Abstract
Background
Treatment of comorbid chronic disease, such as depression, in people living with HIV/AIDS (PLWHA) increasingly falls to HIV treatment providers. Guidance in who will best respond to depression treatment and which patient-centered symptoms are best to target is limited.
Methods
Bivariable analyses were used to calculate hazard ratios for associations between baseline demographic, mental health-related, and HIV-related factors on time to first depression remission among PLWHA enrolled in a randomized trial of measurement-based antidepressant management. Time-updated factors also were analyzed at time of antidepressant (AD) initiation/adjustment and 8 weeks post AD initiation/adjustment.
Results
Baseline comorbid depression and anxiety; comorbid depression, anxiety and substance abuse; and generalized anxiety disorder predicted a slower time to first remission. Being on ART but non-adherent, having panic disorder, having a history of a major depressive episode, or having been in HIV care for > 10 years prior to study initiation predicted a faster time to first remission. Sleep difficulty or fatigue at the time of AD initiation/adjustment predicted a slower time to remission. In non-remitters at 8 weeks post AD initiation/adjustment, sleep difficulty, anxiety, and fatigue each predicted a slower time to remission.
Limitations
Remission was determined by PHQ-9 scores, not diagnostic criteria. The results may apply only to depression recovery in this particular model of treatment. We conducted only exploratory analyses to determine magnitude of effects.
Conclusions
Baseline comorbid anxiety with or without substance abuse predicts slower time to depression remission among PLWHA treated in HIV clinics. Targeting anxiety or fatigue at the time of AD initiation/adjustment or sleep difficulty, anxiety, and fatigue at 8 weeks post AD initiation/adjustment could shorten time to depression remission in this model.
Keywords: HIV, Depression, Remission predictors, Measurement-based care, Depression treatment
1. Background
The number of pepole living with HIV/AIDS (PLWHA) has grown rapidly over the past decade, largely due to increased access to and effectiveness of antiretroviral therapies (ART) (UNAIDS, 2012). As a result, life expectancies have increased and the burden of opportunistic infections has decreased (Bor et al., 2013; Jahn et al., 2008; Mills et al., 2011). An unforeseen and unwelcome consequence of these results is that the number of PLWHA who develop noncommunicable diseases has increased (Mocroft et al., 2002; Palella et al., 2006). Included in this group are a spectrum of mental illnesses, including major depressive disorder. HIV infection is associated with an elevated prevalence of depression, affecting 20–30% of PLWHA (Bing et al., 2001; Ciesla and Roberts, 2001). Various factors contribute to this higher prevalence, including high viral load, direct effects of the virus on the immune system, symptom burden, emotional reaction to the diagnosis, and social stigma (Atkinson et al., 2008; Bing et al., 2001; Lyketsos et al., 1996). Worldwide, depression in PLWHA is associated with decreased quality of life, reduced adherence to anti-retroviral medications, increased use and abuse of substances, and poor treatment outcomes, including increased utilization of services, more rapid decline in CD4 count, and increased mortality (Ickovics et al., 2001; Kacanek et al., 2010; Pence et al., 2007; Rubin et al., 2011; Uthman et al., 2014). Treatments for depression in PLWHA are similar to those in the general population, and they are similarly effacacious. Meta-analyses of randomized control trials of pharmacologic and group-therapy based treatments of depression in PLWHA showed effect sizes that are similar to those in the general population (Himelhoch and Medoff, 2005; Himelhoch et al., 2007).
While there has been an increased demand for mental health care services for PLWHA, the supply of specialty mental health care providers has not kept pace. As a result, the identification and management of depression in PLWHA often falls to HIV care providers. The challenges of identifying, effectively treating, and monitoring depressive symptoms in these clinical settings can require significant resources. Consequently, models of care that integrate medical and psychiatric care have the potential to provide cost-effective care in HIV clinic settings (Hyle et al., 2014). Indeed, the National AIDS Policy discusses the need for making the HIV clinic the “medical home” where patients can receive all of their medical and mental health care in one setting (Adams et al., 2012; Policy, 2010). Integrated care approaches are cost-effective in treating depression with resultant improvements in other noncommunicable diseases in other populations (Bogner and de Vries, 2008; Katon et al., 2010; Katon et al., 2004; Pyne et al., 2011). However, some PLWHA who have depression may still require specialty level care. Early identification of individuals who may not benefit from integrated care approaches and who will require specialty-level mental health care could lead to more cost-effective treatment. Among PLWHA who are receiving depression care, response to depression treatment has been associated with employment status, viral load at initial treatment, increase in CD4 count during treatment, and lower mean baseline CD4 count at initiation of treatment (Primeau et al., 2013), although these factors have not been consistent across studies. More patient-centered measures of distress have not been examined in this population in regards to response to depression treatment. Such symptom-level factors (i.e. sleep difficulty, fatigue, anxiety, anhedonia, appetite, etc.) could provide clinicians with targets for treatment that could improve response to depression interventions.
Here we present a secondary analysis of data from a randomized controlled trial to evaluate the effectiveness of evidence-based antidepressant management integrated into HIV care. Our focus here is on baseline characteristics that might distinguish those individuals who will remit or respond to depression treatment sooner and those who may be more treatment resistant. We also examine factors at important time frames during the course of depression treatment in an effort to identify targets for more focused interventions. This information could help guide clinicians to identify such characteristics when making decisions about how to optimally treat an individual or when to refer a patient for more specialized mental health care over the course of depression treatment. Given this is a secondary analysis, our aim with this study was to identify potentially interesting questions that could be the topic of future, prospective, appropriately-powered studies.
2. Methods
Data for the present analysis come from a randomized controlled trial to evaluate the effectiveness of evidence-based antidepressant management integrated into HIV care in improving antiretroviral adherence (the SLAM DUNC Study), described in detail elsewhere (Pence et al., 2012). Briefly, HIV-infected patients receiving medical care at one of four U.S. infectious diseases clinics were eligible to participate if they were English-speaking, ages 18 – 65, screened positive for depression (score ≥ 10) on the Patient Health Questionnaire-9 (PHQ-9) (Spitzer et al., 1999), and were confirmed to have a current major depressive disorder on the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Exclusion criteria included history of bipolar or psychotic disorder, failure of two or more adequate antidepressant trials in the current major depressive episode, or psychiatric presentation requiring immediate hospitalization or other acute intervention (Pence et al., 2012).
Eligible individuals who agreed to participate were randomized to receive either enhanced usual care for depression or a depression treatment model called Measurement-Based Care (MBC) (Adams et al., 2012). Data for the present analysis come only from individuals randomized to MBC. In the MBC arm, a clinically supervised depression care manager (DCM) completed regular assessments of depressive severity and antidepressant tolerability using standardized tools. The DCM then followed an evidence-based algorithm to provide antidepressant treatment recommendations (e.g. increase dose, maintain dose, switch medication) to the HIV provider, who made final decisions on the depression treatment plan. Participants randomized to the enhanced usual care arm could also receive depression treatment from their HIV provider or other sources, but no in-clinic decision support was provided by the DCM. All participants provided written informed consent, and ethical approval was provided by Duke University, the University of North Carolina at Chapel Hill, and the University of Alabama at Birmingham.
2.1. Measures
Depressive severity over time was measured using the PHQ-9 (Kroenke et al., 2001). The PHQ-9 was administered monthly to participants in the MBC arm during the acute phase of treatment. Patients who achieved remission (PHQ-9 < 5) entered a maintenance phase, with PHQ-9 assessment every 3 months. Patients could also move to the maintenance phase if they and their provider were “satisfied” with their improvement and thought that further dose adjustments were unlikely to lead to further gains. We defined three antidepressant treatment outcomes: remission (our primary outcome, defined as PHQ-9 < 5), response (≥ 50% improvement in PHQ-9 score from baseline), and clinically acceptable improvement (either remission or patient/provider “satisfaction”, as described above).
We investigated associations of all three depression treatment outcomes with three categories of predictors: demographic, mental health-related, and HIV-related. Demographic predictors considered were gender, marital status and education. Mental health-related predictors considered were depressive severity (PHQ-9 score), presence of psychiatric comorbidities (depression only; depression and anxiety only; depression and substance abuse only; or depression, anxiety and substance abuse), history of recurrent major depressive episode, specific types of anxiety diagnoses (panic disorder, post-traumatic stress disorder or generalized anxiety disorder), sleep difficulty, and fatigue. Analyses that included time-updated predictors also evaluated antidepressant side effect burden. Anxiety and substance use diagnoses and past depressive episodes were measured using the MINI (Sheehan et al., 1998). Presence or absence of self-reported sleep difficulty, anxiety, and fatigue were measured with the Patient-Reported Inventory of Side Effects (Rush et al., 2004). Antidepressant side effect burden was measured using the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) on a scale from 0 to 6, with 0 indicating no side effects, 1–5 indicating minimal, mild, moderate, marked and severe side effects, and 6 indicating inability to function due to side effects (Wisniewski et al., 2006). HIV predictors considered were ART use and adherence (on ART and adherent [no missed pills in the previous week], on ART and not adherent [any missed pills in the previous week], off ART), viral suppression (< 50 copies/mL), CD4 count and the number of years in care prior to the study start (≤ 1, 4 1– 5, > 5–10 and > 10 years).
2.2. Statistical analyses
We used Cox proportional hazards models to estimate bivariable hazard ratios relating predictors to depression treatment outcomes. We conducted exploratory analyses focused on measuring the magnitude of the association of a given variable on treatment outcomes, rather than the statistical significance of the variables (whether the 95% confidence intervals of the hazard ratios crossed the null). In this exploratory analysis, we considered HRs to be of a meaningful magnitude if they were ≤ 0.7 or ≥ 1.3. We felt that below and above these values suggest a potentially clinically meaningful association between the predictor and the outcome. While we determined all factors with HRs above or below these cutoffs to be clinically meaningful, we also delineated those factors that had 95% CIs that excluded the null from those which had 95% CIs that did not exclude the null where appropriate. In the primary analysis, following the logic of an “intent-to-treat” analysis, participants were followed from study entry through the end of the study (one year), or until they experienced the outcome for that analysis, withdrew from the study (n = 11), or died (n = 2) (whichever date occurred first). In a second analysis, following the logic of an “as-treated” analysis, participants were followed from the date of antidepressant treatment initiation until outcome, withdrawal, death, or study end. In this analysis, participants who entered the study on depression treatment were followed from the date of their first antidepressant dose increase or switch after enrollment. In a third analysis, we restricted our attention to participants who had not remitted by 8 weeks after antidepressant treatment initiation or first adjustment, and assessed baseline and time-updated predictors of subsequent time to depression remission. We investigated the proportional hazards assumption and examined model fit using df betas to determine if there were influential observations.
2.3. Analysis sample
All study participants randomized to MBC were included in the primary analysis (n = 149). Only participants who initiated depression treatment or received their first treatment adjustment prior to experiencing the outcome for a particular analysis were included in the second analysis (n = 122–125, depending on the outcome). Finally, only participants who initiated depression treatment or received a treatment adjustment and did not remit within 8 weeks were included in the third analysis (n = 104).
3. Results
3.1. Demographic characteristics of the study population
The demographic and clinical variables at baseline in the study population are shown in Table 1. Our population was predominantly male and most individuals were on ART. Almost 27% of patients on ART were non-adherent at baseline. The majority of patients had a viral load that was suppressed at baseline, but 31% did not. In terms of comorbidities, depression alone was present in 28% of patients, 40% had comorbid depression and anxiety, 13% had comorbid depression and substance abuse, and 19% had comorbid depression, anxiety, and substance abuse. Over half (57%) reported a prior major depressive episode. The median PHQ9 score at baseline was 15, indicating moderate to moderately severe depression. Large numbers of patients reported sleep difficulty (85%), anxiety (72%), and fatigue (86%) as prominent symptoms on the PRISE rating scale.
Table 1.
Sociodemographic and mental health characteristics of 149 people enrolled in the SLAM-DUNC intervention arm.
| Predictors of treatment response | N (%) or median (IQR) |
|---|---|
| Psychiatric comorbidities | |
| Depression only | 42 (28.2) |
| Depression and anxiety | 60 (40.3) |
| Depression and substance abuse | 19 (12.8) |
| Depression, anxiety and substance abuse | 28 (18.8) |
| Baseline ART and adherence | |
| On ART and adherent | 94 (63.1) |
| On ART and non-adherent | 40 (26.9) |
| Not on ART | 15 (10.1) |
| VL suppressed at baseline (<50 copies/mL) | |
| No | 91 (68.9) |
| Yes | 41 (31.1) |
| CD4 count (/100) at baseline | 541 (360, 786) |
| Gender | |
| Male | 111 (75.5) |
| Female | 36 (24.5) |
| Marital status | |
| Married/cohabitating | 31 (21.1) |
| Never married | 85 (57.8) |
| Divorced, widowed, separated | 31 (21.1) |
| Education | |
| ≤12 years | 81 (55.5) |
| >12 years | 65 (44.5) |
| Baseline PHQ-9 | 15 (12, 19) |
| History of major depressive episode | 85 (57.1) |
| Current panic disorder | 21 (14.2) |
| Current PTSD | 28 (18.9) |
| Current GAD | 67 (45.3) |
| Sleep difficulty-PRISE | 119 (85.0) |
| Anxiety-PRISE | 101 (72.1) |
| Fatigue-PRISE | 121 (86.4) |
| Years in HIV care (prior to study start) | |
| ≤ 1 year | 35 (23.5) |
| > 1–5 years | 53 (35.6) |
| > 5–10 years | 43 (28.9) |
| > 10 years | 18 (12.1) |
Abbreviations: ART = antiretroviral therapy; GAD = generalized anxiety disorder; HIV = Human immunodeficiency virus; IQR = Interquartile range; N = number of subjects; PHQ-9 = Patient Healthcare Questionnaire-9; PTSD = posttraumatic stress disorder; PRISE = Patient Rated Inventory of Side Effects; VL = Viral load.
3.2. Outcome experiences for patients
Over the 12 months of the study, 73 of 149 individuals achieved remission of their depression. The cumulative probability of first remission was 0.35, 0.63, and 0.78 at 3, 6, and 12 months, respectively. A total of 99 of 149 individuals achieved response over the 12 months of the study. The cumulative probability of first response was 0.59, 0.79, and 0.96 at 3, 6, and 12 months, respectively. A total of 79 individuals achieved first clinically acceptable improvement over the 12 months of the study. The cumulative probability of reaching clinically acceptable improvement was 0.36, 0.65, and 0.82 at 3, 6, and 12 months, respectively.
3.3. Predictors of time to first remission
In an analysis of all patients in the treatment arm, when compared to patients with just depression alone, having comorbid anxiety and depression (HR 0.56, 0.32–0.98) or having comorbid depression with anxiety and substance abuse (HR 0.55, 0.27–1.13) at baseline predicted a slower time to first remission (Table 2). Further, having current generalized anxiety disorder (HR 0.60, 0.37–0.96) at baseline also predicted a slower time to first remission. Of these factors, having comorbid anxiety and depression and current generalized anxiety disorder had both a clinically meaningful magnitude of association and a 95% CI that excluded the null. Baseline factors that predicted a faster time to first remission included being on ART but being non-adherent (compared to those on ART and adherent; HR 1.53, 0.93–2.53), having current panic disorder (HR 1.34, 0.73–2.49), having a history of a major depressive episode (HR 1.33, 0.83–2.12), or having been in HIV care for > 1–5 years (HR 1.32, 0.7–2.46) or for >10 years (HR 1.65, 0.74–3.66) prior to study initiation (compared to those in HIV care ≤ year). Of note, we found no evidence of violation of the proportional hazards assumption or of overly influential individual observations.
Table 2.
Bivariable results for the association between baseline predictors and hazard of depression outcomes in all subjects (N = 149).
| Baseline predictors of depression outcome | First remission N = 74 HR (95% CI) |
First Response N = 99 HR (95% CI) |
First clinically acceptable N = 79 HR (95% CI) |
|---|---|---|---|
| Slower course | |||
| Depression and anxiety | 0.56 (0.32, 0.98) | 0.64 (0.39, 1.04) | 0.59 (0.35, 1.01) |
| Depression, anxiety, and substance abuse | 0.55 (0.27, 1.13) | 0.62 (0.34, 1.12) | 0.52 (0.26, 1.04) |
| Current PTSD | 0.73 (0.39, 1.36) | 0.73 (0.43, 1.24) | 0.68 (0.37, 1.26) |
| Current GAD | 0.60 (0.37, 0.96) | 0.72 (0.48, 1.08) | 0.61 (0.38, 0.96) |
| Never married | 0.78 (0.44, 1.39) | 0.87 (0.53, 1.44) | 0.69 (0.40, 1.18) |
| Not on ART | 1.06 (0.47, 2.35) | 0.65 (0.30, 1.42) | 0.92 (0.42, 2.04) |
| Sleep difficulty, first contact-PRISE | 0.85 (0.44, 1.62) | 0.70 (0.41, 1.21) | 0.80 (0.43, 1.48) |
| Anxiety, first contact-PRISE | 0.75 (0.45, 1.26) | 0.69 (0.44, 1.06) | 0.76 (0.46, 1.26) |
| Faster course | |||
| On ART and non-adherent | 1.53 (0.93, 2.53) | 1.22 (0.79, 1.88) | 1.49 (0.91, 2.41) |
| History of major depressive episode | 1.33 (0.83, 2.12) | 1.52 (1.01, 2.28) | 1.32 (0.84, 2.08) |
| Current panic disorder | 1.34 (0.72, 2.49) | 1.35 (0.79, 2.32) | 1.19 (0.64, 2.20) |
| Years in HIV care prior to study start | |||
| >1–5 years | 1.32 (0.70, 2.46) | 0.96 (0.57, 1.62) | 1.45 (0.78, 2.69) |
| >5–10 years | 1.17 (0.60, 2.29) | 0.89 (0.51, 1.56) | 1.53 (0.80, 2.92) |
| >10 years | 1.65 (0.74, 3.66) | 1.09 (0.55, 2.19) | 1.66 (0.75, 3.70) |
Bold = HR ≤ 0.7 or ≥ 1.3.
Abbreviations: AD = antidepressant; ART = antiretroviral therapy; CI = confidence interval; HR = Hazard ratio; GAD = generalized anxiety disorder; N = number of subjects; PTSD = posttraumatic stress disorder; PRISE = Patient Rated Inventory of Side Effects.
In our study population, not all patients were receiving active depression treatment. Of the 149 patients followed, 130 patients started antidepressant (AD) treatment or had an adjustment in their AD during the course of the study. Most of these patients (122) did not achieve first remission prior to initiation or adjustment of AD. Amongst those 122 individuals, a bivariable analysis showed that when compared to those individuals who had depression only, having comorbid anxiety with depression (HR 0.51, 0.29–0.92) or having comorbid depression, anxiety, and substance abuse (HR 0.46, 0.21–1.01) led to a slower time to first remission in those who were in active treatment for depression (Table 3). Having comorbid anxiety with depression not only had a clinically meaningful magnitude of association with slower remission, but also a 95% CI that excluded the null. Having current generalized anxiety disorder (HR 0.64, 0.39–1.07) also predicted slower time to first remission. Baseline factors that predicted a faster time to first remission in those receiving active treatment included having been in HIV care for >1–5 years (HR 1.35, 0.68–2.7), >5–10 years (HR 1.49, 0.73–3.04) or for more than 10 years (HR 2.54, 1.11–5.82) (compared to those in HIV care ≤1 year), having more than 12 years of education (HR 1.56, 0.94–2.6), being on ART and non-adherent with treatment (compared to those on ART and adherent; HR 1.58, 0.93–2.71), or having panic disorder (HR 1.36, 0.73–2.57). Of these, being in HIV care for >10 years had both a clinically meaningful magnitude of association, but also a 95% CI that excluded the null. We also examined symptoms at the time of AD initiation or adjustment that might predict course of treatment. Sleep difficulty (HR 0.55, 0.32–0.94) or fatigue (HR 0.63, 0.37–1.08) at this time point predicted slower time to remission (Table 3). Sleep difficulty had both a clinically meaningful magnitude of association, but also a 95% CI that excluded the null.
Table 3.
Bivariable results for the association between baseline or time-updated predictors (at time of antidepressant initiation or adjustment) and hazard of depression outcomes in patients in active treatment (N = 122–125).
| Baseline predictors of depression outcome | First remission N = 63 HR (95% CI) |
First response N = 83 HR (95% CI) |
First clinically acceptable N = 67 HR (95% CI) |
|---|---|---|---|
| Slower course | |||
| Depression and anxiety | 0.51 (0.29, 0.92) | 0.59 (0.35, 0.99) | 0.56 (0.32, 0.98) |
| Depression, anxiety and substance abuse | 0.46 (0.21, 1.01) | 0.53 (0.27, 1.02) | 0.44 (0.20, 0.95) |
| Not on ART | 1.09 (0.46, 2.61) | 0.65 (0.28, 1.52) | 0.95 (0.40, 2.26) |
| Never married | 0.75 (0.41, 1.40) | 0.86 (0.50, 1.45) | 0.62 (0.35, 1.11) |
| Divorced, widowed, separated | 0.74 (0.36, 1.53) | 0.66 (0.34, 1.27) | 0.66 (0.33, 1.30) |
| Current PTSD | 0.72 (0.38, 1.38) | 0.72 (0.41, 1.25) | 0.66 (0.35, 1.26) |
| Current GAD | 0.64 (0.39, 1.07) | 0.78 (0.50, 1.20) | 0.67 (0.41, 1.10) |
| Sleep difficulty-PRISE | 0.78 (0.38, 1.60) | 0.64 (0.35, 1.16) | 0.75 (0.38, 1.49) |
| Faster course | |||
| On ART and non-adherent | 1.58 (0.93, 2.71) | 1.21 (0.76, 1.93) | 1.52 (0.91, 2.55) |
| Education>12 years | 1.56 (0.94, 2.60) | 1.65 (1.06, 2.56) | 1.50 (0.92, 2.45) |
| History or a recurrent major depressive episode | 1.19 (0.72, 1.97) | 1.44 (0.93, 2.24) | 1.26 (0.77, 2.04) |
| Current panic disorder | 1.36 (0.73, 2.57) | 1.37 (0.79, 2.37) | 1.20 (0.64, 2.25) |
| Years in HIV care prior to study start | |||
| >1–5 years | 1.35 (0.68, 2.70) | 1.02 (0.58, 1.79) | 1.45 (0.73, 2.87) |
| >5–10 years | 1.49 (0.73, 3.04) | 1.08 (9.58, 1.96) | 2.01 (1.01, 4.01) |
| >10 years | 2.54 (1.11, 5.82) | 1.75 (0.86, 3.58) | 2.65 (1.16, 6.07) |
| Predictors at time of AD initiation/first adjustment | |||
| Sleep difficulty-PRISE | 0.55 (0.32, 0.94) | 0.67 (0.41, 1.09) | 0.58 (0.34, 0.98) |
| Anxiety-PRISE | 0.71 (0.42, 1.19) | 0.77 (0.49, 1.22) | 0.65 (0.39, 1.07) |
| Fatigue-PRISE | 0.63 (0.37, 1.08) | 0.86 (0.52, 1.40) | 0.55 (0.33, 0.93) |
Bold = HR ≤ 0.7 or ≥ 1.3.
Abbreviations: AD = antidepressant; ART = antiretroviral therapy; CI = confidence interval; HR = Hazard ratio; GAD = generalized anxiety disorder; N = number of subjects; PTSD = posttraumatic stress disorder; PRISE = Patient Rated Inventory of Side Effects.
Of those 122 individuals who were in active treatment, 104 did not achieve first remission within 8 weeks of initiation or adjustment of AD. A total of 43 of these individuals eventually achieved remission during the study. Baseline factors that predicted a slower time to first remission in this group included having co-morbid depression and anxiety (HR 0.68, 0.33–1.41) or comorbid depression, anxiety and substance abuse (HR 0.64, 0.25–1.64), having a suppressed viral load (<50 copies/mL) (HR 0.55, 0.24– 1.24), having generalized anxiety disorder (HR 0.62, 0.33–1.16), or having fatigue (HR 0.58, 0.26–1.26) (Table 4). Baseline factors that predicted a faster time to first remission included being on ART and non-adherent to treatment (HR 1.30, 0.67–2.55), having >12 years of education (HR 1.58, 0.85–2.93), having panic disorder (HR 1.63, 0.78–3.42), and having been in HIV care >10 years (compared to those in HIV care ≤1 year; HR 1.78, 0.62–5.13). Examination of symptoms (as measure on PRISE) at the time of AD initiation or first adjustment showed that having sleep difficulty at that time predicted a slower time to first remission (HR 0.51, 0.26– 0.98) with both clinically meaningful magnitude of association and a 95% CI that excluded the null (Table 4). Examination of symptoms at 8 weeks after AD initiation or first adjustment showed that having sleep difficulty (HR 0.68, 0.36–1.27), anxiety (HR 0.57, 0.31–1.07), or fatigue (HR 0.52, 0.28–0.96) at that time predicted a slower time to first remission (Table 4). However, fatigue was the only factor that had both clinically meaningful magnitude of association and a 95% CI that excluded the null.
Table 4.
Bivariable results for the association between baseline or time-updated predictors and hazard of remission in subjects who did not remit at 8 weeks post-antidepressant (AD) initiation/first adjustment.
| Baseline predictors of depression outcome | First remission N = 43 HR (95% CI) |
|---|---|
| Slower course | |
| Depression and anxiety | 0.68 (0.33, 1.41) |
| Depression, anxiety and substance abuse | 0.64 (0.25, 1.64) |
| VL suppressed at baseline (<50 copies/mL) | 0.55 (0.24, 1.24) |
| Current GAD | 0.62 (0.33, 1.16) |
| Fatigue-PRISE | 0.58 (0.26, 1.26) |
| Faster course | |
| On ART and non-adherent | 1.30 (0.67, 2.55) |
| Education>12 years | 1.58 (0.85, 2.93) |
| Current panic disorder | 1.63 (0.78, 3.42) |
| HIV care >10 years | 1.78 (0.62, 5.13) |
| Predictors at time of AD initiation/first adjustment | |
| Sleep difficulty-PRISE | 0.51 (0.26, 0.98) |
|
Predictors at 8 weeks after AD initiation/first adjustment |
|
| Sleep difficulty-PRISE | 0.68 (0.36, 1.27) |
| Anxiety-PRISE | 0.57 (0.31, 1.07) |
| Fatigue-PRISE | 0.52 (0.28, 0.96) |
Abbreviations: AD = antidepressant; ART = antiretroviral therapy; CI = confidence interval; HR = Hazard ratio; GAD = generalized anxiety disorder; N = number of subjects; PRISE = Patient Rated Inventory of Side Effects; VL = viral load.
3.4. Predictors of time to first response or clinically acceptable improvement
In the analysis of all patients in the treatment arm, having comorbid depression and anxiety, or having comorbid depression, anxiety, and substance abuse both predicted a slower time to first response or clinically acceptable improvement (Table 2). Not being on ART or having sleep difficulty or fatigue at baseline predicted a slower time to first response, while having never been married, or having PTSD or GAD at baseline predicted a slower time to first clinically acceptable improvement. A baseline factor that predicted a faster time to first response or clinically acceptable improvement included having a history of a prior major depressive episode. Having panic disorder at baseline predicted a faster time to first response, while being on ART but non-adherent to treatment or being in HIV care for >1–5 years, >5–10 years, or >10 years at baseline predicted a faster time to first clinically acceptable improvement.
Amongst those patients who did not achieve first response or first clinically acceptable improvement prior to AD initiation or adjustment, bivariable analysis showed that baseline predictors of slower time to first response or to first clinically acceptable improvement included having comorbid depression and anxiety or having comorbid depression, anxiety, and substance abuse, having been in HIV care for >10 years, or being divorced, widowed, or separated (Table 3). Baseline factors in this population that predicted a slower time to response only included not being on ART (compared to those on ART and adherent to treatment) or having sleep difficulty. Having never been married, having PTSD, having GAD, or having been in HIV care for >1–5 years or > 5–10 years at baseline predicted a slower time to first clinically acceptable improvement only. Baseline factors that predicted a faster time to first response or first clinically acceptable improvement in those receiving active treatment included having > 12 years of education or having been in HIV care for >10 years. Having panic disorder or having a history of a prior major depressive episode at baseline predicted a faster time to first response. Being on ART but non-adherent to treatment, or having been in HIV care for >1–5 years or >5 years predicted a faster time to clinically acceptable improvement. Examination of symptoms at the time of AD initiation or first adjustment showed that having sleep difficulty at that time predicted a slower time to response or clinically acceptable improvement, while having anxiety or fatigue at that time predicted a slower time to clinically acceptable improvement only (Table 3).
4. Discussion
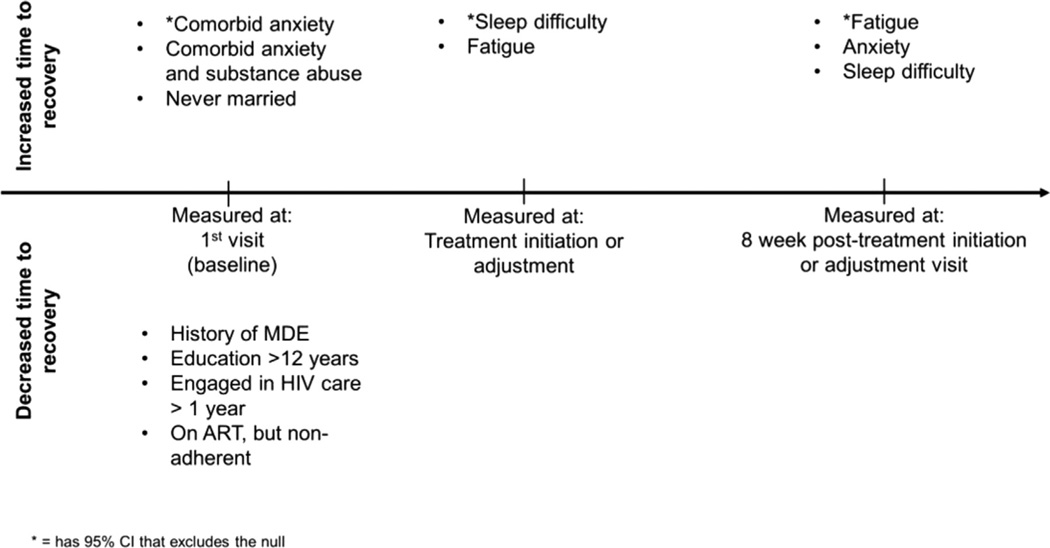
Our study shows predictors of outcomes for HIV-infected individuals with depression enrolled in a MBC protocol from typical infectious disease clinics. As summarized in Fig. 1, several factors negatively affected recovery from depression in response to our intervention. We note that our study is an exploratory analysis and may not have been powered to determine predictors of recovery with clear statistical significance. However, we were still able to identify some predictors which had HRs with 95% CIs that excluded the null. We further identified other predictors that appeared to have clinically meaningful HRs, but had 95% CIs that failed to exclude the null. While we cannot say with complete assurance that these factors truly predict slower or faster depression recovery, we highlight them as potential predictors that warrant further study.
Fig. 1.
Summary of factors associated with recovery from depression symptoms (remission, response or clinically acceptability), by time point at which they were measured. Abbreviations: MDE = Major Depressive Episode, HIV = Human Immunodeficiency Virus, ART = Antiretroviral Therapy.
As described previously, our population showed high levels of comorbid anxiety and/or substance abuse at baseline (Gaynes et al., 2015). Having comorbid anxiety and depression at baseline predicted a slower time to remission in all subjects and those in active depression treatment with a 95% CI that excluded the null (Fig. 1). In HIV-infected individuals, having comorbid anxiety with depression is significantly associated with a greater number of reported HIV symptoms, as well as worse mental health functioning (Gaynes et al., 2015). Further, having “anxious depression” was associated with lower remission and response rates in the STAR*D trial (Fava et al., 2008). Other studies have shown that patients with depression and “high anxiety” or who had an “anxious subtype” of depression showed either no difference or slightly higher remission and response rates than those with lower levels of anxiety (Arnow et al., 2015; Russell et al., 2001; Uher et al., 2011). However, in at least one study, this difference didn’t appear until after 6 weeks in treatment, and up until 6 weeks of treatment, the high anxiety group was actually less likely to achieve response, suggesting high levels of anxiety were associated with a slower initial response (Russell et al., 2001). A major caveat to some of these latter studies is that most of them specifically excluded individuals with DSM-diagnosed anxiety disorders, while our study and the STAR*D trial did not. Thus, our study may be more applicable to a more typical population in a HIV clinic. Interestingly, in our study, having GAD at baseline was predictive of a slower time to remission or clinically acceptable improvement, while having panic disorder at baseline was predictive of a faster time to remission or response (although the latter had a 95% CI that did not exlude the null). This distinction suggests that the quality of the anxiety a person experiences affected their response to depression treatment in this model. While our study is only an exploratory analysis, our findings suggest that identification of those patients with generalized anxiety at baseline in this model could trigger clinicians to be more aggressive about treatment of their depression or the need for early referral to specialized mental health care providers for these patients.
Substance abuse is prevalent in PLWHA. In one US-based study, as many as 40% of HIV-infected individuals admitted to illicit drug use in the prior 12 months (Bing et al., 2001). Alcohol use is also quite prevalent, with some estimates showing that 8–15% of HIV-infected individuals in the US are classified as heavy drinkers, with 53% reporting a 1-month prevalence of any current alcohol use (Galvan et al., 2002). Approximately 24% of PLWHA in the U.S. are in need of treatment for alcoholism or other substance abuse (Kumar et al., 2015). Not only is substance abuse highly prevalent in PLWHA, but it also has been associated with decreased anti-retroviral adherence (Arnsten et al., 2002; Berg et al., 2004; Braithwaite et al., 2005; Howard et al., 2002). Unfortunately, there have been few studies to date on outcomes in PLWHA and co-occurring substance abuse and depression. What has been published looked at injection-drug users specifically and has focused on depression as an independent variable affecting HIV outcomes (Arnsten et al., 2002; Carrieri et al., 2003). Depression is associated with poor adherence to antiretroviral therapy in this population (Avants et al., 2001; Waldrop-Valverde and Valverde, 2005), although one study suggested depression predicted HIV clinical progression among injection-drug users regardless of adherence (Bouhnik et al., 2005). Interestingly, in our study, the presence of substance abuse alone in depressed individuals did not appear to affect the rate of recovery from depression. This was somewhat surprising, given that the co-occurrence of substance abuse can negatively impact response to depression treatment in the general population (Bagby et al., 2002). However, the co-occurrence of both substance abuse and anxiety didoes appear to predict slower recovery from depression, although the 95% CI did not exclude the null. This result could be due to the effects of anxiety, which independently has a negative effect on the rate of depression recovery. However, given that comorbid psychiatric disorders can exacerbate substance abuse and vice versa, it is not surprising that the combination of all three diagnoses (depression, anxiety, and substance abuse) might lead to worse outcomes. A larger, prospective study is needed to definitively explore the effects of co-morbid substance abuse and anxiety on depression recovery.
Fatigue is one of the most common complaints of PLWHA, with an estimated prevalence between 55 and 65% (Barroso et al., 2010; Breitbart et al., 1998; Henderson et al., 2005; Voss, 2005). Fatigue can lead to difficulties with work, social activities, and activities of daily living. It is also associated with poor antiretroviral adherence and virologic failure (Al-Dakkak et al., 2013; Marconi et al., 2013). Several studies have shown that several psychosocial variables are consistently correlated with fatigue in PLWHA, including depression, anxiety, PTSD, stressful life events, being unemployed, not being on antiretroviral therapy, and having fewer years since HIV diagnosis (Barroso et al., 2010; Jong et al., 2010). In our study, fatigue predicted a slower time to recovery in those involved in active treatment, as well as in non-remitters at 8 weeks post-treatment initiation/adjustment. Given that adherence to ART treatment in our study was quite high, our findings suggest that fatigue contributes to poor treatment response regardless of treatment adherence. Targeting fatigue for treatment through psychological or pharmacologic treatment thus has the potential to improve depression treatment outcomes. There is little direct evidence in the literature specifically regarding treatment of fatigue in PLWHA, although it has been suggested that psychological treatments based in cognitive-behavioral therapy may be successful (Barroso et al., 2015; Jong et al., 2010). Modafanil and armodafanil appear to have the most evidence in the pharmacologic treatment of fatigue in PLWHA and seem to have beneficial effects (McElhiney et al., 2010; Rabkin et al., 2011a,b, 2004, 2010). Incorporation of these therapeutic interventions could potentially improve the rate of depression recovery in this population, although further study is needed.
Sleep difficulty at baseline in all patients and in just those getting active treatment predicted a slower time to depression response, although the 95% CIs failed to exclude the null. This suggests that sleep difficulty could affect an individual’s depression recovery in our model. This is not necessarily surprising, given sleep difficulty is known to be associated with a longer course of a major depressive episode and less response to pharmacologic or psychotherapeutic treatments (Dew et al., 1997; Franzen and Buysse, 2008; Pigeon et al., 2008). Sleep difficulty as reported at the time of AD initiation or adjustment remained a predictor of slower remission, response, or clinically acceptable improvement, although again the 95% CIs failed to exclude the null. In addition, fatigue at the time of AD initiation or adjustment also predicted a slower recovery in patients receiving active treatment. These symptoms could represent targets for treatment that could potentially improve outcomes. For example targeting sleep hygiene, using conjunctive sedative-hypnotic medications, or selecting a sedating AD might be reasonable clinical decisions to potentially speed up recovery in this population, although further study is needed to confirm our results.
We also took a closer look at those individuals who did not enter remission 8 weeks after AD initiation or adjustment to see what factors might predict remission in this population, and therefore inform clinical decisions at 8 weeks about how best to proceed with treatment. None of the baseline factors had 95% CIs that excluded the null, but those that showed a clinically meaningful HR and predicted a slower time to remission were similar to those seen in the study population as a whole: namely comorbid depression and anxiety, comorbid depression, anxiety and substance abuse, or current GAD. Interestingly, sleep difficulty was the only symptom at the time of AD treatment initiation or adjustment that predicted a slower time to remission in this population. However, at 8 weeks after AD treatment initiation or adjustment, the presence of sleep difficulty, fatigue, or anxiety all predicted a slower time to remission, although only fatigue had a 95% CI that excluded the null (Fig. 1). These results could help guide clinicians in making treatment decisions at different points along the course of treatment. Using our treatment model, targeting sleep difficulty at the time of AD initiation or adjustment may quicken the time to recovery, while if patients have not responded by 8 weeks after AD initiation or adjustment, targeting sleep difficulty, anxiety and fatigue may be helpful. Further, those individuals who exhibit these symptoms may represent a more treatment-resistant population that could benefit from early referral to specialty mental health providers to optimize care. As such, focusing on these patient-centered measurements of symptoms during care could potentially lead to overall better outcomes in this population.
Several baseline factors were predictive of a faster time to improvement in depression, although none were consistently found to have 95% CIs that excluded the null across the conditions examined (Fig. 1). Having had a prior major depressive episode was predictive of a faster time to remission, response, or clinically acceptable improvement in all patients, as well as a faster time to response or clinically acceptable improvement in those receiving active treatment. It is unclear why patients with a history of depression might have a faster clinical improvement in response to our intervention. In our study, these patients did not appear to have higher rates of baseline comorbid anxiety disorders and did not have a faster or slower time to first AD initiation or adjustment. Further, we do not have any data that would suggest these individuals have a more “treatment-resistant” form of depression, only that they have a prior depressive episode. It may be that those individuals with a prior MDE in this study had developed better skills for dealing with depression due to their prior experience, or their provider was able to more quickly identify an efficacious medication strategy given prior response history. However, we are unable to definitively determine if these suggestions are true given our collected data.
Interestingly, being on ART but not being adherent to treatment at baseline was predictive of a faster time to remission, response, or clinically acceptable improvement in all subjects, as well as in the subset of those receiving active treatment. This result seems counterintuitive at first, but could be explained if by enrolling in the study, patients subsequently became more adherent to treatment, leading to a greater reduction in HIV symptom burden and improvement in mood. Another baseline factor that predicted a faster improvement in depression symptoms was if the patient had been engaged in HIV care for a longer period of time than 1 year. There is no clear reason for this effect, but potentially, those who have had HIV for longer periods of time may have less life stressors than those who are adjusting to a more recent diagnosis. Further, those engaged in long-term HIV treatment are likely to have formed strong relationships with providers and support staff that could be drawn on when treatment for depression is needed. Interestingly, amongst those patients receiving active treatment, having more than 12 years of education predicted a faster time to remission, response, or clinically acceptable improvement. However, a longer duration of education did not predict faster improvement in the analysis of all patients in the study. It is unclear why this might be the case, although it might be that those with more education may engage more fully in treatment or have more resources to utilize when involved in treatment. Unfortunately, there were no symptoms at the time of AD initiation or adjustment that predicted a faster time to any of the clinical outcomes. Amongst those individuals who did not remit by 8 weeks after AD initiation or adjustment, there were no symptoms at the 8 week time point that predicted a faster time to ultimate remission.
Our study has limitations. Our analysis was conducted only within the MBC treatment arm of our study due to the availability of more monitoring with PHQ-9 screens in this arm. We were unable to assess whether our intervention had any effects on the rate of remission, response or clinically acceptable improvement relative to usual care. Further, there may be something about our intervention that could have affected the rate of recovery from depression in this population. We used PHQ-9 scores only to determine rates of remission or response, not true diagnostic criteria. Several items on the PHQ-9 are related to somatic symptoms (sleep, fatigue, appetite) that can be affected by several medical issues, including HIV infection or side effects from ART. In the palliative care population, for example, the presence of somatic symptoms has a very low positive predictive value for depression and scores on the PHQ-9 are not likely to respond to depression treatment alone (Rayner et al., 2011). Thus, it is possible that in our population, some patients with high PHQ-9 scores do not truly have depression, and their response to our intervention targeting the disease would be limited. A further limitation is that we conducted only exploratory analyses of our data to calculate hazard ratios, but did not do further statistical analyses to determine the statistical significance of these findings. However, given the relatively small size of our study, we felt focusing on the magnitude of effects may be more instructive, as the study was likely not powered to show statistical significance in these variables. In addition, we conducted only bivariable analyses, and multivariate analysis could potentially further delineate what baseline and time-updated factors are independently important to the rate of recovery from depression. Another limitation was out inability to determine if differences in depression recovery existed based on the specific antidepressant medication used or the dose of the medications. This was due to the fact that several different medications were used, and our numbers for any one medication were too small to produce meaningful statistical analysis. Finally, while we speculate that intervening on particular patient-reported symptoms at certain time points in treatment may lead to faster recovery, only a prospective interventional study could inform us if that is true.
In summary, our study presents important data on factors that could predict the rate of depression recovery in patients enrolled in a MBC treatment program. We have shown that comorbid psychiatric illness, as well as certain patient-centered factors play important roles in how quickly patients respond or remit with treatment. Further, targeting these patient-centered symptoms at certain times during treatment, or using them as a signal for early referral to more specialized care could lead to improvements in patient outcomes. These data can help guide clinicians in their thinking about how best to treat patients in MBC models to target a more rapid recovery.
Acknowledgments
Funding/support
This work was supported by grant R01MH086362 of the National Institute of Mental Health and the National Institute for Nursing Research, National Institutes of Health, Bethesda, MD, USA. Support for the design and conduct of the study was also provided by the NIH-funded Centers for AIDS Research at the University of North Carolina at Chapel Hill, Duke University, and the University of Alabama at Birmingham (P30- AI50410, P30-AI064518, and P30-AI027767). BNG is supported by NC TRACS Institute, which is supported by grants UL1RR025747, KL2RR025746, and TLRR025745 from the NIH National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health.
We gratefully acknowledge the contributions of the SLAM DUNC study staff, providers, and participants. We also would like to acknowledge the contributions of Julie O’Donnell PhD, who provided additional data analysis support for this manuscript.
Role of the sponsors
None of the financial sponsors were directly responsible for the design, conduct or reporting of the study.
Abbreviations
- AD
antidepressant
- ART
antiretroviral therapy
- DCM
depression care manager
- GAD
generalized anxiety disorder
- HIV
Human immunodeficiency virus
- IQR
Interquartile range
- MBC
measurement-based care
- MINI
Mini International Neuropsychiatric Interview (MINI)
- N
number of subjects
- PHQ-9
Patient Healthcare Questionnaire-9
- PLWHA
people living with HIV/AIDS
- PTSD
posttraumatic stress disorder
- PRISE
Patient Rated Inventory of Side Effects
- VL
Viral load
Footnotes
Author contributions
NAS participated in review of data for analysis and wrote, edited, and submitted the manuscript. AB conducted all data analyses and reviewed and edited the manuscript. BNG and BWP were involved in the design and execution of the original SLAM-DUNC study, as well as in the design of data analyses and manuscript review.
The authors report no conflicts of interest.
References
- Adams JL, Gaynes BN, McGuinness T, Modi R, Willig J, Pence BW. Treating depression within the HIV “medical home”: a guided algorithm for antidepressant management by HIV clinicians. AIDS Patient Care STDS. 2012;26:647–654. doi: 10.1089/apc.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2013;25:400–414. doi: 10.1080/09540121.2012.712667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, Etkin A, Kulkarni J, Luther JF, Rush AJ. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D trial. Am. J. Psychiatry. 2015;8:743–750. doi: 10.1176/appi.ajp.2015.14020181. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J. Gen. Intern. Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, Summers J, Sciolla A, Gutierrez R, Ellis RJ, Abramson I, Hesselink JR, McCutchan JA, Grant I. Two-year prospective study of major depressive disorder in HIV-infected men. J. Affect. Disord. 2008;108:225–234. doi: 10.1016/j.jad.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Warburton LA, Hawkins KA, Shi J. Predictors of nonadherence to HIV-related medication regimens during methadone stabilization. Am. J. Addict./Am. Acad. Psychiatr. Alcohol Addict. 2001;10:69–78. doi: 10.1080/105504901750160501. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Cristi C. Psychosocial and clinical predictors of response to pharmacotherapy for depression. J. Psychiatry Neurosci.: Jpn. 2002;27:250–257. [PMC free article] [PubMed] [Google Scholar]
- Barroso J, Hammill BG, Leserman J, Salahuddin N, Harmon JL, Pence BW. Physiological and psychosocial factors that predict HIV-related fatigue. AIDS Behav. 2010;14:1415–1427. doi: 10.1007/s10461-010-9691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, Leserman J, Harmon JL, Hammill B, Pence BW. Fatigue in HIV-infected people: a three-year observational study. J. Pain Symptom Manag. 2015;50:69–79. doi: 10.1016/j.jpainsymman.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to anti-retroviral therapy. J. Gen. Intern Med. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bogner HR, de Vries HF. Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Ann. Fam. Med. 2008;6:295–301. doi: 10.1370/afm.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhnik AD, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, Gastaut JA, Moatti JP, Spire B. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir. Ther. 2005;10:53–61. [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JF, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol.: Clin. Exp. Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Breitbart W, McDonald MV, Rosenfeld B, Monkman ND, Passik S. Fatigue in ambulatory AIDS patients. J. Pain. Symptom Manag. 1998;15:159–167. doi: 10.1016/s0885-3924(97)00260-1. [DOI] [PubMed] [Google Scholar]
- Carrieri MP, Chesney MA, Spire B, Loundou A, Sobel A, Lepeu G, Moatti JP. Failure to maintain adherence to HAART in a cohort of French HIV-positive injecting drug users. Int. J. Behav. Med. 2003;10:1–14. doi: 10.1207/s15327558ijbm1001_01. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am. J. Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF, 3rd, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch. Gen. Psychiatry. 1997;54:1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am. J. Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialog. Clin. Neurosci. 2008;10:473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and services utilization study. J. Stud. Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, O’Donnell J, Nelson E, Heine A, Zinski A, Edwards M, McGuinness T, Riddhi MA, Montgomery C, Pence BW. Psychiatric comorbidity in depressed HIV-infected individuals: common and clinically consequential. Gen. Hosp. Psychiatry. 2015;37:277–282. doi: 10.1016/j.genhosppsych.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M, Safa F, Easterbrook P, Hotopf M. Fatigue among HIV-infected patients in the era of highly active antiretroviral therapy. Hiv. Med. 2005;6:347–352. doi: 10.1111/j.1468-1293.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: a systematic review and meta-analysis. AIDS Patient Care STDS. 2005;19:813–822. doi: 10.1089/apc.2005.19.813. [DOI] [PubMed] [Google Scholar]
- Himelhoch S, Medoff DR, Oyeniyi G. Efficacy of group psychotherapy to reduce depressive symptoms among HIV-infected individuals: a systematic review and meta-analysis. AIDS Patient Care STDS. 2007;21:732–739. doi: 10.1089/apc.2007.0012. [DOI] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Lo Y, Vlahov D, Rich JD, Schuman P, Stone VE, Smith DK, Schoenbaum EE. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- Hyle EP, Naidoo K, Su AE, El-Sadr WM, Freedberg KA. HIV, tuberculosis, and noncommunicable diseases: what is known about the costs, effects, and cost-effectiveness of integrated care? J. Acquir. Immune Defic. Syndr. 2014;67(Suppl. 1):S87–S95. doi: 10.1097/QAI.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. J Am. Med. Assoc. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, Munthali F, McGrath N, Mwafilaso J, Mwinuka V, Mangongo B, Fine PE, Zaba B, Glynn JR. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong E, Oudhoff LA, Epskamp C, Wagener MN, van Duijn M, Fischer S, van Gorp EC. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS. 2010;24:1387–1405. doi: 10.1097/QAD.0b013e328339d004. [DOI] [PubMed] [Google Scholar]
- Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the Nutrition for Healthy Living study. J. Acquir. Immune Defic. Syndr. 2010;53:266–272. doi: 10.1097/QAI.0b013e3181b720e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N. Engl. J. Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch. Gen. Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rao PS, Earla R, Kumar A. Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert. Opin. Drug Metab. Toxicol. 2015;11:343–355. doi: 10.1517/17425255.2015.996546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Hutton H, Fishman M, Schwartz J, Treisman GJ. Psychiatric morbidity on entry to an HIV primary care clinic. AIDS. 1996;10:1033–1039. doi: 10.1097/00002030-199610090-00015. [DOI] [PubMed] [Google Scholar]
- Marconi VC, Wu B, Hampton J, Ordonez CE, Johnson BA, Singh D, John S, Gordon M, Hare A, Murphy R, Nachega J, Kuritzkes DR, del Rio C, Sun-path H. Early warning indicators for first-line virologic failure independent of adherence measures in a South African urban clinic. AIDS Patient Care STDS. 2013;27:657–668. doi: 10.1089/apc.2013.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhiney M, Rabkin J, Van Gorp W, Rabkin R. Modafinil effects on cognitive function in HIV+ patients treated for fatigue: a placebo controlled study. J. Clin. Exp. Neuropsychol. 2010;32:474–480. doi: 10.1080/13803390903201769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, Nachega JB, Dybul M, Hogg RS. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann. Intern. Med. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, Francioli P, D’Arminio Monforte A, Fox Z, Lundgren JD. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- Pence BW, Gaynes BN, Williams Q, Modi R, Adams J, Quinlivan EB, Heine A, Thielman N, Mugavero MJ. Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: rationale and design of the SLAM DUNC Study. Contemp. Clin. Trials. 2012;33:828–838. doi: 10.1016/j.cct.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2007;44:159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Hegel M, Unutzer J, Fan MY, Sateia MJ, Lyness JM, Phillips C, Perlis ML. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31:481–488. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policy, T.W.H.O.o.N.A. National HIV/AIDS Strategy for the United States. 2010. [Google Scholar]
- Primeau MM, Avellaneda V, Musselman St D, Jean G, Illa L. Treatment of depression in individuals living with HIV/AIDS. Psychosomatics. 2013;54:336–344. doi: 10.1016/j.psym.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Pyne JM, Fortney JC, Curran GM, Tripathi S, Atkinson JH, Kilbourne AM, Hagedorn HJ, Rimland D, Rodriguez-Barradas MC, Monson T, Bottonari KA, Asch SM, Gifford AL. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch. Intern. Med. 2011;171:23–31. doi: 10.1001/archinternmed.2010.395. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Rabkin R. Modafinil and armodafinil treatment for fatigue for HIV-positive patients with and without chronic hepatitis C. Int. J. STD AIDS. 2011a;22:95–101. doi: 10.1258/ijsa.2010.010326. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Rabkin R. Treatment of HIV-related fatigue with armodafinil: a placebo-controlled randomized trial. Psychosomatics. 2011b;52:328–336. doi: 10.1016/j.psym.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Rabkin R, Ferrando SJ. Modafinil treatment for fatigue in HIV+ patients: a pilot study. J. Clin. Psychiatry. 2004;65:1688–1695. doi: 10.4088/jcp.v65n1215. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ. Modafinil treatment for fatigue in HIV/AIDS: a randomized placebo-controlled study. J. Clin. Psychiatry. 2010;71:707–715. doi: 10.4088/JCP.09m05171bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner L, Lee W, Price A, Monroe B, Sykes N, Hansford P, Higginson IJ, Hotopf M. The clinical epidemiology of depression in palliative care and the predictive value of somatic symptoms: cross-sectional survey with four-week follow-up. Palliat. Med. 2011;25:229–241. doi: 10.1177/0269216310387458. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Grey DD, Weber K, Wells C, Golub ET, Wright RL, Schwartz RM, Goparaju L, Cohan D, Wilson ML, Maki PM. Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. J. Womens Health (Larchmt) 2011;20:1287–1295. doi: 10.1089/jwh.2010.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control. Clin. trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Russell JM, Koran LM, Rush J, Hirschfeld RM, Harrison W, Friedman ES, Davis S, Keller M. Effect of concurrent anxiety on response to sertraline and imipramine in patients with chronic depression. Depression Anxiety. 2001;13:18–27. doi: 10.1002/1520-6394(2001)13:1<18::aid-da3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:34–57. [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. J. Am. Med. Assoc. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Uher R, Dernovsek MZ, Mors O, Hauser J, Souery D, Zobel A, Maier W, Henigsberg N, Kalember P, Rietschel M, Placentino A, Mendlewicz J, Aitchison KJ, McGuffin P, Farmer A. Melancholic, a typical and anxious depression subtypes and outcome of treatment with escitalopram and nor-triptyline. J. Affect. Disord. 2011;132:112–120. doi: 10.1016/j.jad.2011.02.014. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Together we will end AIDS. 2012 [Google Scholar]
- Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr. HIV/AIDS Rep. 2014;11:291–307. doi: 10.1007/s11904-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JG. Predictors and Correlates of Fatigue in HIV/AIDS. J. Pain. Symptom Manag. 2005;29:173–184. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Waldrop-Valverde D, Valverde E. Homelessness and psychological distress as contributors to antiretroviral nonadherence in HIV-positive injecting drug users. AIDS Patient Care STDS. 2005;19:326–334. doi: 10.1089/apc.2005.19.326. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J. Psychiatr. Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]