Abstract
Background
To compare dementia incidence of African American and Yoruba cohorts age 70 or older enrolled in 1992 and 2001.
Methods
African Americans residing in Indianapolis and Yoruba in Ibadan, Nigeria without dementia were enrolled in 1992 and 2001 and evaluated every two to three years until 2009. The cohorts consist of 1440 African Americans, 1774 Yoruba in 1992 and 1835 African Americans and 1895 Yoruba in the 2001 cohorts age 70 or older.
Results
In African Americans, dementia and AD incidence rates were significantly lower in 2001 than 1992 for all age groups except the oldest group. The overall standardized annual dementia incidence rates were 3.6% (95% CI: 3.2–4.1%) in the1992 cohort and 1.4% (95% CI: 1.2–1.7%) in the 2001 cohort. There was no significant difference in dementia or AD incidence between the Yoruba cohorts.
Conclusions
Future research is needed to explore the reasons for the differential changes in incidence rates in these two populations.
Keywords: dementia, Alzheimer’s disease, incidence, African Americans, Nigerians
Introduction
With the aging of the population the dementing disorders including Alzheimer’s disease (AD) are widely recognized as a major public health problem worldwide. There have, however, been reports of significant decline in dementia prevalence.1–4 It is not clear whether this decline in dementia prevalence was caused by lower dementia incidence or shorter survival of patients diagnose with dementia.1 A few studies have reported on dementia incidence trends over the past two decades with some reporting no change5,6 while others report a decline in dementia incidence.7 Few studies to date have examined changes in dementia incidence in African Americans and none were reported in African populations.
This study was part of the Indianapolis-Ibadan Dementia Project. In this analysis, we compare age-specific incidence rates for dementia and Alzheimer’s disease (AD) between cohorts assembled in 1992 to those enrolled in 2001 in African Americans in Indianapolis and Yoruba in Ibadan, Nigeria.
Methods
Study Participants
Participants were from the Indianapolis-Ibadan Dementia Project (IIDP), a longitudinal study comparing dementia prevalence, incidence, and other health outcomes in two community-based cohorts. Recruitment to the study was conducted at two time points. In the first recruitment in 1992, cohorts of African Americans age 65 or older living in Indianapolis and Yoruba age 65 or older living in Ibadan, Nigeria, were enrolled in the study. In Indianapolis, interviewers went door-to-door to randomly sampled addresses to invite African Americans (self-identified) age 65 years and over to participate. In 1992, 2,212 African Americans were enrolled while 249 (9.6%) refused, and 121 (4.7%) were too sick to participate. In Ibadan, the study was carried out in the Idikan area and adjacent wards and a complete enumeration and census were conducted for study enrollment. In 1992, 2,486 Yoruba individuals were enrolled while 41 (1.6%) individuals were too sick or refused.
In 2001, the IIDP conducted another wave of enrollment in both populations. In Indianapolis, community-dwelling subjects were randomly selected from Medicare records, who identified themselves as African-Americans, and were at least 70 years of age. The age cut-off for the 2001 cohorts was chosen in order to maintain comparability with the survivors in the 1992 cohort since the youngest participants in the 1992 cohort had since turned 70. Out of 7,583 eligible individuals, interviewers were able to contact 4,433 by telephone or home visit. Of those contacted, 100 were deceased, 54 had moved to nursing homes, and 14 were not African American. Of the remaining 4265 eligible, 1,892 (44%) were enrolled, 2,020 (47%) refused, and 369 (9%) were too ill. In Ibadan, a house-to-house census was conducted in 2001 in which 34,733 individuals were enumerated in 3,452 households, of which 3,144 were age ≥70 years. Of those eligible, 866 were already enrolled in the 1992 cohort, and 1,939 were enrolled in the 2001 cohort. There were no refusals in Ibadan during the 2001 enrollment.
All participants agreed to undergo regular follow-up cognitive assessment and clinical evaluations. The study was approved by the Institutional Review Boards of Indiana University-Purdue University of Indianapolis and University of Ibadan. All enrolled participants provided informed consent. In Ibadan, the consenting process involved reading the consent form to study participants, answering any questions they might have had, and obtaining a signature. For illiterate participants a thumbprint was obtained. Details on the assembling of the original cohorts and the enrichment cohort were described elsewhere.8,9
Study Design
A prospective cohort design was used with a baseline evaluation followed by regular evaluations scheduled two to three years apart in both populations using identical assessment instruments. Participants in the 1992 cohorts were evaluated for up to 7 times, in 1992, 1995, 1998, 2001, 2004, 2007 and 2009. Participants in the 2001 cohort were evaluated for up to 4 times, in 2001, 2004, 2007 and 2009.
A two-stage design was used at each evaluation with in-home cognitive and functional evaluations for all participants followed by a full diagnostic workup of selected participants based on the performance of stage one cognitive tests. After each stage one evaluation, study participants were divided into three performance groups (good, intermediate and poor) based on their cognitive and functional scores obtained during the in-home assessment and changes in scores from previous evaluations.10 Percentages sampled from each performance category were chosen to ensure that participants with the highest probability of dementia would be clinically assessed. All participants in the poor performance group were invited to be clinically assessed. Participants were randomly sampled from the intermediate performance group until 50% had clinical assessments and from the good performance group until 5% had clinical assessments.
Each clinically assessed participant was evaluated for the diagnosis of dementia or normal cognition, with further subtypes for those diagnosed with dementia (see section on Clinical Evaluation below). All individuals diagnosed as demented were no longer followed for in-person evaluations.
Cognitive Instruments
The Community Screening Interview for Dementia (CSID) was used during the first stage in-home assessment with a cognitive assessment of the study participant and an interview with a close relative evaluating the daily functioning of the participant. The CSID was developed by our group specifically for use in comparative epidemiological studies of dementia in culturally disparate populations.11,12 The cognitive assessment in CSID evaluates multiple cognitive domains (language, attention and calculation, memory, orientation, praxis, and comprehension and motor response) and details of its content and development are described elsewhere.12–14
Clinical Evaluation
Clinical evaluations included (1) a neuropsychological battery adapted from the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD);13 (2) a standardized neurologic and physical exam, and functional status review (The Clinician Home-based Interview to assess Function CHIF);14 and (3) a structured interview with an informant familiar with the participant (most often a close relative) adapted from the Cambridge Examination for Mental Disorders of the Elderly informant interview (CAMDEX).15,16 Diagnosis was made in a consensus diagnostic conference of clinicians reviewing the neuropsychological test battery, the physician’s assessment, the informant interview, and available medical records. Dementia was diagnosed with both the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R)17 and International Classification of Diseases, 10th Revision (ICD-10)18 criteria. AD was diagnosed using criteria proposed by NINCDS/ADRDA.19
Other Information
Demographic information including age, sex and education were available on all study participants. Information was also collected on whether the participant ever consumed alcohol or smoked regularly. In addition, medical history of coronary heart disease (CHD), cancer, diabetes, heart attack, hypertension, Parkinson’s disease, stroke and depression was collected from self or informant reports as affirmative answers to whether the participants had ever been diagnosed or treated for these conditions. In addition, medication use was also ascertained at each evaluation. In the Yoruba, due to the lack of regular medical care, self-reported medical history cannot be reliably verified. Thus data from self-reported medical history were not presented for the Yoruba cohorts.
Statistical Analyses
Comparisons of baseline demographic characteristics and medical history among participants with incident dementia and those not demented were conducted using Chi-square tests for categorical variables and t-tests for continuous variables for both the 1992 cohort and the 2001 cohort within each population.
To account for the two-stage sampling design, weighted logistic regression models were used to model the probability of incident dementia from a particular evaluation time to the next follow-up. Sampling probabilities were calculated as the numbers of participants who were clinically evaluated divided by the total numbers in each sampling stratum for each evaluation wave. Sampling weights were the reciprocals of these sampling probabilities calculated at each evaluation. Weighted logistic models for incident dementia included age at the time of evaluation, gender, years of education and stage one performance groups as independent variables. Predicted probability of dementia was derived from these logistic regression models. The person-years method was used to estimate age specific incident rates from non-dementia to dementia. For each individual in each of the cohorts, we first determined the observation time contributed to a given age group using times of evaluation for each individual. We then summed individual observation times for a given age group over all cohort members so as to obtain the total number of person-years of observation in that category. Since not all participants received clinical evaluation at the second stage, both the estimated incident dementia cases and the person-years at risk were further adjusted by the predicted probabilities of dementia for those not clinically evaluated in a previous wave. Incident dementia rates for a specific age group were derived as the total estimated number of incident dementia cases divided by the total estimated person-years at risk for the age group. For estimated age-specific incidence rates, 95% confidence intervals were derived using the inverse of the χ2-distributions evaluated with the predicted dementia cases to approximate Poisson distributions.20
Age-standardized overall incident rates were obtained by applying the estimated age specific rates to the age distribution of African American residents in Marion County (Indianapolis) observed in the 2000 census. The variance of the overall age-standardized rate was calculated as a weighted mean of the variances of the age and gender specific rates. Ninety-five percent confidence intervals for the incident rates were constructed based on asymptotic normality of the estimates. Significant difference between two rates in comparison was established by non-overlapping 95% confidence intervals. A number of sensitivity analyses were carried out to examine the robustness of the rate estimates to changes in cohort composition and length of follow-up. We include description of the sensitivity analyses in the online supplemental materials.
Results
For comparison with the 2001 cohort, this analysis excluded participants in the 1992 cohorts who were younger than 70. The 1992 African American cohort included 1440 participants, 191 of whom developed incident dementia. The 2001 African American cohort included 1835 African Americans without dementia at 2001, of these, 94 were diagnosed with dementia during follow-up. There were 1174 Yoruba without dementia in the 1992 cohort and 108 were diagnosed with dementia during follow-up. The 2001 Yoruba cohort included 1895 Yoruba without dementia in 2001, of which 82 were diagnosed with dementia during follow-up.
In Table 1, we present baseline demographic characteristics for the 1992 and 2001 African American cohorts. African Americans in the 1992 cohort were slightly but significantly older at baseline than those in the 2001 cohort, had significantly fewer years of education, and had significantly lower cognitive scores at baseline. However, cohort differences in cognitive scores were non-significant after adjusting for age, gender and education (p=0.4978). The two cohorts have similar gender distributions. Participants in the 2001 cohort had significantly higher rates of cancer, diabetes, hypertension, stroke and depression and they also had significantly higher percentages of using antihypertensive, antidiabetic and lipid-lower medications than those in the 1992 cohort after controlling for medical conditions (p<0.0001).
Table 1.
Baseline characteristics of African American participants (age 70 or older) enrolled in 1992 and 2001: Participants diagnosed as incident dementia during the course of the follow-up were included in the dementia group.
| 1992 African American Cohort | 2001 African American Cohort | |||||
|---|---|---|---|---|---|---|
| Variables | Dementia (n=191) |
Non-demented (n=1249) |
Overall (n=1440) |
Dementia (n=94) |
Non-demented (n=1741) |
Overall (n=1835) |
| Mean Age (SD) | 78.5 (6.1) | 77.6 (5.9)* | 77.7 (5.9) | 79.4 (6.4) | 77.1 (5.4)* | 77.2 (5.5)# |
| Female, % | 72.8 | 64.4* | 65.5 | 74.5 | 65.0 | 65.5 |
| Years of education (SD) | 9.2 (3.3) | 9.4 (3.1) | 9.4 (3.1) | 10.6 (3.1) | 11.4 (2.6)* | 11.4 (2.7)# |
| Alcohol, % | 28.0 | 39.1* | 37.6 | 33.3 | 38.3 | 38.0 |
| Smoking, % | 50.8 | 64.1* | 62.4 | 41.9 | 56.8* | 56.1# |
| Cognitive score | 29.8 (2.9) | 30.4 (2.4)* | 30.3 (2.5) | 30.2 (2.2) | 30.9 (2.1)* | 30.9 (2.2)# |
| Medical History of | ||||||
| CHD, % | 28.8 | 27.9 | 28.1 | 24.5 | 31.0 | 30.7 |
| Cancer, % | 12.0 | 12.1 | 12.1 | 8.5 | 17.3* | 16.8# |
| Diabetes, % | 18.3 | 25.7* | 24.7 | 28.7 | 29.2 | 29.2# |
| Heart attack, % | 13.1 | 16.1 | 15.7 | 9.6 | 14.2 | 14.0 |
| Hypertension, % | 66.5 | 64.9 | 65.1 | 71.0 | 75.8 | 75.6# |
| Parkinson’s, % | 0.0 | 1.3 | 1.1 | 1.1 | 0.9 | 0.9 |
| Stroke, % | 10.0 | 12.6 | 12.2 | 21.7 | 14.8 | 15.2# |
| Depression, % | 5.8 | 6.5 | 6.4 | 10.9 | 11.0 | 11.0# |
| Medication Use | ||||||
| Antihypertensive, % | 40.3 | 45.3 | 44.7 | 66.3 | 77.5* | 76.9# |
| Anti-diabetic, % | 12.0 | 15.1 | 14.7 | 21.7 | 21.9 | 21.9# |
| Lipid-Lowering medications | 1.6 | 1.5 | 1.5 | 19.6 | 25.2 | 24.9# |
: p<0.05 comparing participants with incident dementia to those who remained non-demented within each cohort
: p<0.0001 comparing participants between the 1992 cohort and the 2001 cohort
The Yoruba participants in the 1992 cohort were significantly older at baseline than those in the 2001 cohort (Table 2). The 1992 Yoruba cohort also had significantly lower proportions of female participants, those who consumed alcohol or smoked, higher proportions of participants who had attended school, and also significantly higher cognitive screening scores at baseline. The difference in cognitive screening scores between the two Yoruba cohorts remained significant after adjusting for age, gender and whether participants had attended school (p<0.0001).
Table 2.
Baseline characteristics of Yoruba participants (age 70 or older) enrolled in 1992 and 2001: Participants diagnosed as incident dementia during the course of the follow-up were included in the dementia group.
| 1992 Yoruba Cohort | 2001 Yoruba Cohort | |||||
|---|---|---|---|---|---|---|
| Variables | Dementia (n=108) |
Non-demented (n=1066) |
Overall (n=1174) |
Dementia (n=82) |
Non-demented (n=1813) |
Overall (n=1895) |
| Mean Age (SD) | 79.6 (8.1) | 77.8 (7.9)* | 77.9 (8.0) | 78.0 (6.4) | 75.6 (5.3)* | 75.7 (5.4)# |
| Female, % | 75.9 | 57.2* | 58.9 | 81.7 | 66.3* | 67.0# |
| Ever attended school, % | 12.0 | 17.6 | 17.1 | 3.7 | 13.2 | 12.8# |
| Alcohol, % | 17.0 | 28.8* | 27.7 | 31.7 | 40.5 | 40.2# |
| Smoking, % | 25.9 | 29.0 | 28.7 | 51.2 | 41.2 | 41.6# |
| Cognitive score | 27.3 (3.6) | 27.8 (4.0) | 27.8 (3.9) | 23.2 (3.2) | 25.8 (3.3)* | 25.6 (3.3)# |
: p<0.05 comparing participants with incident dementia to those who remained non-demented within each cohort
: p<0.0001 comparing participants between the 1992 cohort and the 2001 cohort
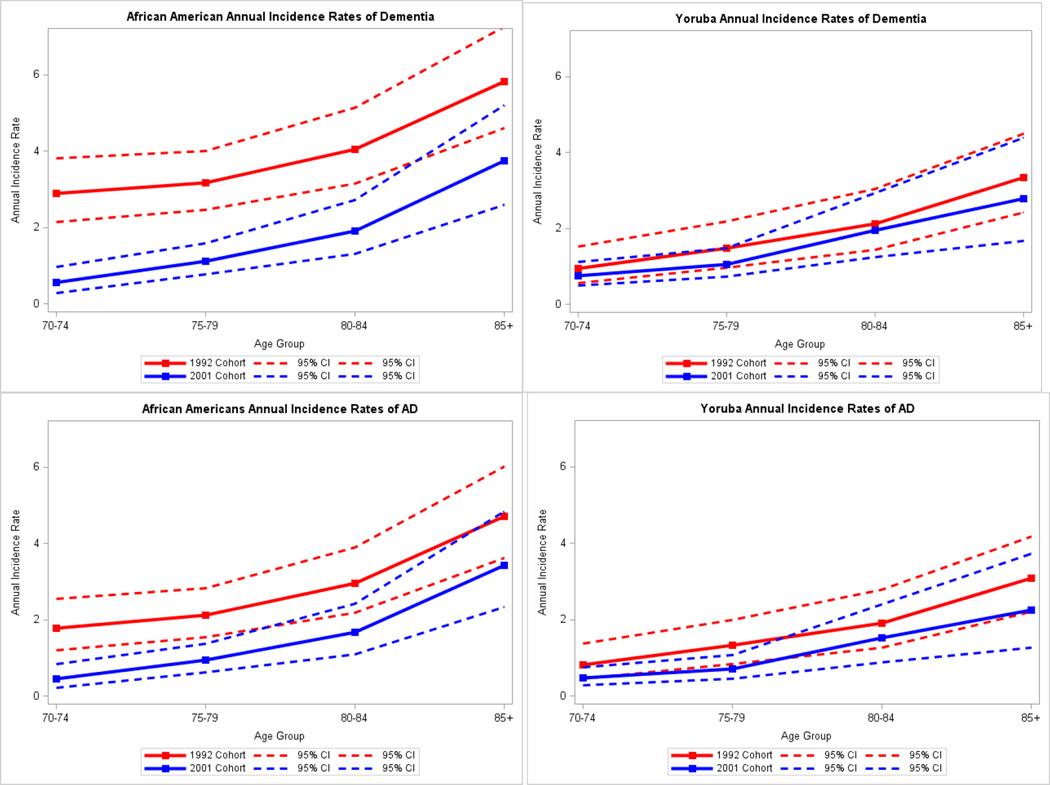
In Table 3, we present age-specific incidence rate estimates for dementia for the 1992 and 2001 cohorts in each population. The 2001 African American cohort had significantly lower age-specific incidence rates for all age groups except for age 85 or older and lower overall standardized incidence rate than the 1992 African American cohort. However, there were no significant differences in dementia incident rates for any age groups in the Yoruba cohorts. Estimated annual incidence rates for AD are presented in Table 4. The 2001 African American cohort showed lower overall AD incidence rate than the 1992 cohort while the Yoruba cohorts showed no significant differences in age-specific incidence rates or in the overall rates. Figure 1 presents the age specific incidence rates for dementia and AD along their 95% confidence intervals for the two populations.
Table 3.
Estimated annual incidence rates of dementia with 95% confidence intervals (CI) for African Americans and Yoruba
| 1992 Cohort | 2001 Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Population |
Age Group | Predicted Cases |
Person-year | Rate (%) | 95% CI | Predicted Cases |
Person-year | Rate (%) | 95% CI | ||
| African Americans | 70–74 | 49 | 1705 | 2.9 | 2.1 | 3.8 | 12 | 2220 | 0.6* | 0.3 | 1.0 |
| 75–79 | 70 | 2194 | 3.2 | 2.5 | 4.0 | 33 | 2907 | 1.1* | 0.8 | 1.6 | |
| 80–84 | 69 | 1699 | 4.1 | 3.2 | 5.1 | 31 | 1634 | 1.9* | 1.3 | 2.7 | |
| 85+ | 78 | 1340 | 5.8 | 4.6 | 7.3 | 35 | 926 | 3.7 | 2.6 | 5.2 | |
| Overall^ | -- | -- | 3.6 | 3.2 | 4.1 | -- | -- | 1.4* | 1.2 | 1.7 | |
| Yoruba | 70–74 | 17 | 1748 | 1.0 | 0.6 | 1.5 | 27 | 3574 | 0.8 | 0.5 | 1.1 |
| 75–79 | 25 | 1699 | 1.5 | 1.0 | 2.2 | 35 | 3315 | 1.1 | 0.7 | 1.5 | |
| 80–84 | 30 | 1404 | 2.1 | 1.4 | 3.0 | 23 | 1162 | 2.0 | 1.2 | 2.9 | |
| 85+ | 44 | 1307 | 3.3 | 2.4 | 4.5 | 18 | 661 | 2.8 | 1.7 | 4.4 | |
| Overall^ | -- | -- | 1.7 | 1.4 | 2.0 | -- | -- | 1.4 | 1.1 | 1.6 | |
denotes significant difference in rates between the 1992 and 2001 African American cohorts.
Overall rates were standardized using the age distribution of 1990 United States census data for African Americans in Marion County.
Table 4.
Estimated annual incidence rates of Alzheimer’s disease with 95% confidence intervals (CI) for African Americans and Yoruba
| 1992 Cohort | 2001 Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Population |
Age Group | Predicted Cases |
Person-year | Rate (%) | 95% CI | Predicted Cases |
Person-year | Rate (%) | 95% CI | ||
| African Americans | 70–74 | 30 | 1705 | 1.8 | 1.2 | 2.5 | 10 | 2220 | 0.5* | 0.2 | 0.8 |
| 75–79 | 46 | 2194 | 2.1 | 1.6 | 2.8 | 27 | 2907 | 0.9* | 0.6 | 1.4 | |
| 80–84 | 50 | 1699 | 3.0 | 2.2 | 3.9 | 27 | 1634 | 1.7 | 1.1 | 2.4 | |
| 85+ | 63 | 1340 | 4.7 | 3.6 | 6.0 | 32 | 926 | 3.4 | 2.3 | 4.8 | |
| Overall^ | -- | -- | 2.5 | 2.1 | 2.9 | -- | -- | 1.3* | 1.0 | 1.5 | |
| Yoruba | 70–74 | 14 | 1748 | 0.8 | 0.5 | 1.4 | 17 | 3574 | 0.5 | 0.3 | 0.8 |
| 75–79 | 22 | 1699 | 1.3 | 0.8 | 2.0 | 24 | 3315 | 0.7 | 0.5 | 1.1 | |
| 80–84 | 27 | 1404 | 1.9 | 1.3 | 2.8 | 18 | 1162 | 1.5 | 0.9 | 2.4 | |
| 85+ | 40 | 1307 | 3.1 | 2.2 | 4.2 | 15 | 661 | 2.3 | 1.3 | 3.7 | |
| Overall^ | -- | -- | 1.5 | 1.2 | 1.8 | -- | -- | 1.0 | 0.7 | 1.2 | |
denotes significant difference in rates between the 1992 and 2001 African American cohorts.
Overall rates were standardized using the age distribution of 1990 United States census data for African Americans in Marion County.
Figure 1.
Estimated annual incidence rates of dementia and Alzheimer’s disease (AD) in African Americans and Yoruba
Results of the sensitivity analyses were presented in Supplemental Tables. Estimated dementia incidence rates from two sub-cohorts in the 1992 cohorts with shortened length of follow-up were similar to those estimated in the original 1992 cohorts in both populations (Table 1s). Results from simulation studies indicate that even if we assume that all participants who refused to enroll in the 2001 cohort had the same dementia rate as those in the 1992 cohort, we would still observe significantly lower dementia incidence rate than in the 1992 cohort. In addition, we estimate that for the 2001 African American cohort to have the same dementia incidence rate as observed in the 1992 cohort, potential participants who refused to enroll at 2001 would have to have 48% higher dementia incidence rate than those in the 1992 cohort.
Discussion
In this prospective community-based study of two populations, we found that dementia and AD incidence rates were significantly lower in African Americans enrolled in 2001 than in the cohort enrolled in 1992. However, in the Yoruba cohorts, we found that dementia and AD incidence rates were similar between the 1992 and 2001 cohorts.
There have been reports of decreasing dementia or AD prevalence rates in the United States1–3 or European populations.4 However, since there were also reports of increased mortality in patients diagnosed with cognitive impairment,1 disease incidence is a more appropriate measure for determining temporal changes in disease risk over time. Studies comparing dementia incidence over the past two decades have not been entirely consistent. A community-based study of participants age 65 or older in a biracial, geographically defined area in Chicago with up to 11 years of follow-up found no change in the risk of incident AD.5 A study using Medicare inpatient records from 1984–2001 linked to the National Long-Term Care Survey reported increased age-adjusted rates of incident dementia and AD from 1984–1990 to 1991–2000.21 A study in an elderly urban population in Beijing, China, found higher dementia incidence rates in a cohort assembled in 1997 compared to incidence from a historic cohort assembled 10 years earlier.6 However, the Rotterdam Study, a population-based study in the Netherlands, compared dementia incidence rates in a cohort assembled in 1990 to a second cohort in 2000 and found that age-adjusted dementia incidence rates were consistently, but not significantly, lower in the 2000 cohort with borderline significance in the overall analysis (incidence rate ratio 0.75, 95% confidence interval [CI]: 0.56–1.02).6,7 Our study is the first to demonstrate significantly decreased dementia and AD incidence rates in an African American cohort from one enrolled a decade earlier.
Two other studies had reported incidence rates of dementia or AD in African American cohorts assembled during the early 1990’s. The Northern Manhattan study reported an overall AD incidence rate of 3.0% (95% CI: 2.5–3.5%) in 610 African American participants age 65 or older followed from 1992 to 1999.22 The Cardiovascular Health Study reported an overall dementia incidence rates of 3.1% (95% CI: 2.4–3.8%) in 492 African Americans age 65 or older with baseline between 1992–1994 who were followed for an average of 5.4 years.23 The incident rates from both studies were similar to what we found in our 1992 African American cohort. We could not identify any studies with enrollment in the 2000’s reporting dementia incidence in African Americans to compare with our results in the 2001 African American cohort.
The reason for the lower dementia incidence in the 2001 African American cohort is not yet entirely clear. We had previously found no differences in the prevalence of dementia or AD for African Americans living in Indianapolis between the 1992 and 2001 cohorts.24 It is worth noting that African Americans in the 2001 cohort had significantly higher rates of medical conditions including diabetes, hypertension and stroke, but they also had significantly higher treatment rates than those in the 1992 cohort after adjusting for the differences in the underlying conditions. These differences are consistent with national trends for African Americans over this period.25 It is possible that medications for cardiovascular conditions contributed to the reduced dementia incidence rates despite of the higher rates for these conditions.
Despite of our effort in employing identical assessment procedures in all cohorts over time, it is likely that African Americans in the 2001 cohort are different in composition from the 1992 cohort. The 2001 cohort was assembled using the Medicare enrollee list using telephone as the initial recruitment contact which resulted in much higher refusal rates than the 1992 cohort. It is possible that healthier participants were enrolled in 2001. Nevertheless, we found no significant difference in baseline cognitive scores, APOE genotype distributions, and the severity of diagnosed dementia cases between the 1992 and 2001 African American cohorts. Those in the 2001 cohort actually had higher comorbidities than African Americans in the 1992 cohort. Even accounting for potential higher dementia rates in the refused subjects, our simulation study still found significantly lower dementia rate in the 2001 cohort (see supplementary online material). We estimate that potential participants who refused to enroll at 2001 would have to have 48% higher dementia incidence rate than those in the 1992 cohort for the 2001 African American cohort to have the same rate as in the 1992 cohort. Thus the lower incidence rates in the 2001 cohort were unlikely due to the differences in cohort composition.
Using identical assessment procedure and follow-up schedules, we also found no change in dementia or AD incidence rates in the Yoruba over the last two decades. Our report of trends in incident dementia or AD is the first one conducted in an African population. It is interesting to observe a significant decline in dementia incidence in the African American population, but similar incidence rates in the Yoruba population for every age group between the 1992 and 2001 cohorts. Self-reported cardiovascular disease rates in the Yoruba remained low in both the 1992 and 2001 cohorts and they were lower than those reported in developed countries, although it is not clear whether the lower disease rates were due to detection bias or true disease rates in the Yoruba. A potential explanation for the lower rates of self-reported cardiovascular disease in Yoruba than in the African Americans is survivor bias, as it is hypothesized that survivors of difficult child and adulthood are likely to be more resilient to diseases later in life. However, we will not be able to test this hypothesis due to the lack of early life data in our cohorts.
Our study has the strength of long follow-ups in large dynamic cohorts of both African Americans and Yoruba. Identical diagnostic criteria were used throughout the entire study period in both populations, ensuring diagnostic consistency. In addition, random sampling was used for the selection of clinical assessment, thus permitting the use of weighted models to appropriately adjust for the sampling schemes and for the use of predicted probability of transitions.26
However, our study also has limitations. The study included only African American participants or Yoruba living in Ibadan, thus it is not known whether the rates reported here are generalizable to other elderly populations. Since not all study participants received the extensive clinical assessment at the second stage, our estimated incidence rates may not be as accurate as they would be if every participant had received the extensive clinical assessment. Nevertheless, the two stage sampling design and our model-based approach have been used in many epidemiologic studies to estimate both prevalence and incidence rates.8,10,27 This approach has been shown to provide unbiased and accurate estimates under various assumptions.26
Our rate estimates could also be affected by selection biases from participants’ refusal to enroll in the study, in particular, the relatively high refusal rate in the 2001 African American cohort. We had previously reported comparisons made between those who enrolled and those who did not at 2001 using information from the Medicare rolls.9 Those who were not enrolled were significantly older (mean age, 79.06 years; SD, 7.42), compared with those who did enroll (mean age, 76.81 years; SD, 5.58; P <0.0001). A higher proportion of women enrolled (64.75%) compared with the unenrolled group (59.63%; P=0.0006). No other information was available from the Medicare rolls. Despite differences in enrollment strategies, our previous analyses found no differences in prevalence rates of dementia or AD between the 1992 and 2001 African American cohorts.9 However, selection bias based upon differential refusal rates cannot be entirely ruled out. Another source of potential selection bias comes from refusals to the second stage clinical assessment. Our estimation provides unbiased rate estimates even if those participants who refused to enroll differed in age, gender, education, or cognitive performance from those evaluated. However, potential bias may be introduced if the two groups differed on other characteristics not controlled in the logistic models.
In summary, using similar study design and procedures, we found a decline in dementia and AD incidence rates in the African American cohort enrolled in 2001 compared to those enrolled in 1992, but almost identical incidence rates between the Yoruba 1992 and 2001cohorts. The reasons for this differential decline in incidence rates in the 2 populations are unclear at this time and need to be further explored in future research.
Supplementary Material
Acknowledgments
The research is supported by NIH grants R01 AG09956, R01 AG019181, and P30 AG10133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No disclosures to report.
Statistical analyses were completed by Sujuan Gao and Kathleen A. Lane from the Department of Biostatistics, Indiana University School of Medicine.
The authors have no conflict of interest with the content of this manuscript.
References
- 1.Langa KM, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manton KC, Gu XL, Ukraintseva SV. Declining prevalence of dementia in the U.S. elderly population. Advances in gerontology = Uspekhi gerontologii / Rossiiskaia akademiia nauk, Gerontologicheskoe obshchestvo. 2005;16:30–37. [PubMed] [Google Scholar]
- 3.Sheffield KM, Peek MK. Changes in the prevalence of cognitive impairment among older Americans, 1993–2004: overall trends and differences by race/ethnicity. Am J Epidemiol. 2011;174:274–283. doi: 10.1093/aje/kwr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews FE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert LE, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, et al. Is the dementia rate increasing in Beijing? Prevalence and incidence of dementia 10 years later in an urban elderly population. Acta Psychiatr Scand. 2007;115:73–79. doi: 10.1111/j.1600-0447.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 7.Schrijvers EM, et al. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–1463. doi: 10.1212/WNL.0b013e3182553be6. [DOI] [PubMed] [Google Scholar]
- 8.Hendrie HC, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 9.Hall KS, et al. Prevalence rates for dementia and Alzheimer's disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5:227–233. doi: 10.1016/j.jalz.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrie HC, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 11.Hall KS, et al. A cross-cultural community based study of dementias: methods and performance of the survey instrument: Indianapolis, U.S.A. and Ibadan, Nigeria. Int J Methods Psychiatr Res. 1996;6:129–142. [Google Scholar]
- 12.Hall KS, et al. Community screening interview for dementia (CSI 'D'); performance in five disparate study sites. Int J Geriatr Psychiatry. 2000;15:521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 14.Hendrie HC, et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. Int Psychogeriatr. 2006;18:653–666. doi: 10.1017/S104161020500308X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrie HC, et al. The CAMDEX: a standardized instrument for the diagnosis of mental disorder in the elderly: a replication with a US sample. J Am Geriatr Soc. 1988;36:402–408. doi: 10.1111/j.1532-5415.1988.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 16.Roth M, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Assocation; 1987. Rev. [Google Scholar]
- 18.American Psychiatric Association Press. ICD-10. The International Statistical Classification of Diseases and Related Health Problems: 1 and 2. Report No.: V.3. 1992 [Google Scholar]
- 19.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Johnson NL, Kotz S, Kemp AW. Univariate Discrete Distributions. New York: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 21.Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing rates of dementia at time of declining mortality from stroke. Stroke. 2006;37:1155–1159. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- 22.Tang MX, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick AL, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 24.Rocca WA, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health care use and expenditures—personal health care expenditures of Medicare beneficiaries by chronic conditions and type of service, ages 65+: US, 1992–2005 (Source: MCBS) Available at http://www.cdc.gov/nchs/hdi.htm.
- 26.Beckett LA, Scherr PA, Evans DA. Population prevalence estimates from complex samples. J Clin Epidemiol. 1992;45:393–402. doi: 10.1016/0895-4356(92)90040-t. [DOI] [PubMed] [Google Scholar]
- 27.Evans DA, et al. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.