Abstract
Positively-charged chitosan gauzes stop bleeding from wounds by electrostatically interacting with negatively-charged cell membranes of erythrocytes to cause erythrocyte agglutination and by sealing wounds through tissue adhesion. In the following work, nonwoven chitosan gauze was impregnated with PolySTAT, a synthetic polymer that enhances coagulation by cross-linking fibrin, to generate PolySTAT/chitosan gauzes with improved hemostatic efficacy. When comparing nonwoven chitosan and PolySTAT/chitosan to a commercially-available chitosan-containing gauze (Celox® Rapid), no appreciable differences were observed in fiber size, morphology, and pore size. However, PolySTAT/chitosan demonstrated more rapid blood absorption compared to Celox® Rapid. In a rat model of femoral artery injury, PolySTAT/chitosan gauzes reduced blood loss and improved survival rate compared to non-hemostatic controls and Celox® Rapid. While Celox® Rapid had stronger adherence to tissues compared to PolySTAT/chitosan gauzes, blood loss was greater due to hematoma formation under the Celox® dressing. Animals treated with PolySTAT/chitosan gauzes required less saline infusion to restore and maintain blood pressure above the target blood pressure (60 mmHg) while other treatment groups required more saline due to continued bleeding from the wound. These results suggest that PolySTAT/chitosan gauzes are able to improve blood clotting and withstand increasing arterial pressure with the addition of a fibrin cross-linking hemostatic mechanism.
Keywords: Chitosan gauze, fibrin, hemostasis, polymer, trauma, PolySTAT
Graphical abstract
1. Introduction
Hemorrhage is the leading cause of death on the battlefield and is the second leading cause of prehospital death in the civilian trauma population [1]. Reduced circulating volumes after severe hemorrhage leads to insufficient oxygen delivery to tissues (i.e. shock) and complications such as hypothermia, coagulopathy, and acidosis, which are associated with increased morbidity and mortality [2]. Therefore, early control of hemorrhage is necessary to minimize blood loss. The body normally produces blood clots in response to blood vessel injury. During clot formation, platelets adhere to the exposed subendothelial matrix and aggregate to form an initial platelet plug which is then reinforced by a hydrogel matrix made of fibrin biopolymers generated by the coagulation cascade [3]. However, medical intervention is needed to stop bleeding in more severe traumatic injuries.
External hemorrhage is most commonly treated by application of tourniquets, direct pressure, and/or hemostatic dressings. The need for improved hemostasis in combat casualties, in particular, has driven the development of more effective hemostatic dressings beyond standard gauze [4]. Early generations of hemostatic gauzes, such as dry fibrin sealant (DFS) dressing and combat gauze (CG), enhance coagulation by providing clotting factors and coagulation-activating minerals (e.g. kaolin) at the site of injury [5]. In QuikClot®, zeolite incorporation is used to rapidly absorb the fluid in blood to concentrate clotting factors, platelets, and erythrocytes. More recently, dressings made from chitosan have been used for hemostasis.
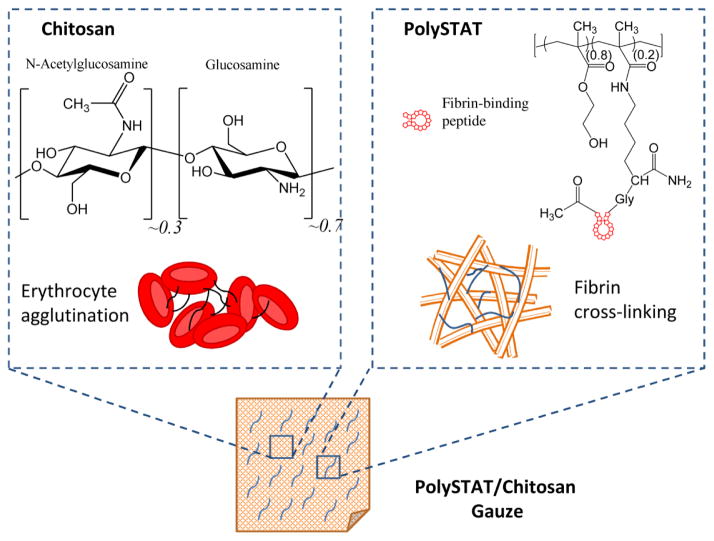
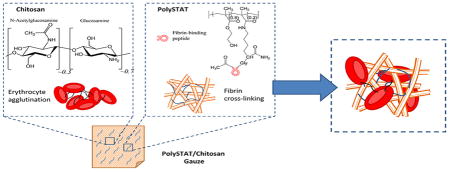
Chitosan is a biodegradable, positively-charged polysaccharide derived from the deacetylation of chitin, a polymer of N-acetylglucosamine found in the exoskeleton of crustaceans [6]. Due to its biocompatibility, mucoadhesivity, and impressive range of therapeutic functions including hemostatic, antimicrobial, anti-tumor, and anti-inflammatory activity [6–9], chitosan has been used in numerous biomaterials for tissue engineering and drug delivery [10,11]. Chitosan’s hemostatic property is thought to arise from its electrostatic interaction with negatively-charged cell membranes of erythrocytes leading to erythrocyte agglutination and formation of a hemostatic plug at the site of injury [4] (Figure 1). In dressing form, chitosan further prevents blood loss by adhering to tissues and injured vessels to seal off the wound. Furthermore, the hemostatic mechanism of chitosan is independent of innate clotting mechanisms and can therefore act in the presence of anticoagulants [12,13]. Chitosan gauzes, such as HemCon® and Celox® Rapid, are currently marketed in the US and Europe [4]. However, chitosan-containing dressings have shown variable efficacy when tested in animal injury models [14,15]. In some instances, these gauzes were able to stop low pressure arterial bleeds, but were unable to maintain hemostasis after intravenous administration of fluids to raise blood pressure back to baseline [14]. Recovering blood pressure caused rebleeding due to detachment of the bandage, suggesting that the erythrocyte aggregates formed by chitosan are mechanically unstable. We hypothesize that mechanical reinforcement of erythrocyte aggregates via a secondary hemostatic mechanism can further improve hemostasis.
Figure 1.
Hemostatic mechanisms of PolySTAT/chitosan gauze components. PolySTAT structure reproduced with permission from [16].
In the present work, we aim to improve hemostatic efficacy through impregnation of chitosan gauzes with a recently-reported synthetic hemostatic polymer (PolySTAT) which reduced blood loss and improved survival in animal injury models when injected intravenously [16]. PolySTATs are linear poly(HEMA) polymers grafted with multiple fibrin-binding peptides [17]. The proposed hemostatic mechanism of PolySTAT is non-covalent binding of multiple fibrin monomers during fibrin polymerization to form a highly cross-linked, stable network within the blood clot (Figure 1). PolySTAT/chitosan gauzes are therefore expected to stabilize chitosan-induced erythrocyte aggregates through additional fibrin crosslinks. In the following work, PolySTAT/chitosan gauzes were evaluated in a rat femoral artery injury model. Application of PolySTAT/chitosan gauzes reduced blood loss as well as reduced resuscitative requirements compared to commercially available Celox® Rapid.
2. Materials and Methods
2.1. Preparation of nonwoven PolySTAT/chitosan gauzes
Chitosan staple fibers (length 32 mm, crimp 10 ea/inch, 3 denier, degree of deacetylation: 87%, MW ~1,000 kDa) were purchased from NTPIA Corp Co. Ltd. (Korea). Polyethylene terephthalate (PET) filament fibers (POY, 120den/36fila) was purchased from Hyosung Co. Ltd, crimped, and cut to 51 mm length for negative controls. Chitosan and PET nonwoven fabrics were prepared by a needle punching process [18,19] using a pilot nonwoven system (Samhwa Machinery Co., Ltd., Korea). Briefly, 5 kg of chitosan and PET staple fibers were opened, mixed, carded, and then formed into a web. The web was cross-lapped and needle punched into a nonwoven with a base weight of 120 g/m2. The nonwoven fabrics were then calendered through heated rollers at 100°C using a 3-bowl calender machine (DWNBC3-2400, Dong Won Roll Co. Ltd, Korea). PolySTAT was synthesized as previously described [16] and dissolved in a 0.1% solution in PBS. Nonwoven chitosan was plasma-treated in the presence of O2 for 10 min at 50 W, 50 sccm, and 10 mTorr using a Europlasma surface treatment system (CD 400 MC, Belgium) to create a hydrophilic surface for quick absorption of PolySTAT solution. 5 cm × 5 cm chitosan gauze was saturated with a 3-mL volume of PolySTAT solution for 0.12 mg PolySTAT/cm2 loading and was air-dried before characterization and use in animal studies.
2.2. Measurement and visualization of gauze pore size, porosity, and topology
Pore size was measured using an automated capillary flow porometer (CFP-1200-AEL, Porous Materials Inc., USA). The samples (2.5 cm × 2.5 cm) were soaked in a liquid of known surface tension (Porwick, proprietary product of PMI, surface tension 16 dynes/cm) to fill the pores and air pressure was applied on one side of the samples to force the liquid out of the pores. Flow rate was used to calculate the pore diameter [20,21]. Internal pore structure was determined with mercury-intrusion porosimetry, a method suitable for measuring pores with diameters ranging from 5 nm to 360 μm; mercury penetration through gauze samples under 0.5 to 61,000 psi applied pressure was measured and used to calculate porosity using a pore-size analyzer (Autopore IV 9500, Micromeritics Instrument, USA). Gauze topology was visualized by cold field emission scanning electron microscopy (FE-SEM; Hitachi SU-8010, Hitachi High Technologies Co. Japan). Prior to imaging, the samples were sputter-coated with gold for 200 s using a 15 mA current.
2.3. Characterization of gauze absorptive properties
Water contact angle on nonwoven chitosan gauze was measured immediately after plasma treatment using a drop shape analysis system (DSA100, KRÜSS, Germany). Blood absorption time was measured after application of 200 μL whole blood to 1 cm × 1 cm gauze samples. Absorption time was defined as the time for complete absorption of the 200 μL volume. Liquid absorption tests were then performed based on modified BS EN 13726-1 [22] test methods for evaluating primary wound dressings in accordance with British and European standards. Pieces of dry gauze (2 cm × 2 cm) were pre-weighed and subsequently added to a 0.9% saline solution for 10 min at room temperature. After hydration, the specimen mass was measured. The liquid absorption ratio (LAR) was calculated as follows:
| (1) |
| (2) |
where W1 is the mass (g) of dry gauze, W2 is the mass (g) of wet gauze, and A is the gauze area.
Liquid retention ratio under pressure (LRRP) was determined by pre-weighing wet gauze samples and measuring mass of samples after application of 40 mmHg for 1 min.
LRRP was calculated using the following equations:
| (3) |
| (4) |
where W1 is the mass (g) of dry gauze, W3 is the mass (g) of wet gauze after application of pressure, and A is the gauze area
2.4. In vitro clotting assay
PolySTAT/chitosan gauze samples (1 cm × 1cm) with 0, 0.1, 0.2, 0.4, and 1.0 mg/cm2 PolySTAT loading were prepared by addition of 200 μL 0%, 0.05%, 0.1%, 0.2%, and 0.5% PolySTAT solution (PolySTAT dissolved in PBS), respectively, to nonwoven chitosan. Gauzes were air-dried overnight. Citrated whole blood (9:1 whole blood to 3.8% sodium citrate) was collected from human donors at the University of Washington Medical Center with the approval from the University of Washington Institutional Review Board (IRB). Modified in vitro clotting assays were then completed with the dried gauze samples [23,24]. Briefly, 100 μL citrated whole blood was pipetted onto each gauze sample followed by 10 μL 0.2 M CaCl2 solution and then incubation for 5 min at 37°C. Each gauze sample was then placed in a 50 mL conical tube containing 12.5 mL DI water. The tube was inverted 3 times to rinse unclotted blood from the sample, and absorbance at 540 nm from the hemoglobin in the unclotted blood was measured using a Tecan Infinite M1000 platereader.
2.5. Hemostasis in a rat femoral artery injury model
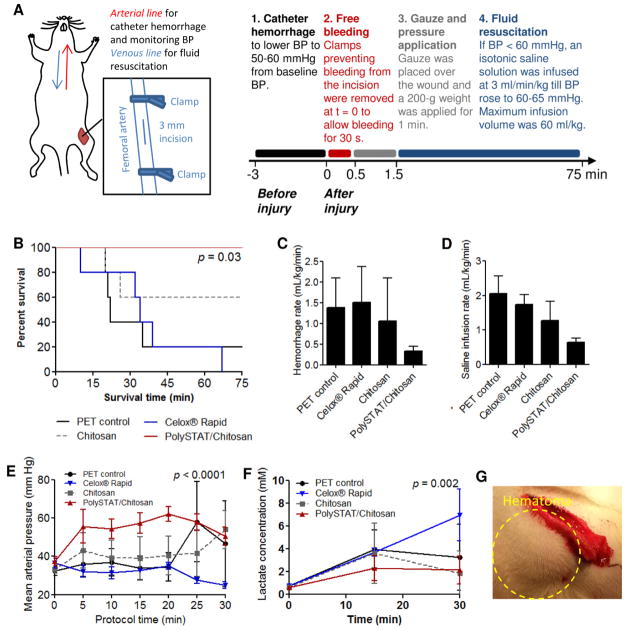
PolySTAT/chitosan gauzes were applied to a rat model of femoral artery injury to evaluate hemostatic efficacy. The protocol was approved by the University of Washington Institutional Animal Care and Use Committee (IACUC). Animals weighing 330–370g were anesthetized with ketamine and xylazine and maintained under isoflurane for the duration of the 75-minute protocol. Before injury, the carotid artery and jugular vein were catheterized for monitoring blood pressure (BP) and for saline infusion, respectively. A tracheotomy was performed and a small polyethylene tube was inserted to aid respiration using a mechanical ventilator. The left femoral artery was isolated and clamped proximally and distally. 50 μL 2% lidocaine was applied on the artery to minimize vasoconstriction and reduce variation in the initial free bleeding rate. A 3-mm longitudinal incision was then made in the femoral artery segment between the clamps to simulate injury. Prior to releasing the clamps, a catheter hemorrhage was performed to standardize starting blood pressure to 50–60 mmHg. Following catheter hemorrhage, clamps were removed to initiate bleeding from the wound (t = 0 min). After 30 s of bleeding, a 1 cm × 3 cm piece of gauze (polyethylene terephthalate negative control, PET; unmodified chitosan gauze containing no PolySTAT; PolySTAT/chitosan gauzes; or Celox® Rapid) with a 2.5 cm × 2.5 cm cotton gauze backing was applied to the wound. A 200-g weight was applied over the gauze for 1 min. After careful removal of the weight, fluid resuscitation was initiated. 0.9% Saline was infused at 3 mL/kg/min to raise and maintain blood pressure above 60 mmHg. Pre-weighed cotton gauze was used to collect blood spilling over from the dressed wound to track blood loss over time. Blood lactate concentration at baseline and 15, 30, and 60 min from the start of fluid resuscitation was measured using a radiometer. Animals dying before 10 min of fluid resuscitation were excluded to remove those who expired for reasons other than blood loss. Animals with blood loss greater than or less than two standard deviations of blood loss in PET controls during the 30 sec after clamp release and before gauze application were also excluded from analysis to remove significant outliers in the initial bleeding response to injury. These outliers may be attributed to differences in blood volumetric flow rate caused by either vasodilation (an effect of anesthesia) or vasoconstriction (the body’s natural response to injury).
2.6. Statistical analysis
One-way ANOVAs with Tukey-Kramer post hoc tests were completed for in vitro clotting assay data and for hemorrhage rates and saline infusion rates from animal studies. A log-rank Mantel-Cox test was completed to determine statistical significance of survival for animal studies. Two-way ANOVAs were completed for blood pressure and lactate levels to determine statistical significance in the first 30 min after initiation of blood loss.
3. Results
3.1. Gauze porosity and topology
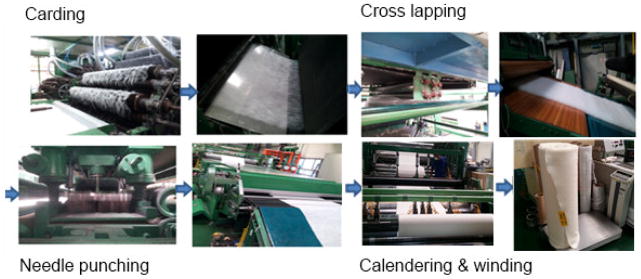
In this work, the following gauzes were characterized and evaluated: commercially available Celox® Rapid (a nonwoven gauze with heat-bonded chitosan-containing Celox® granules for hemostasis and Chito-R agent for adhesion to the wound), nonwoven PET, nonwoven chitosan, and PolySTAT-coated nonwoven chitosan (PolySTAT/chitosan). Nonwoven materials were prepared using standard needle-punching, a technique that mechanically interlocks a web of fibers through the repeated passing of barbed needles in and out of the web (Figure 2) [18]. The physical properties of the gauze materials were then characterized by field emission SEM and by pore diameter and percent porosity measurements (Table 1).
Figure 2.
Nonwoven gauze manufacturing process.
Table 1.
Mean pore size, porosity, and SEM images of nonwoven PET, nonwoven chitosan, PolySTAT/chitosan, and Celox® Rapid.
| Sample | Mean pore diameter (μm) | Porosity (%) | Magnification 100X | Magnification 300X |
|---|---|---|---|---|
| Nonwoven PET | 59±47 | 85.9 |

|

|
| Nonwoven Chitosan | 55±43 | 89.7 |

|

|
| Chitosan nonwoven (plasma-treated) | 63±49 | 91.6 |

|

|
| PolySTAT/Chitosan | 48±37 | 85.9 |

|

|
| Celox® Rapid | 47±34 | 73.2 |

|
|
SEM imaging showed that all gauzes were comprised of fibers with similar morphology and diameters. Chitosan granules were observed in the Celox® Rapid samples as well as uniform PolySTAT coating on individual chitosan fibers in PolySTAT/chitosan gauze. Mean pore diameters measured using a capillary flow porometer ranged from 47–63 μm with no significant difference between gauze type (Table 1). Nonwoven chitosan had ~90% porosity with a slight reduction to 85.9% after PolySTAT incorporation. Celox was the least porous (73.2% porosity).
3.2. Contact angle and fluid absorption properties
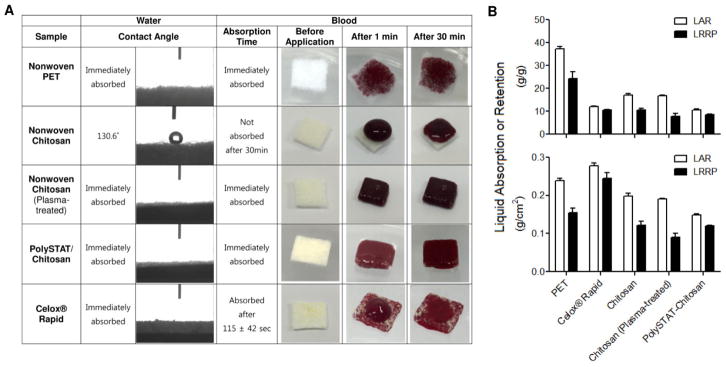
Nonwoven chitosan is relatively hydrophobic with a water contact angle of 130.6° and no absorption of blood 30 min after application (Figure 3A). Water and blood was immediately absorbed in all other samples. Plasma treatment of nonwoven chitosan created a hydrophilic surface resulting in quick absorption of PolySTAT solution. After PolySTAT loading, the surface remained hydrophilic for rapid absorption of blood. Per gram of gauze, PET absorbed and retained the greatest volume of saline (37±0.91 and 24±2.9 g/g, respectively; Figure 3B). PolySTAT/chitosan gauzes and Celox® Rapid absorbed and retained comparable volumes of saline (10–12 g/g and 8.5–11 g/g, respectively). When normalized to gauze area instead of mass, Celox® Rapid had the greatest absorption and retention (0.28±0.01 and 0.25±0.01, respectively). In this instance, PolySTAT/chitosan gauzes were more similar to the nonwoven chitosan.
Figure 3.
Fluid absorption behavior of gauze samples. (A) Water contact angle and blood absorption time. (B) Liquid absorption ratio (LAR) and liquid retention ratio under pressure (LRRP) normalized to gauze mass (top) and area (bottom).
3.3. In vitro clotting assay
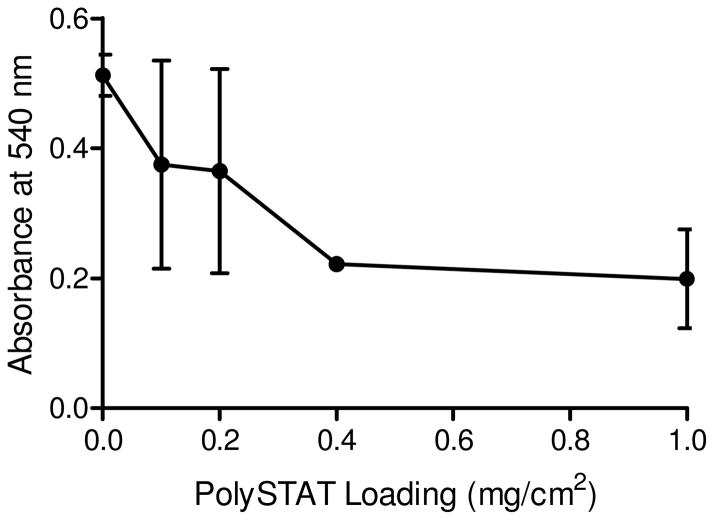
Recalcified whole blood was applied onto nonwoven chitosan and PolySTAT/chitosan gauze with 0.1–1 mg/cm2 PolySTAT loading to determine whether addition of PolySTAT could further promote clot formation on the gauze and the effect of PolySTAT content on in vitro clotting using coated gauze. Absorbance at 540 nm of DI water from the wash step correlates to the amount of unclotted blood (Figure 4). Nonwoven chitosan (containing no PolySTAT) had the greatest average absorbance value, indicating a lesser extent of clotting and clot retention compared to blood on PolySTAT/chitosan gauzes. Whole blood on PolySTAT/chitosan gauze demonstrated progressively greater clotting with increasing PolySTAT loading. PolySTAT/chitosan with 0.1 g/cm2 demonstrated ~27% greater clotting than nonwoven chitosan alone and a similar loading (0.12 g/cm2) was evaluated in subsequent animal studies.
Figure 4.
In vitro clotting assay results. Hemoglobin absorbance from unclotted blood was measured after incubation of recalcified blood with gauze samples containing 0–1 mg/cm2 PolySTAT loading.
3.4. Hemostasis in a rat femoral artery injury model
Hemostatic efficacy of PolySTAT/chitosan gauze relative to PET, nonwoven chitosan, and Celox® Rapid was evaluated in a rat model of femoral artery injury and fluid resuscitation. Fluid resuscitation is the infusion of isotonic solution (i.e. saline solution or lactated ringer) into circulation to increase circulating volumes in hypotensive patients. In this model, starting blood pressure in all animals was standardized using a catheter hemorrhage, gauze was applied to the wound area 30 s after commencement of bleeding for 1 min followed by fluid resuscitation to maintain blood pressure above 60 mmHg (Figure 5A). Gauzes were applied to injured arteries with minute-long application of pressure as instructed on Celox® Rapid packaging. To control for pressure applied on the wound, a 200 g weight was placed on the back of a 1 cm × 3 cm piece of gauze over the wound site. Significantly greater survival was observed in animals treated with PolySTAT/chitosan gauzes compared to animals treated with PET and Celox® Rapid (100% versus 0–20% survival; Figure 5B). No significant difference in survival was observed between PolySTAT/chitosan gauzes and unmodified chitosan gauze (60% survival) although a general trend in improved survival was observed with PolySTAT incorporation. Animals treated with PolySTAT/chitosan gauzes bled on average less than animals treated with other dressings (Figure 5C) and required smaller volumes of infused saline to maintain BP above the targeted 60 mmHg (Figure 5D). The PolySTAT/chitosan gauze group was most responsive to fluid resuscitation and maintained BPs ~60 mmHg, while BP in other treatment groups generally remained between 30–45 mmHg due to continued bleeding during fluid infusion (Figure 5E). Correspondingly, lower blood lactate levels were measured in animals treated with PolySTAT/chitosan gauzes, indicating better tissue perfusion (Figure 5E). Only the first 30 min is shown in Figures 5D–E due to measurement bias towards surviving animals at later time points. Noticeably, Celox® Rapid had better adhesion to tissues surrounding the injured artery compared to unmodified nonwoven chitosan and PolySTAT/chitosan gauzes. However, large hematomas (i.e. pool of blood outside the blood vessel) consistently formed under the Celox® gauze, indicating continued bleeding underneath the dressing (Figure 5G).
Figure 5.
Hemostatic efficacy of PolySTAT/chitosan gauzess in rat femoral artery injury models. (A) Workflow schematic and timeline. Modified with permission from [16]. (B) Survival curve for animals treated with PET gauze, Celox® Rapid, chitosan gauze, and PolySTAT/chitosan gauzes. (C) Cumulative hemorrhage volume normalized to survival time. (D) Total infused saline volume normalized to survival time. (E) Mean arterial pressure in the first 0–30 min of saline infusion. (F) Blood lactate concentration in the first 0–30 min of saline infusion. (G) Image of hematoma forming underneath Celox® Rapid gauze.
4. Discussion
PolySTAT was initially engineered for intravenous administration to stop bleeding from internal injuries that could not be accessed and treated in a prehospital setting [16]. In the following work, we hypothesized that PolySTAT incorporation into hemostatic gauzes was a possible method for localized delivery to enhance coagulation in external injuries. Chitosan gauzes are the most current generation of hemostatic dressings and have shown promising rates of hemostasis, time to hemostasis, blood loss volumes, and survival in swine injury models and in combat operations [15,25–27]. However, variable performance in injury models with high pressure arterial bleeds necessitates further strengthening of the resulting hemostatic plug. In the present study, PolySTAT was incorporated into nonwoven chitosan gauzes with the goal to further improve hemostatic efficacy by combining fibrin cross-linking with erythrocyte agglutination. Fibrin fibers, the product of the coagulation cascade, form a viscoelastic matrix within a blood clot, allowing clots to deform reversibly under the shear forces of blood flow [28]. Fibrin is naturally cross-linked by the transglutaminase, factor XIIIa (FXIIIa), at the end of the coagulation cascade, and this process has been shown to be increase fibrin mechanical stability [29].
Plasma treatment facilitated the absorption of PolySTAT solutions by relatively hydrophobic nonwoven chitosan, resulting in even PolySTAT coating of chitosan fibers (Table 1). Oxygen plasma treatment introduces oxygen-containing polar groups to the nonwoven chitosan, mainly C-OH, which are then able to attract polar water molecules for increased wettability [30]. These effects have also previously been observed after plasma treatment of other nonwoven materials made from polyester [31] and polypropylene [32]. Improved hydrophilicity was transient and dissipated rapidly within a week when nonwoven chitosan was stored under non-vacuum conditions. However, PolySTAT coating improved liquid absorption of nonwoven chitosan. With respect to dressing structure, nonwoven gauzes are beneficial because they can conform to uneven wound sites much more so than wafer and sponge-type materials [33], they are highly absorbent, are easily mass produced at low cost, and can be easily modified three-dimensionally with additional layers containing the same or different material composition [18].
Factors such as absorbency, flexibility, and hemostatic properties were previously identified as critical factors in effective dressings [33]. PET negative controls had significantly greater fluid absorption than other tested materials. However, PET application to femoral artery injuries resulted in the greatest volume blood loss, which is consistent with the observation that absorbency alone is not sufficient to control bleeding [33]. Similarly, standard gauze made of loosely-woven cellulose is able to absorb 15 times its weight in blood without stopping bleeding [33]. Therefore, additional hemostatic mechanisms are necessary beyond blood absorption. PolySTAT/chitosan gauzes demonstrated comparable saline absorption and retention to Celox® Rapid and even faster blood absorption, likely due to its higher porosity (i.e. void volume available to hold fluid) (Figure 3). In vitro clotting studies demonstrated that PolySTAT coating of chitosan fibers was able to further improve hemostasis likely due to the combination of erythrocyte agglutination and fibrin cross-linking or fibrin binding to chitosan fiber surfaces (Figure 4). Therefore, PolySTAT/chitosan gauzes fulfill all three characteristics that are required of effective hemostatic dressings.
PolySTAT/chitosan gauze demonstrated improved clot retention in vitro, suggesting better clot stability within the bandage. These results translated in vivo, where application of PolySTAT/chitosan gauzes to injured femoral arteries reduced average blood loss resulting in an improved survival rate compared to PET and Celox® Rapid. However, there was no significant difference in survival between chitosan with and without PolySTAT, which may be attributed to small sample size (n = 5). Additional studies with larger sample sizes and in large animal bleeding models are needed to reproduce and confirm our results. Although Celox® Rapid adhered strongly to tissues adjacent to the injured artery to seal the wound, bleeding continued underneath the dressing leading to hematoma formation in the wound cavity. This phenomenon has been well-documented with standard gauze and other hemostatic gauzes in large animal studies and is likely due to high pressure from arterial bleeds which limit the formation of durable direct adhesion between the gauze and injured vessel [34,35].
5. Conclusions
In summary, mechanical stabilization of red blood cell aggregates via fibrin cross-linking and anchoring of fibrin to gauze fiber surfaces may be a viable strategy to further improve the hemostatic efficacy of chitosan gauzes.
Statement of Significance.
Blood loss remains one of the leading causes of death after traumatic injury in civilian populations and on the battlefield. Advanced biomaterials that interact with blood components and/or accelerate the clotting process to form a hemostatic plug are necessary to staunch bleeding after injury. Chitosan-based gauzes, which stop bleeding by causing red blood cell aggregation, are currently used on the battlefield and have shown variable performance under high pressure arterial blood flow in animal studies, suggesting that red blood cell aggregates require further mechanical stabilization for more reliable performance. In this work, we investigate the binding and cross-linking of fibrin, a major component in blood clots, on chitosan gauze fiber surfaces to structurally reinforce red blood cell aggregates.
Acknowledgments
This work was supported by NIH 1R21EB018637, KITECH grant JE140004 and EO150029, grant KL2 TR000421 from the NIH National Center for Advancing Translational Sciences (N.J.W.), and the Bioengineering Cardiovascular Training grant (NIH 2T32EB001650-06A2) (L.W.C.).
Footnotes
Disclosures
The University of Washington has applied for a patent for PolySTATs with S.H.P., N.J.W., and L.W.C. listed as the inventors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Mikhail J. The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues. 1999;10:85–94. [PubMed] [Google Scholar]
- 3.Hawiger J. Formation and regulation of platelet and fibrin hemostatic plug. Hum Pathol. 1987;18:111–122. doi: 10.1016/s0046-8177(87)80330-1. [DOI] [PubMed] [Google Scholar]
- 4.Bennett BL, Littlejohn L. Review of new topical hemostatic dressings for combat casualty care. Mil Med. 2014;179:497–514. doi: 10.7205/MILMED-D-13-00199. [DOI] [PubMed] [Google Scholar]
- 5.Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251:217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 6.Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res. 1997;34:21–8. doi: 10.1002/(sici)1097-4636(199701)34:1<21::aid-jbm4>3.0.co;2-p. http://www.ncbi.nlm.nih.gov/pubmed/8978649. [DOI] [PubMed] [Google Scholar]
- 7.Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010;56:290–9. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015;6:33–49. doi: 10.3390/jfb6010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ta HT, Dass CR, Dunstan DE. Injectable chitosan hydrogels for localised cancer therapy. J Control Release. 2008;126:205–16. doi: 10.1016/j.jconrel.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49:780–792. [Google Scholar]
- 12.Klokkeuold PR, Fukayama H, Sung EC, Bertolami CN. The Effect of Chitosan (poly-N-Acetyl Glucosamine) on Lingual Hemostasis in Heparinized Rabbits. J Oral Maxillofac Surg. 1999;57:49–52. doi: 10.1016/s0278-2391(99)90632-8. [DOI] [PubMed] [Google Scholar]
- 13.Koksal O, Ozdemir F, Etoz BC, Buyukcoskun NI, Sigirli D. Hemostatic effect of a chitosan linear polymer (Celox®) in a severe femoral artery bleeding rat model under hypothermia or warfarin therapy. Turkish J Trauma Emerg Surg. 2011;17:199–204. [PubMed] [Google Scholar]
- 14.Kheirabadi BS, Scherer MR, Estep JS, Dubick Ma, Holcomb JB. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J Trauma. 2009;67:450–9. doi: 10.1097/TA.0b013e3181ac0c99. discussion 459–60. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson SB, Fulkerson P, Bildfell R, Aguilera L, Hazzard TM. Chitosan dressing provides hemostasis in swine femoral arterial injury model. Prehosp Emerg Care. 2006;11:172–8. doi: 10.1080/10903120701205893. [DOI] [PubMed] [Google Scholar]
- 16.Chan LW, Wang X, Wei H, Pozzo LD, White NJ, Pun SH. A Synthetic Fibrin-Crosslinking Polymer for Modulating Clot Properties and Inducing Hemostasis. Sci Transl Med. 2015;7:277ra29. doi: 10.1126/scitranslmed.3010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolodziej AF, Nair SA, Graham P, McMurry TJ, Ladner RC, Wescott C, Sexton DJ, Caravan P. Fibrin specific peptides derived by phage display: characterization of peptides and conjugates for imaging. Bioconjug Chem. 2012;23:548–556. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell SJ. Handbook on Nonwovens. Woodhead Publishing Limited; 2007. [Google Scholar]
- 19.Rawal A, Majumdar A, Anand S, Shah T. Predicting the properties of needlepunched nonwovens using artificial neural network. J Appl Polym Sci. 2009;112:3575–3581. [Google Scholar]
- 20.Jena AK, Gupta KM. In-plane compression porometry of battery separators. J Power Sources. 1999;80:46–52. [Google Scholar]
- 21.Jena BA, Gupta K. Pore Volume of Nanofiber Nonwovens. J Eng Fiber Fabr. 2005:25–30. [Google Scholar]
- 22.Test Methods Prim. Wound Dressings; 2002. BS EN 13726-1, Aspects of Absorbancy. [Google Scholar]
- 23.Gu BK, Park SJ, Kim MS, Kang CM, Kim JI, Kim CH. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr Polym. 2013;97:65–73. doi: 10.1016/j.carbpol.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 24.Shih MF, Shau MD, Chang MY, Chiou SK, Chang JK, Cherng JY. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int J Pharm. 2006;327:117–25. doi: 10.1016/j.ijpharm.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Smith AH, Laird C, Porter K, Bloch M. Haemostatic dressings in prehospital care. Emerg Med J. 2013;30:784–9. doi: 10.1136/emermed-2012-201581. [DOI] [PubMed] [Google Scholar]
- 26.Rall JM, Cox JM, Songer AG, Cestero RF, Ross JD. Comparison of novel hemostatic dressings with QuikClot combat gauze in a standardized swine model of uncontrolled hemorrhage. J Trauma Acute Care Surg. 2013;75:S150–6. doi: 10.1097/TA.0b013e318299d909. [DOI] [PubMed] [Google Scholar]
- 27.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–8. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 28.Lim BBC, Lee EH, Sotomayor M, Schulten K. Molecular basis of fibrin clot elasticity. Structure. 2008;16:449–59. doi: 10.1016/j.str.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM) Thromb Haemost. 2010;104:385–391. doi: 10.1160/TH09-12-0858. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Yin S, Ren L, Zhao L. Surface characterization of the chitosan membrane after oxygen plasma treatment and its aging effect. Biomed Mater. 2009;4:035003. doi: 10.1088/1748-6041/4/3/035003. [DOI] [PubMed] [Google Scholar]
- 31.Šimor M, Ráhel’ J, Černák M, Imahori Y, Štefečka M, Kando M. Atmospheric-pressure plasma treatment of polyester nonwoven fabrics for electroless plating. Surf Coatings Technol. 2003;172:1–6. [Google Scholar]
- 32.Černáková L, Kováčik D, Zahoranová a, Černák M, Mazúr M. Surface Modification of Polypropylene Non-Woven Fabrics by Atmospheric-Pressure Plasma Activation Followed by Acrylic Acid Grafting. Plasma Chem Plasma Process. 2005;25:427–437. [Google Scholar]
- 33.Arnaud F, Parreño-Sadalan D, Tomori T, Delima MG, Teranishi K, Carr W, McNamee G, McKeague A, Govindaraj K, Beadling C, Lutz C, Sharp T, Mog S, Burris D, McCarron R. Comparison of 10 hemostatic dressings in a groin transection model in swine. J Trauma. 2009;67:848–55. doi: 10.1097/TA.0b013e3181b2897f. [DOI] [PubMed] [Google Scholar]
- 34.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15:74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 35.Devlin JJ, Kircher S, Kozen BG, Littlejohn LF, Johnson AS. Comparison of ChitoFlex®, CELOX™, and QuikClot® in control of hemorrhage. J Emerg Med. 2011;41:237–45. doi: 10.1016/j.jemermed.2009.02.017. [DOI] [PubMed] [Google Scholar]