Scholar]
- 131.Frei B, et al. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yun TK, et al. Trial of a new medium-term model using benzo(a)pyrene induced lung tumor in newborn mice. Anticancer Res. 1995;15:839–845. [PubMed] [Google Scholar]
- 133.Cortés-Jofré M, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2012;10:CD002141. doi: 10.1002/14651858.CD002141.pub2. [DOI] [PubMed] [Google Scholar]
- 134.Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;56:630–635. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- 135.Stoner GD, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carlton PS, et al. Failure of dietary lyophilized strawberries to inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene-induced lung tumorigenesis in strain A/J mice. Cancer Lett. 2000;159:113–117. doi: 10.1016/s0304-3835(00)00464-x. [DOI] [PubMed] [Google Scholar]
- 137.Siegfried JM. Early changes in pulmonary gene expression following tobacco exposure shed light on the role of estrogen metabolism in lung carcinogenesis. Cancer Prev Res (Phila) 2010;3:692–695. doi: 10.1158/1940-6207.CAPR-10-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aiyer HS, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev Res (Phila) 2010;3:727–737. doi: 10.1158/1940-6207.CAPR-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schmitt E, Stopper H. Estrogenic activity of naturally occurring anthocyanidins. Nutr Cancer. 2001;41:145–149. doi: 10.1080/01635581.2001.9680625. [DOI] [PubMed] [Google Scholar]
- 140.Larrosa M, et al. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 141.De Flora S, Izzotti A. Modulation of genomic and postgenomic alterations in noncancer diseases and critical periods of life. Mutat Res. 2009;667:15–26. doi: 10.1016/j.mrfmmm.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 142.Gupta SC, et al. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 143.Keith RL, Miller YE. Lung cancer chemoprevention: current status and future prospects. Nat Rev Clin Oncol. 2013;10:334–343. doi: 10.1038/nrclinonc.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barker DJP. Maternal nutrition, fetal nutrition, and diseases in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 145.Anderson LM. Introduction and overview. Perinatal carcinogenesis: growing a node for epidemiology, risk management, and animal studies. Toxicol Appl Pharmacol. 2004;199:85–90. doi: 10.1016/j.taap.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 146.Burns TE, Stabile LP. Targeting the estrogen pathway for the treatment and prevention of lung cancer. Lung Cancer Manag. 2014;3:43–52. doi: 10.2217/lmt.13.67. [DOI] [PMC free article] [PubMed] [Google Scholar]